Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Chaiwat Aneklaphakij and Version 2 by Beatrix Zheng.
Stilbenoids are well-known phytoalexins in the group of polyphenolic compounds. Because of their potent bioactivities, including antioxidant, antityrosinase, photoprotective, and antibacterial activities, stilbenoids are utilized as pharmaceutical active ingredient in cosmetic products. Thus, the demand for stilbenoids in the cosmetic industry is increasing.
- bioactivity
- resveratrol
- oxyresveratrol
- biosynthesis
- polyphenols
1. Introduction
Cosmetic ingredients are generally originated from both chemical synthesis and natural sources [1][2][3][4][1,2,3,4]. Synthetic and/or semi-synthetic polymers are clear examples of chemical substances employed in cosmetics to prolong the release, improve delivery systems of each specific molecule to the target site of action, and decrease the evaporation rate of volatilizable formulations [1]. In some cases, the interaction between polymers and other compounds in the formulation can lead to hazardous effects on human health [1]. Moreover, some chemicals can act as pollutants that harm the environment, for example, parabens, a well-known preservative, from the factory contaminate the air, dust, soil, and water [5]. Thus, the cosmetic agents from nature such as plants, seaweed (macroalgae), ferns, animals, and marine creatures are preferred, and also attract customers [4][6][7][8][9][4,6,7,8,9]. Plants are renowned as enormous sources of pharmaceutical active ingredients since they accumulate diverse metabolites and show numerous biological activities.
Stilbenoids are one of the famous classes of the phyto-polyphenols [10]. The use of phytochemicals in cosmetic products is widespread because of their several prominent bioactivities [11][12][11,12]. For cosmetic purposes, several outstanding bioactivities of stilbenoids have been reported and have attracted the interest of customers, including antioxidant and anti-inflammatory activities, antityrosinase activity (depigmentation), antimicrobial activity, and photoprotective effect, i.e., ultraviolet (UV) protection [10][13][14][15][10,13,14,15]. Remarkably, stilbenoids are conventionally extracted from many plant species by several types of organic solvents such as methanol, ethanol, and acetone [16][17][16,17]. Nevertheless, these are not appropriate for the cosmetic industry since the procedures of extraction and purification are multifaceted, requiring high technical skills, the contamination of residual solvent, and are expensive [18]. Furthermore, the yield fluctuates greatly depending on the quality of raw material, geography, temperature, humidity, rainfall, soil type and season [19]. Although the solvent is intentionally removed from the extract, residual solvents may remain in the extract because of incomplete evaporation processes. Thus, the residual solvents should be identified since they may potentially deteriorate human health. In the United States Pharmacopeia, the classification by risk assessment, limitation, identification, quantification, analytical procedures, and control strategy of residual solvents are described under the topic no. 467. The harvest of plant materials, especially perennial trees, directly from the natural resources is an unsustainable approach and also affects the ecosystem. Furthermore, direct cultivation of plants is not appropriate for the cosmetic industry because of its high cost, and because it is time-consuming and laborious work. Hence, other alternative methods for the extraction and sustainable production of stilbenoids are necessary to be considered for utilization in the future of the cosmetic industry.
2. Stilbenoids
Plants naturally produce chemical compounds called “plant-specialized (secondary) metabolites” to survive and protect themselves from abiotic and biotic stresses [20][21][20,21]. The phytochemical compounds are generally classified into three main groups based on their chemical structures and biosynthesis, including alkaloids, terpenoids, and polyphenols [20]. Polyphenols are abundantly present in daily diets, in foods such as vegetables, fruits, and nuts, and have been associated with health-promoting benefits [20]. The typical chemical structure of polyphenols consists of more than one hydroxyl group which are bound to one or more aromatic ring systems [22]. The most commonly known polyphenols are phenolic acids, flavonoids, tannins, lignans, coumarins, and stilbenoids [20]. Here, three compounds in stilbenoids containing resveratrol, oxyresveratrol, and piceatannol are emphasized and reviewed because of their outstanding bioactivities and high possibilities for utilization in cosmeceuticals.
