PAX5, a member of the Paired Box (PAX) transcription factor family, is an essential factor for B-lineage identity during lymphoid differentiation. Mechanistically, PAX5 controls gene expression profiles, pivotal to cellular processes such as viability, proliferation, and differentiation. Given its crucial function in B-cell development, PAX5 aberrant expression also correlates with hallmark cancer processes leading to hematological and other types of cancer lesion.
- PAX5
- BSAP
- B-cell
- differentiation
- transcription factor
1. Introduction
The Paired Box (PAX) gene family encodes nine transcription factors (PAX1–9), which regulate gene expression programs in tissue development[1] [1]. Although PAX transcription factors share a highly similar paired-box DNA-binding domain, they are classified into four subgroups (I–IV) based on additional functional domains such as the octapeptide and the homeodomain, which are generally located in the protein’s internal and amino-terminal regions respectively[2][3] [2,3]. Given their structural resemblance, PAX members from a particular subgroup account for similar activities and functions. For example, PAX genes in subgroups II (PAX2, PAX5, and PAX8) and III (PAX3 and PAX7) are commonly involved in processes including cell survival, motility, and tumor progression. Conversely, members from subgroup I (PAX1 and PAX9) and IV (PAX4 and PAX6) seem less involved in cancer processes[4] [4]. The expression of PAX family gene products is also generally tissue specific. For instance, PAX2 expression has been described in kidney and optic nerve development[5] [5], whereas PAX5 has mostly been associated with the development of the central nervous system, of B-lymphocytes, and spermatogenesis[6] [6]. Furthermore, the expression patterns of subgroup II members are reported to be altered in various cancer tissues, which suggests a distinctive role for these PAX gene products in the regulation of specific malignancies[1] [1]. Amongst these members, PAX5 has been extensively studied and characterized for its role in cancer pathogenesis.
2. Expression and Tissue Specificity
The human PAX5 gene locus is located on the 9p13 chromosomal region known to undergo a high degree of alterations leading to its implication in cancer development and progression[7] [22]. Structurally, the PAX5 gene is characterized by two known distinct promoters, resulting in two alternative transcriptional initiation sites known as PAX5A and PAX5B[8] [11]. Both transcripts share the same sequence encoded by exon 2 through exon 10. However, they have different sequences in their first exon (1A or 1B), which is dependently linked to their respective promoter regions (PAX5 1A versus PAX5 1B). Both PAX5A and PAX5B protein variants consist of a 52-kD protein known as the B-cell lineage-Specific Activator Protein (BSAP), which was initially identified as an essential regulator of early B-cell differentiation and commitment[6][9] [6,23]. Despite their structural similarities, PAX5A and PAX5B gene products display differential expression signatures and tissue specificity[10][11] [24,25].
3. PAX5 Expression and Regulation
As depicted in Figure 13, PAX5 is widely associated with various cellular processes and pathologies (Figure 3). Given the essential role of PAX5-mediated transactivation of vital genes for cell biology, its deregulation will have consequences on basic cellular processes such as differentiation, viability, and proliferation (reviewed in[4][12][13] [4,40,56]). Investigation of deregulated mechanisms leading to aberrant PAX5 expression and activity is therefore relevant and warranted to provide more insight into the overall comprehension of PAX5 mechanisms of action. Although the literature provides abundant research characterizing PAX5-mediated pathways and interactions, the upstream mechanisms regulating PAX5 expression are much less defined.
