Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Koji Miyabayashi and Version 4 by Koji Miyabayashi.
The microbiome is now known to be associated with cancer development and progression in many types of cancer including pancreatic ductal adenocarcinoma (PDAC). Many observational studies have revealed the association of the oral, gut, and intratumor microbiome with human PDAC. The microbiome may affect the composition of tumor microenvironment via the immune response and generate an immunosuppressive environment. The microbiome could be a biomarker for the prediction of an immunogenic tumor microenvironment and immune-targeted therapies.
- pancreatic ductal adenocarcinoma (PDAC)
- microbiome
- tumor microenvironment
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a deadly cancer worldwide, and it has a five-year survival rate of less than 9% for all the stages combined [1]. More than half of PDAC patients are diagnosed as inoperable with metastatic diseases or advanced diseases. PDAC frequently recurs even after resection, and chemotherapies are frequently ineffective. Early diagnosis methods and new therapeutic strategies are needed.
According to the development of the genetic and molecular characterization of PDAC, tailored therapies have emerged. Recent studies have suggested that up to 25% of PDACs have actionable genetic mutations [2] and three subgroups of PDAC patients are considered to be possibly targeted by tailored therapies. Patients with gene alterations of homologous recombination deficiency (HRD), such as BRCA1 and BRCA2 mutations, benefit from platinum-based therapy and poly (ADP-ribose) polymerase (PARP) inhibitors [3][4][5][6][7][8][3,4,5,6,7,8]. Patients with mismatch repair deficiency (MMR-D), including high microsatellite instability (MSI-H) and high tumor gene mutation burden (TMB-H), can be targeted by immune checkpoint blockade (ICB) therapies [2][9][2,9]. Patients with wild-type KRAS (KRASWT) often have alternative oncogenic mutations, such as BRAF [3][5][3,5], and may be candidates for small-molecule therapies.
Although ICB is a promising therapy in many types of cancers as well as PDAC, biomarkers for the efficacy of treatment are still unknown in PDAC. Clinical trials showed that MMR-D patients in PDAC are resistant to ICB therapies compared to other types of cancer [2][9][2,9]. PDAC is characterized by a dense stromal component that interacts with cancer cells and serves as a tumor-supportive environment [10]. Tumor-infiltrating T cells play an important role in eliminating tumor cells, and these components are regulated by other types of cells in tumor microenvironment (TME) such as fibroblasts, macrophages, and dendritic cells [11]. Cancer cells orchestrate stromal cells to create an immunosuppressive environment that is favorable to cancer cells. Dense stroma creating an immunosuppressive TME is one of the reasons for the complexity of chemoresistance in PDAC. Intrinsic factors in PDAC cells as well as extrinsic factors in non-cancer cells are associated with the formation of immunosuppressive TME. A comprehensive analysis of PDAC using multi-omics studies, including the microbiome, would contribute to the understanding of complexed TME and the establishment of new therapeutic strategies.
The microbiome is now known to be associated with cancer development and progression in many types of cancer [12]. Several studies have revealed the association between PDAC progression and the oral, gut, and intratumor microbiomes, although the identified bacteria differ between reports [13]. These reports have commonly reported that high microbial diversity is associated with favorable outcomes. Bacteria are thought to migrate from the gut to the pancreas, and a recent report has suggested that the gut microbiota modifies the overall microbiome of tumors [14][15][14,15]. Regarding the early detection of PDAC, evidence is accumulating to suggest that the microbiome is associated with premalignant diseases of the pancreas, such as chronic pancreatitis (CP) and intraductal papillary mucinous neoplasia (IPMN) [16][17][16,17]. Although further studies are needed to understand whether the differences in the microbiome in pancreatic precursors of PDAC are a cause or a consequence, the microbiome has a potential role in the early detection of PDAC. Regarding the treatment of PDAC, intratumor CD8 + T cell infiltration may play an important role in microbiome-associated immune modification [15]. The microbiome can be a biomarker of the efficacy of ICB therapies. Furthermore, antibiotic treatment may provide new options to modify the efficacy of chemotherapies as well as ICB therapies.