2.1. Chemical Structures and Biosynthesis
The core structure of stilbene compounds comprises two aromatic rings connected with an ethylene bridge (C6–C2–C6 backbone) and is commonly found as monomers and oligomers in both aglycone and glycoside forms [20]. Phenylalanine and tyrosine are the amino acid precursors for stilbenoids biosynthesis in the phenylpropanoid pathway, although the chemical reactions occur differently [23]. Phenylalanine ammonia lyase (PAL) converts phenylalanine into trans-cinnamic acid and ammonia as by-products, then, cinnamate-4-hydroxylase (C4H) catalyzed trans-cinnamic acid to produce p-coumaric acid [23]. In addition, p-coumaric acid is also synthesized from tyrosine by tyrosine ammonia lyase (TAL) [23]. Hence, p-coumaric acid synthesis from tyrosine requires one step less than phenylalanine. Next, p-coumaric acid is the substrate for 4-coumarate:coenzyme A (CoA) ligase (4CL) to generate p-coumaroyl-CoA [23]. The p-coumaroyl-CoA is the most important intermediate compound for flavonoids and stilbenoids biosynthesis. In the case of stilbenoids, stilbene synthase (STS) or resveratrol synthase, the key enzyme for stilbenoids synthesis, and three molecules of malonyl-CoA are coupled with p-coumaroyl-CoA to yield resveratrol by the aldol reaction [23].
Resveratrol (3,4′,5-trihydroxystilbene) is the parent compound for other derivatives such as hydroxylated, methylated, and prenylated derivatives. Here, thwe researchers focused on two hydroxylated derivatives, i.e., oxyresveratrol (2,3’,4,5’-tetrahydroxystilbene) and piceatannol (3,5,3′,4′-tetrahydroxystilbene), since these compounds have several potential bioactivities, and may be possibly used in the cosmetic industry. An overview of the biosynthesis and chemical structures of stilbenoids is shown in Figure 1.
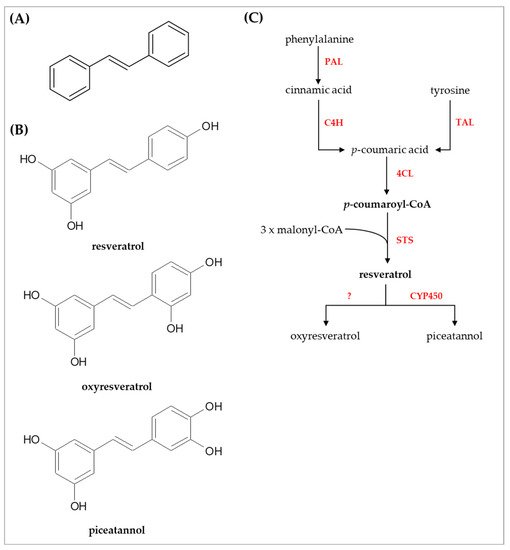
Figure 1. Chemical structures and biosynthesis of stilbenoids. (A) core structure of stilbenoids (B) chemical structures of resveratrol, oxyresveratrol, and piceatannol (C) overview of the biosynthetic pathway of stilbenoids. Abbreviations used: PAL, phenylalanine ammonia lyase; TAL, tyrosine ammonia lyase; C4H, cinnamate-4-hydroxylase; 4CL, 4-coumarate-CoA ligase; STS, stilbene synthase or resveratrol synthase; CYP450, cytochrome P450.
2.2. Plant Sources
To discover current plant sources of resveratrol, oxyresveratrol, and piceatannol, a search was conducted in the well-known phytochemical database “KNApSAcK: A Comprehensive Species-Metabolite Relationship Database”, by chemical names (http://www.knapsackfamily.com/KNApSAcK/ accessed on 5 July 2022 [24]).
It is known that stilbenoids are found ubiquitously in several plant species; however, only some of them are mentioned and discussed in this resviearchw based on quantity in the plant extract, known plant species, and potential application as a raw material for stilbenoids production.