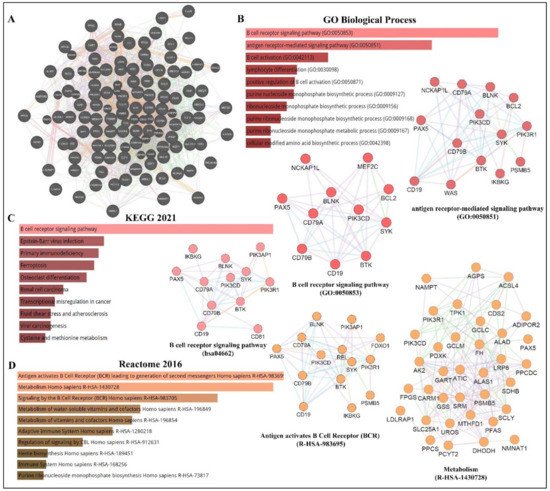
Figure 13. PAX5 interaction networks and related biological pathways. (A) PAX5 gene interaction networks have been mapped using the Cytoscape plugin GeneMANIA (https://genemania.org, accessed on 8 April 2022). Schematic illustrations of functional annotations and biological terms visualization are represented by: (B) PAX5 gene ontology (GO) in terms of functional orthologs and their relative implication in each predicted biological processes; (C) PAX5 pathway analysis using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, which provides an integrated evaluation of genomic, chemical, and biochemical functions; and (D) relative functional association to biological reactomes based on PAX5-related network genes. Annotations were done using the Enrichr algorithms (https://maayanlab.cloud/Enrichr, accessed on 10 April 2022). Significance was considered if p < 0.05.
4.
PAX5
Epigenetic Regulation
Many genomic studies have described the PAX5 locus as a genetic hot-spot susceptible to structural variation[14][15][16][17][18] [57,58,59,123,124]. For example, PAX5 expression and function are altered by various genetic alterations, including somatic mutation, translocation, and duplication/polyploidy[19][8][20][21][22] [10,11,62,78,83]. In addition to genetic mutation, which changes both the transcriptional levels and protein sequences, genes are also submitted to epigenetic deregulation, which impacts overall expression levels[23] [125]. These epigenetic processes include methylation of 5′-cytosine-phosphate-guanine-3′ (CpG) islands, chromatin remodeling via histone modifications, and various RNA-mediated mechanisms, which involve regulatory non-coding RNAs[23][24] [125,126]. A brief description of each regulatory mechanism and its impact on PAX5-mediated function is discussed below.
First, methylation of CpG islands to form 5′-methylcytosine (5mC) is a well-described mechanism to repress transcriptional expression of unwanted genes during fundamental cellular processes such as development and differentiation[25][26][27] [127,128,129]. DNA methylation is catalyzed by a group of DNA methyltransferase (DNMT) enzyme members (e.g., DNMT1, DNMT3a, and DNMT3b)[28][29] [130,131]. DNA methylation can also be reversed by demethylation, which is mediated by Ten-Eleven Translocation (TET) family dioxygenase enzymes, which include TET1, TET2, and TET3[30] [132]. In fact, B-lineage development is coordinated by the well-timed deployment of B-cell fate transcription factors, which are regulated by epigenetic events and post-transcriptional modifications[31][32] [133,134]. For example, DNMT1, DNMT3a, and DNMT3b are required for the maturation of hematopoietic stem cells into CLPs, whereas DNMT1 is particularly essential for pre-B-cell differentiation to immature B-cell[26][33] [128,135]. Subsequent studies have since demonstrated that TET function is required for developing B-cells to transit from the pro-B to pre-B developmental stage[34] [136]. Mechanistically, the B-cell-specific MB-1 (CD79a) promoter is known to be hypermethylated during hematopoietic stem cells transition to CLPs and then progressively demethylated during the expression and assembling of the BCR components. These events upregulate PAX5 expression and concomitant target genes to achieve B-lineage identity[35][36][37][38] [63,137,138,139]. On the other hand, attenuation of PAX5 expression during terminal B-cell differentiation is reported to partly mediated by methylation of PAX5[39] [140]. In support of these events, a study by Danbara et al., (2002) demonstrates that genomic demethylation using 5-aza-2′-deoxycytidine in myeloma cell lines results in the reconstitution of PAX5 expression and its transcriptional target genes (CD19 and MB-1)[39] [140]. Although the regulation of the complex networks of epigenetic modifications governing B-cell differentiation is only partially understood, one aberrant mechanism leading to deregulated PAX5 methylation has been described for the inadequate function of AID[40][41] [41,141]. The PAX5/AID pathway is essential for somatic hypermutation and antibody class switching during Ig production[42] [142]. However, constitutive expression of AID has been associated with lymphomagenesis through its capacity to alter the sequence of non-Ig genes (i.e., PAX5) or through AID-mediated deamination of the PAX5 gene[41][43] [141,143]. As a result, changes in PAX5 gene sequences redefine motif-specific regions marked for epigenetic modifications and subsequent expression control[40][41] [41,141].