2. Mechanisms of Role of Microbiomes in PDAC
2.1. Association of Microbiomes with Molecular Subtypes of Cancer Cell
In recent years, intense genomic analyses have been performed to reveal the mutational landscape of PDAC [18][19][20][21][55,56,57,58]. The frequently reported genetic mutations are concentrated in core signaling pathways including KRAS, WNT, NOTCH, DNA damage repair, RNA processing, cell-cycle regulation, transforming growth factor beta (TGF-β) signaling, switch or sucrose non-fermentable (SWI/SNF), chromatin regulation, and axonal guidance [18][19][20][21][55,56,57,58]. Recent comprehensive sequencing analysis elucidated transcriptional molecular subtypes of the cancer cells of PDAC including basal-like or squamous and classical or progenitor subtypes. Basal-like or squamous tumors are associated with poor outcomes and treatment resistance compared to classical or progenitor subtypes [21][22][23][24][25][26][27][28][29][30][58,59,60,61,62,63,64,65,66,67]. In addition to genome-based precision medicine, tailored therapies based on transcriptomic subtypes have emerged. Recent clinical trials have revealed that basal-like tumors are resistant to FOLFIRINOX-based therapies [30][31][67,68]. These results were supported by a study using patient-derived organoids (PDOs) by Tiriac et al. [32][69], who showed that chemotherapy signatures based on PDO could predict the treatment response in PDAC patients. Although the underlying mechanisms of this chemoresistance in basal-like tumors are still unknown, subgroups in basal-like subtypes characterized by the activation of KRAS, MYC, ∆N isoform of TP63 (∆Np63), and GLI2 [21][23][24][33][34][35][36][37][38][58,60,61,70,71,72,73,74,75] may be the key to solving the problem. An association between the molecular subtypes and microbiome was recently reported, identifying a high abundance of Acinetobacter, Pseudomonas, and Sphingopyxis in basal-like human PDAC, and the analysis of microbial genes suggested the potential of the microbiome in inducing pathogen-related inflammation [39][76].2.2. Role of Microbiomes in TME
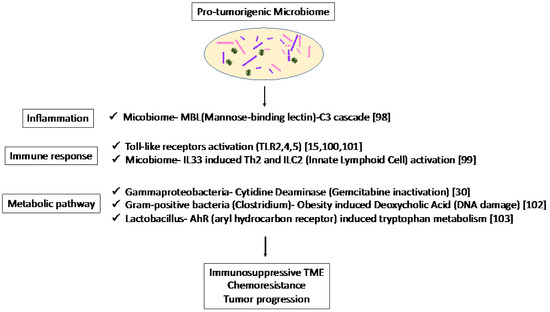
In recent studies, single-cell RNA sequencing has been used to reveal the heterogeneity of stromal components [40][41][42][43][51,52,53,54]. CAFs play an important role in the regulation of the TME, and it has been reported that cancer-derived IL-1 or TGF-β can differentiate surrounding fibroblasts into inflammatory and myofibroblastic CAFs, respectively [41][52]. IL-6 secreted by inflammatory CAFs promote tumor growth, while myofibroblastic CAFs produce surrounding stroma. Since cancer cells create a microenvironment favorable to themselves, these stromal subtypes are related to the cancer-cell subtypes described above. Maurer et al. [26][63] reported CAF subtypes by RNA sequencing separately harvested PDAC epithelium and adjacent stroma using laser capture microdissection. The researcheuthors identified two subtypes that reflect ECM deposition and remodeling (ECM-rich) versus immune-related processes (immune-rich). ECM-rich stroma was strongly associated with basal-like tumors, while immune-rich stroma was more frequently associated with classical tumors [26][44][63,77]. Thus, the cancer cell subtypes and stromal subtypes were partially related, suggesting that they can be potential biomarkers for therapies targeting stroma in PDAC. Dense stroma with desmoplastic reaction may act as a physical barrier and affect the infiltration of MDSCs and T cells in TME [45][46][78,79]. In addition, PDAC shows substantial immunological heterogeneity influencing T-cell infiltration [47][48][49][50][51][52][53][80,81,82,83,84,85,86], the level of T-cell infiltration is important in predicting the efficacy of ICB therapies, and patients with MSI-H tumors show abundant TILs and sensitivity to