Resveratrol was first discovered in the root of V. grandiflorum O. Loes. (white hellebore) and then detected in more than seventy plant species [25][26][25,26]. Grapes, mulberries, and peanuts are well-recognized as rich sources of resveratrol. Fruits of grapes (V. vinifera L.), especially a part of the skin and seeds contain 3.66 × 10−2 g/kg of resveratrol [27]. Numerous parts of mulberries (M. alba L.), i.e., root, fruit, aerial part, and leaves, are comprised of resveratrol in the range from 1.6 × 10−3 to 7.95 × 10−3 g/kg [27]. Resveratrol is found in peanut (A. hypogaea L.) stem and fruit from 1.1 × 10−2 to 1.5 × 10−2 g/kg. In addition, resveratrol is also detected in peanut skin and other nut species, such as whole almond seeds and pistachio kernel [20]. Apart from the KNApSAcK database, a list of plant sources containing resveratrol was also compiled by Tian and Liu in 2020 [27].
Oxyresveratrol is believed to be generated by hydroxylation at the C-2 position of resveratrol, although there is no reliable evidence to support this hypothesis [13]. Additionally, the enzyme involved in hydroxylation is still unknown (Figure 1). Mostly, oxyresveratrol is the major compound of plant species in the Moraceae family. The dried aqueous extract (so-called “Puag-Haad”) of heartwood extract of a well-known Thai medicinal plant named “Mahat” (A. lacucha Buch.-Ham.) is comprised of approximately 80% w/w oxyresveratrol [28][29][30][28,29,30]. Another source of oxyresveratrol is mulberries (M. alba L.). Oxyresveratrol is detected in the root, stem, and twig of mulberries [31][32][33][31,32,33]. In 2021, Likhitwitayawuid also well-summarized both gymnosperms and angiosperms which consist of oxyresveratrol [13].
Piceatannol is another hydroxylated derivative of resveratrol. It is formed by cytochrome P450 (CYP450) metabolism by adding a hydroxyl group to the C-3’ position [34]. Piceatannol is also found in grapes, passion fruits, and blueberries. Both red and white grapes contain piceatannol but in different quantities [34]. Piceatannol is approximately nine times more accumulated in red than white grapes (red grapes: 374 ng/g; white grapes 43 ng/g) [34][35][34,35]. Passion fruit seeds are very rich in piceatannol, containing 4.8 mg/g [34][36][34,36]. The amount of piceatannol in blueberries is reported as 138–422 ng/g at dry concentration [34][37][34,37].
2.3. Bioactivities
As mentioned above, stilbenoids show various bioactivities that are beneficial to human health. In cosmeceuticals, five bioactivities, i.e., antioxidant, anti-aging—either as photoprotective or in terms of autophagy—MMP inhibitory, antityrosinase, and antibacterial activities are mainly recognized to produce high-quality cosmetic products. Here, thwe researchers ssummarized the bioactivities of resveratrol, oxyresveratrol, and piceatannol as follows.
Recently, stilbenoids have been promoted not only as a potential antioxidant, but also for skin aging protection. Skin aging is a complex physiological and pathological process, including a series of continuous changes, which leads to wrinkles, loss of elasticity, laxity, and rough-textured appearance [38]. It is caused by both intrinsic and extrinsic factors. Exposure to UV radiation is the primary factor of extrinsic skin aging by stimulating the generation and accumulation of reactive oxygen species (ROS), impairing the skin’s antioxidant status, which causes damage to deoxyribonucleic acid (DNA), and proteins that lead to photocarcinogenesis and photoaging [39].