Given the importance of adequate methylation processes regulating PAX5-induced B-cell development, deregulated methylation results in the destabilization of B-cell homeostasis and cancer phenotypes[38][44] [139,144]. This phenomenon has been further substantiated by the demonstration that PAX5 methylation status directly correlates with overall survival rates of cancer patients[45][46][47] [72,74,105]. Furthermore, studies profiling methylation signatures in pediatric ALL patients have correlated PAX5 hypermethylation to the pathogenesis of B-ALL and T-ALL subtypes[48][49] [145,146]. These findings have also prompted Nordlund et al. (2015) to propose that PAX5 methylation status combined with the mapping of PAX5 gene recombinations with other partner genes represent an effective diagnostic tool to classify heterogeneous and cytogenetically undefined ALL subtypes[50] [147].
PAX5 aberrant methylation is not a tissue-specific phenomenon. In fact, PAX5 hypermethylation has been described in many non-hematological cancers, particularly where PAX5 is characterized as a tumor suppressor (e.g., hepatocellular carcinoma[51] [100], ovarian carcinoma[51] [107]; head and neck cancer[52] [76], gastric cancer[47] [105], lung and breast cancer malignancies[53][53] [108,148]). Mechanistically, many of these latter studies demonstrate that silencing of PAX5 expression by hypermethylation leads to the inadequate transactivation of Tp53 expression, thus ensuing uncontrolled proliferation or decreased chemosensitivity to anticancer treatment regimens[45][54][55] [72,73,149].
Gene expression profiles are also epigenetically regulated by multiple histone-modifying enzymes, which change chromatin structure to alter promoter region accessibility and recruit other modifications[56] [150]. Histones, which assemble the nucleosomes, are prone to modifications, which include acetylation, methylation, ubiquitination, phosphorylation, and sumoylation[56] [150]. The most common modifications consist of arginine methylation and/or lysine acetylation, where acetylation generally promotes gene expression whereas methylation elicits the opposite effects. Many histone modifying enzymes have been characterized including histone acetyltransferases (HATs), histone deacetylases (HDACs), histone demethylases, and various methyltransferases (e.g., Euchromatic Histone-Lysine N-Methyltransferase-2/EHMT2 and Lysine Methyltransferase-2A[26][44] [128,144]). Like CpG island methylation, chromatin modifications represent an intrinsic part of B-cell activation and differentiation. For example, during early B-cell development, PAX5 secures B-cell commitment through activating B-cell specific genes. In addition, PAX5 PAX5 concomitantly inhibits B-lineage inappropriate genes through the recruitment of HDACs to modify and silence promoter activation of these genes[38] [139]. Studies show that the PAX5 locus is also continuously regulated by histone modifications throughout B-cell maturation. Specifically, the PAX5 promoter in pro-B-cells are modified by HDACs, whereas EHMT2 regulates mature B-cells located in germinal centers[26] [128]. Another example is the EBF transcription factor, which is shown to be implicated in PAX5 and CD19 transactivation through the silencing of Lysine Methyltransferase-2A during early B-cell development[32][57][58] [134,151,152]. Another example is the previously mentioned PAX5/BLIMP-1 axis during terminal B-cell differentiation into plasma cells. It is reported that BLIMP-1 suppresses PAX5 expression through the recruitment of histone demethylases and EHMT2 activities on the PAX5 promoter[59][60] [153,154]. Furthermore, transcription factor Forkhead Box Protein-O1, which is essential for B-cell development beyond the pro-B-cell stage[61] [155], is only activated upon histone methylation of TCF3, which only then can elicit histone modifications and silence PAX5 to enable the progression of B-cell development[62] [156]. Another study conducted by Danbara et al., (2002) has specifically demonstrated that the upstream PAX5 promoter (exon 1A) is predominantly inactivated by DNA methylation, whereas the downstream promoter (exon 1B) is repressed by histone deacetylation during the final stages of B-cell terminal differentiation[39] [140]. Comprehensively, deregulation of histone modifying events on PAX5 or its upstream regulators lead to aberrant PAX5 transcript levels and the development of diseases[44] [144]. Accordingly, a recent study from Jin et al. (2021) has not only shown that PAX5 is hypermethylated in retinoblastoma tumors but also, the treatment of patients with cyclophosphamide (a common antineoplastic agent to treat retinoblastoma) increases PAX5 expression via gene demethylation and concomitant DNMT inhibition, which result in tumor regression[63][64] [104,157].