immune-targeted therapies [54][55][56][57][58][59][60][87,88,89,90,91,92,93]. Studies in mouse models have revealed potential targets, such as colony-stimulating factor 1 receptor, cytotoxic T-lymphocyte-associated protein 4 [61][62][94,95], and CXC chemokine receptor 2 [63][64][96,97] in combination with ICB, which have been tested in clinical trials. These results suggest that both the quality and quantity of CD8 + T cells in tumors are important in predicting the efficacy of immunotherapy, and that new biomarkers are needed to predict the status of infiltrating CD8 + T cells in tumors. As an association between microbiome and CAF subtypes was not clear, the microbiome was associated with the inflammatory and immunosuppressive TME in mice. The association of the mycobiome with the complement system has been reported [65][98]. The mycobiome promoted tumor growth due to mannose-binding lectin(MBL)–C3 cascade in a genetically engineered mouse model (GEMM) [65][98]. The complement system is an important component of the inflammatory response, which is involved in tumorigenesis and the adaptive immune response, which modulates T cell activation. The mycobiome has also been reported to enhance oncogenic KrasG12D-induced IL-33 secretion from PDAC and activates TH2 and ILC2 cells, which contribute to tumor progression using GEMM [66][99]. Anti-IL-33 or anti-fungal treatment decreases TH2 and ILC2 infiltration and increases the survival in GEMM. Microbiota-induced activation of toll-like receptors (TLRs), especially TLR9, activate pancreatic cancer stellate cells and attract immunosuppressive T regulatory cells and MDSCs to the tumor environment, which contribute to the suppression of innate and adaptive immunity in PDAC progression in mice [67][100]. Lipopolysaccharide and TLR4 ligation induce a dendritic-cell-dependent immune response in the pancreas and increase pancreatic tumorigenesis, where Myd88 inhibition induced fibroinflammation via dendritic cells andTh2-derived CD4 T cells [68][101]. In addition, microbiota-mediated TLR2 and TLR5 ligation alters macrophages into an immunosuppressive phenotype and suppresses the T-cell-mediated antitumor immune response in mice [15]. Furthermore, microbial metabolism and metabolites can alter the TME. Obesity alters the gut microbiota and increases the level of the microbial metabolite deoxycholic acid (DCA), which induces DNA damage in obesity-associated hepatocellular carcinoma development in mice [69][102]. This metabolite may also be a risk factor for obesity-induced PDAC. Hezaveh et al. [70][103] showed that the aryl hydrocarbon receptor (AhR), which is a sensor of products of tryptophan metabolism, modulates immunity due to tumor-associated macrophage (TAM) function in murine PDAC. TAMs AhR activity was dependent on Lactobacillus metabolization of dietary tryptophan to indoles. Inhibition of AhR in myeloid cells reduced PDAC growth due to increased infiltration of IFNγ + CD8 + T cells in murine PDAC tumor [70][103]. Moreover, cytidine deaminase, an enzyme expressed by many bacteria, converts active gemcitabine into an inactive metabolite in colon cancer mouse models. Gamma Proteobacteria are reported to present in PDAC tumors and induce resistance to gemcitabine via cytidine deaminase [71][30]. Therefore, when antibiotics are used to reduce the bacteria, resistance to gemcitabine is eliminated. Thus, microbiome-based therapy may be useful not only for the suppression of carcinogenesis but also for preventing resistance to treatment. Thus, the microbiome plays a pro-tumorigenic role via inflammation, immune response, and metabolic pathways (Figure 12). These results suggest the potential of the microbiome as a biomarker in immunotherapy and microbiome-targeted therapies.
Figure 12.
Specific microbiota and associated metabolic and biochemical pathways.