Resveratrol has been described as a potent antioxidant [40]. The capacity of antioxidant activity, including free radical scavenging and metal ion chelation, of resveratrol depends on the position and number of hydroxyl groups in the chemical structure [10]. Based on this activity, resveratrol is reported to protect cells from UV irradiation-induced cell death, and contains photoprotective effects [10][25][10,25]. Besides, resveratrol promotes the activity of antioxidant enzymes in the skin, i.e., glutathione S-transferase (GST), and superoxide dismutase (SOD). This activity leads to the reduction of superoxide ion production from UV-A and UV-B irradiation as well as lipid peroxidation activity [14]. Oxyresveratrol is also claimed as a potent antioxidant based on several reported models of antioxidant testing, such as DPPH (1,1-diphenyl-2-picrylhydrazyl radical), superoxide anion, hydroxyl radical, 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) radical, etc. [13][41][13,41]. Combinations of resveratrol and oxyresveratrol result in the synergism of antioxidant activities [42]. Free radical scavenging of piceatannol has been described as it obviously reduces intracellular reactive oxygen species (ROS) levels in human keratinocytes (HaCat) cells irradiated by UV-B. Moreover, oxyresveratrol and piceatannol mostly contain antioxidant activity stronger than that of resveratrol due to an additional hydroxyl group [10][13][43][44][10,13,43,44]. However, resveratrol exhibits stronger inhibition of peroxy oxygen radical absorbance capacity (ORAC), lipopolysaccharide (LPS)-induced production of nitric oxide (NO) in murine BV-2 microglial cells, and cyclooxygenase 1 and 2 (COX-1, COX-2) than oxyresveratrol [13].
To date, the matrix metalloproteinase (MMP) inhibitory effect of stilbenoids is interesting to investigate for its known anti-aging effects. The MMPs are induced by extrinsic factors such as UV irradiation, inflammation, or toxins. Members of the MMP group show an important role in the degradation of corneocyte desmosomes (collagen and elastin) in the extracellular matrix of skin, such as MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, and MMP-13 [45]. This degradation results in the induction of wrinkle formation [46][47][46,47]. Among them, MMP-1 (collagenase) plays a major role in the specific degradation of collagen types I and III during the aging process of the human dermis, suggesting that inhibition of MMP-1 induction reduces UV-induced photoaging [48][49][48,49]. Meanwhile some MMPs are known to be involved in the degradation of elastin resulting in skin aging [50]. Resveratrol, oxyresveratrol, and its acetylated derivatives show markedly strong inhibition of UV-B-induced MMP-1 activity and expression in human dermal fibroblast cells, indicating that stilbenoids can prevent the degradation of collagens [51]. Moreover, it has been found that a reduction of UV-B-induced MMP-1 expression was inhibited via mitogen-activated protein kinases (MAPKs) and Akt/mammalian targeting of rapamycin (mTOR) signaling pathways. Chuang et al. demonstrated that autophagy is normally induced by the inhibition of the mTOR signaling pathway, which contributes to preventing the aging process [52]. There are fewer studies on anti-aging in terms of autophagy, although it has been found that resveratrol can enhance autophagy, which may be able to suppress oxidative stress and thus greatly to improve the aging process [53][54][53,54].
Resveratrol is able to inhibit melanogenesis causing skin-whitening effects or depigmentation via several mechanisms [14]. Melanogenesis is the process of melanin synthesis that utilizes L-tyrosine as a precursor [13]. Tyrosinase is the crucial enzyme for the conversion of L-tyrosine to L-DOPA (L-3,4-dihydroxyphenylalanine), dopaquinone, followed by cyclization, oxidation, and polymerization, until finally melanin is produced [13]. The metabolite from the biotransformation of resveratrol by tyrosinase inhibits dopa oxidase activity and competes with tyrosine and L-DOPA as a substrate for melanogenesis [10][14][25][10,14,25]. In addition, resveratrol diminishes gene expression of melanogenesis-related proteins such as tyrosinase-related protein (TRYP) 1, TRYP2, and microphthalmia-associated transcription factor (MITF) in melanoma cells [10][14][25][10,14,25]. Oxyresveratrol shows potent tyrosinase inhibitory effects after testing with several methods, as concluded by Likhitwitayawuid [13]. Oxyresveratrol is a prominent compound for skin-whitening since its activity in this respect is obviously higher than that of resveratrol [13]. The anti-tyrosinase activity of piceatannol has also been studied, and its activity has been shown as stronger than that of resveratrol [43][55][43,55]. The mechanisms of action of piceatannol are the reduction of ROS and increasing the glutathione/oxidized glutathione ratio [55]. In addition, molecular targets of stilbenoids in skin cell lines are presented in Table 12.