To add complexity and appreciation for epigenetic mechanisms, different ATP-dependent chromatin remodeling complexes (CRC) capable of moving, ejecting, or restructuring nucleosomes (events often associated with DNA repair) have also been associated with PAX5 regulation and function[37][65] [138,158]. For example, SWItch/Sucrose Non-Fermentable and the Nucleosome Remodeling Deacetylase CRCs are known to mediate PAX5-dependant induction or repression respectively of MB-1 (CD79a) gene expression during BCR assembly[37] [138]. Therefore, the opposing functions of CRCs provide another layer of PAX5 function during B-cell development[37] [138]. Another example is the histone modifying enzymes HATs, which can acetylate other cellular proteins (e.g., transcription factors) besides histones. A study by He et al., (2011) has found that histone acetyltransferase E1A binding protein p300 interacts with the C-terminal region of PAX5 to acetylate multiple lysine residues of the paired box DNA binding domain[66] [19]. They also demonstrate that acetylation of the PAX5 transcription factor dramatically enhances the transactivation potential of its target genes[66] [19]. This interaction was also investigated in B-cell lymphoma, where the Metastasis-Associated Protein-1 represents a substrate for acetylation upon its interaction with the HAT p300[67] [159]. This study found that Metastasis-Associated Protein-1 acetylation leads to the direct transactivation and overexpression of PAX5, a widespread phenomenon in human DLBCL[67] [159].
The final contributing mechanism in epigenetic control is mediated by non-coding RNAs, which include small interfering RNAs, microRNAs (miRNAs), piwi-interacting RNAs, long non-coding RNAs, and circular RNAs (circRNAs)[68][69][70][71] [160,161,162,163]. In comparison to DNA and histone modifications, only a paucity of studies has directly elucidated ncRNA-mediated mechanisms governing PAX5 expression and function. A recent study from Harquail et al., (2019) has used a bioinformatic approach to establish a causal link between differentially expressed miRNAs in cancer cells in relation to their putative targeting of PAX5-dependent cancer processes and identified miRs-484 and 210 as directly regulators for PAX5 expression and function[72] [164]. Interestingly, miR-210 has been extensively studied as a potent oncogenic miRNA, which targets critical tumor suppressors such as E2F3 and Tp53[73][74] [165,166]. It is also well established that miR-210 is upregulated during hypoxia to induce EMT and tumor progression[75][76][77] [167,168,169]. Given the prevalent role of PAX5 in epithelialization and EMT-MET processes in breast cancer cells[78][79] [75,101], it has been suggested that miR-210 likely targets PAX5 during tumor neoplasm and hypoxia to produce a robust, comprehensive shift from epithelial to mesenchymal phenotypic features to evade hypoxic insult[72] [164]. PAX5 has also been reported to be part of a regulatory feedback loop with miR-155 in cancer cells[80] [170]. MiR-155 is known to play a vital role in the differentiation of memory B-cells where it targets PU.1 and AID necessary for B-cell commitment into plasma cell[81][82] [171,172]. Despite the rapidly growing field of non-coding RNA function in biological processes, the elucidation of non-coding RNA-dependent control of PAX5 expression and function in B-cell development and disease is still under investigation. As our knowledge expands on the deregulation of miRNA profiles and its impact on biological processes, researchers notice that changes to the mRNA sequences targeted by miRNAs will also have significant consequences, including miRNA motif accessibility and disruption of translational control. Accordingly, the next section will discuss PAX5 post-transcriptional modifications and editing, which alter miRNA-specific targeting and impede the potential binding capacity of any motif-specific interacting partners of PAX5 products.
5. PAX5 Post-Transcriptional Regulation
Similar to most human gene transcripts, PAX5 mRNAs undergo alternative splicing processes, which translate into altered translational reading frames and often multiple protein isoforms[83][84][85][86] [15,16,17,173]. To date, alternative splicing events of PAX5 transcripts in humans and other species result in translated products with deleted regions corresponding to single or multiple coding exons[83][85][86] [15,17,173]. Specifically, studies have shown that alternative splicing of the 5′ or 3′ end of PAX5 mRNA leads to structural and functional alterations of the PAX5 transcription factor in the DNA binding (exons 2-3) and transactivation domains (exons 8-9) respectively[85][87][88] [17,174,175]. A study performed by Robichaud et al., (2004) has characterized alternatively spliced PAX5 transcripts in CD19+ peripheral blood lymphocytes from healthy adult donors and found that B-cells simultaneously co-express multiple isoforms, including full-length mRNA (exons 1-10), in addition to transcripts lacking either exon 7 (∆7); exon 8 (∆8); exon 9 (∆9); exons 7-8 (∆7/8); or exons 7-8-9 (∆7/8/9)[85] [17]. Interestingly, this study also demonstrates that each PAX5 protein variant elicits a unique transactivation potential upon downstream target genes[85] [17]. Other studies have since reported additional C-terminal isoforms lacking exons 6-7-8-9 (∆6/7/8/9); exons 6-7-8 (∆6/7/8); exons 8-9 (∆8/9); and finally, a transcript containing a partial intronic sequence (intron 6) in healthy B-cells and lymphoma[88] [175]. These findings underscore the complexity of potential dominant-negative effects and the outcome of downstream target gene expression due to a network of multiple PAX5 transcription factor variants. Despite various reports characterizing the expression of alternatively spliced PAX5 variants, the specific role of each isoform and their capacity to compete for putative PAX5 targets are still undefined. However, one study conducted by Sadakane et al., (2007) has correlated a specific expression profile comprising of the wild-type and the ∆8 PAX5 variants in over 90% of childhood acute lymphoblastic leukemia samples tested[89] [176]. These findings suggest a possible role for individual PAX5 alternatively spliced isoforms in the regulation (or deregulation) of PAX5 function.
More recently, PAX5 transcripts have also been characterized to undergo 3′end shortening[90] [18]. This type of transcriptional modification has significant repercussions on translational fate given that mRNA untranslated regions (UTRs), notably at the 3′end, harbor multiple binding sites for RNA binding proteins and other translational regulatory elements (e.g., miRNAs), which control transcript stability and translation efficiency[91][92] [177,178]. A study by Beauregard et al., (2021) has recently reported that although 3′-editing of PAX5 transcripts is prevalent in healthy peripheral B-cells, shortening of the 3′UTR is directly linked to increased translation of PAX5 and correlates with leukemic disease progression[93] [18]. Mechanistically, the study reveals that PAX5 3′UTR shortening is mainly due to sequence excision (up to 86%) by alternative splicing events. PAX5 mRNA shortening was also investigated in non-hematological cancers. Interestingly, conversely to 3′UTR splicing in B-cells, PAX5 3′UTR shortening in breast cancer cells is primarily manifested by alternative polyadenylation (APA)[94] [18]. APA is another type of post-transcriptional modification where gene transcription is prompted to use alternative polyadenylation motifs (transcription termination signals), which alter the overall length of the mRNA sequences at their 3′ end. In fact, APA motifs are prevalent in more than half of all human transcripts, notably in oncogenes, to evade translational control at their 3′UTR, resulting in increased mRNA stability and translation[95][96][97] [179,180,181]. To further elucidate the impact of PAX5 3′UTR shortening on miRNA targeting and regulation, a bioinformatic approach was used to identify predicted miRNAs targeting the excised 3′UTR in truncated PAX5 transcripts from cancer cells[98] [18]. The study then experimentally validated that miR-181a, miR-217, and miR-1275 represent the most impactful tumor suppressors lost during PAX5 3′UTR shortening in cancer cell models[99] [18]. Nevertheless, more studies are required to fully understand the impact of regulatory elements (e.g., miRNAs) and the accessibility of their corresponding binding sites deleted from truncated PAX5 transcripts in oncogenic processes and disease.