Table 12. Studies of molecular targets of stilbenoids in skin cells.
| Compounds | Biological Activities | Molecular Mechanism | Type of Cell Cultures | References |
|---|---|---|---|---|
| Resveratrol | antioxidative stress | ↑ GST and SOD activities | HaCat | [56] |
| MMP inhibition | ↓phosphorylation of MAPKs and Akt/mTOR signaling pathways | HDF | [51] | |
| antioxidant | ↑ SOD, and GSH-Px activities; ↓ lipid peroxidation | HaCat | [57] | |
| anti-tyrosinase | ↓ melanin pigmentation | B16 F10 melanoma cells | [58] | |
| Oxyresveratrol | suppression of UV-B-induced MMP-1 | ↓phosphorylation of MAPKs and Akt/mTOR signaling pathways | HDF | [51] |
| Piceatannol | antioxidant | ↑ GSH activity; ↓ intracellular ROS level | HaCat | [59] |
Abbreviation: ROS, Reactive Oxygen Species; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; GSH, Glutathione; GST, Glutathione S-transferase; Akt/mTOR, protein kinase B/mammalian target of rapamycin; MAPKs, mitogen-activated protein kinases; HaCat, human keratinocyte cell; HDF, human dermal fibroblast cells; B16 F10 melanoma cells, murine melanoma cell; ↑, increase; ↓: decrease.
Resveratrol has antimicrobial activity against numerous types of microorganisms [15][26][15,26]. One of the most concerning dermatological diseases is acne vulgaris. This disease is caused not only by sebum overproduction, hyperkeratosis of the hair follicles (epidermal hyperproliferation), but also the growth of Cutibacterium acnes (formerly Propionibacterium acnes) [25][60][25,60]. Resveratrol contains antibacterial activity against C. acnes and also decreases sebum production. The potency of resveratrol is comparable to conventional anti-C. acnes drug i.e., benzyl peroxide, as well as having none of its cytotoxicity [25][60][25,60]. In addition, resveratrol is reported to have a higher inhibitory effect on quorum-sensing of Chromobacterium violaceum, which is the motile gram-negative bacillus, than oxyresveratrol [61][62][61,62]. Piceatannol relieves C. acnes-induced HaCaT cell proliferation and migration via its antioxidant and anti-inflammatory activities [63]. Until now, there has been no study on the anti-C. acnes of oxyresveratrol. However, oxyresveratrol plays other roles in antibacterial activity, such as the inhibition of the periodontal pathogenic bacteria, Staphylococcus aureus, and Bacillus subtilis [13]. Oxyresveratrol has been associated with antifungal activity against human pathogenic fungi such as Microsporum gypseum, Microsporum canis, Trichophyton mentagrophytes, and its antistaphylococcal effects (inhibition of S. aureus) are more potent than those of resveratrol [13][64][13,64].
At present, resveratrol is extensively utilized as an ingredient in numerous cosmetic formulations, particularly moisturizing cream and serum. In Thailand, the heartwood extract of A. lacucha containing oxyresveratrol as the major active compound is used as an ingredient in moisturizing cream, serum, toner, and soap [29].
2.4. Safety
At present, there are few data on the safety of stilbenoids for use in humans. Resveratrol is non-toxic, safe, and well tolerated for oral and dermal administration since the 50% lethal dose (LD50) is high (reported as 2 g/day), and irritation of the skin and eyes have not been recorded [25]. In a study with mice, resveratrol did not induce carcinogenesis, and reproductive and developmental toxicity [25]. However, a high dose of resveratrol consumption may inhibit systemic P450 and can interact with numerous drugs [65]. Thus, additional studies of simultaneous medication with resveratrol must be investigated. Furthermore, adverse effects from the long-term application of resveratrol in both oral and dermal routes also have to be studied in order to limit usage or provide precautions to consumers. The safety information on oxyresveratrol for human use is still not reported, but has not been found to generate irritation, edema, or erythema when tested in a white guinea pig model [66]. Toxicological studies of piceatannol are very few and lacking in vivo studies. Based on current data, piceatannol does not cause any severe adverse effects; however, further in-depth studies to discover the safety of piceatannol are necessary [67].