The previous sections describe multiple regulatory mechanisms, which lead to very diverse PAX5 transcripts, proteins, and functions. More recently, researchers have also characterized a new class of transcriptional PAX5 products, circular PAX5 RNAs (circPAX5)[100][101][102] [12,13,14]. Circular RNAs (circRNAs) represent a relatively new category of non-coding RNAs characterized by a covalent phosphodiester bond between the 5′ and 3′ extremities of the transcript[103][104] [182,183]. After being discovered in viruses in 1976, circular RNA was first observed in humans in 1991, when it was initially thought to be the product of improper post-translational editing[105] [184]. Since then, circRNAs have been shown to be abundantly expressed and play essential roles in cell biology and disease[71][103] [163,182]. Accordingly, circular RNAs can encode proteins through cap-independent translation pathways, regulate gene transcription, regulate gene translation, interact with proteins, and even mop up (sponge) small RNAs such as miRNAs[106][107][108] [185,186,187]. Due to their essential role in cellular processes, circular RNA aberrant expression and function are consequently associated with diseases, including cancer[105][109][110] [184,188,189]. In fact, a study by Gaffo et al. (2019) describes circPAX5 as one of the most differentially overexpressed products in pediatric B-ALL patients[100] [12]. They also demonstrate that circPAX5 directly binds to miR-124-5p in B-cell precursors to promote B-ALL progression through the interference of the B-cell maturation process[100] [12]. More recently, researchers have mapped multiple circPAX5 isoforms in B-cells including: circPAX5_2-3 (containing exons 2 and 3); circPAX5_2-4 (exons 2, 3 and 4); circPAX5_2-5 (exons 2, 3, 4 and 5); circPAX5_2-6 (exons 2, 3, 4, 5 and 6); circPAX5_2-7 (exons 2, 3, 4, 5, 6, and 7); circPAX5_2-8 (exons 2, 3, 4, 5, 6, 7, and 8); circPAX5_8 (exon 8); circPAX5_7-8 (exons 7 and 8); circPAX5_5-8 (exons 5, 6, 7, 8); and finally, circPAX5_2-6+intron 5 (exons 2, 3, 4, 5, partial intron 5, and exon 6)[111][112] [13,14]. Furthermore, using TaqMan probes designed to target each unique circPAX5 junction region created by both extremities, researchers demonstrate that circPAX5_2-5 and circPAX5_2-6 are overexpressed in chronic lymphocytic leukemia patients in comparison to peripheral B-cells from healthy individuals. Mechanistically, researchers demonstrate that circPAX5 products interact with important microRNAs such as miR-146a and the miR-17-92 cluster. Previous reports demonstrate that miR-146a is a critical regulator of BLIMP-1 during B-cell differentiation[113] [190], whereas microRNAs from the miR-17-92 cluster mediate the developmental transition of pro-B to pre-B-cells[114][115] [191,192]. In addition, the miR-17-92 cluster is also associated with many oncogenic processes and phenotypes of hematopoietic cancers, notably in Burkitt lymphoma[116] [193]. Altogether, these findings not only identify a new class of PAX5 products (i.e., circPAX5) but also provide new potential signaling avenues for PAX5-mediated function in B-cell development and disease.
6. Post-Translational Regulation of PAX5
Post-translational modifications and regulation of PAX5 function have not been extensively characterized. However, a few studies have reported specific PAX5-interacting regulators, which modify the PAX5 transcription factor to regulate its transactivation potential. As described earlier, PAX5 can be acetylated by HATs on multiple lysine residues, which enhances its transcriptional activation of downstream target genes[66] [19]. Another example is how the PAX5 transcription factor can be regulated through phosphorylation events. Accordingly, studies show that PAX5 phosphorylation is responsible for the BLIMP-1/PAX5 regulatory axis during the critical stages of plasma cell differentiation[117][118] [20,21]. Specifically, upon BCR engagement of pro-B cells, PAX5 is phosphorylated on serine and tyrosine residues by Extracellular Regulated Kinases-1/2 and Spleen Associated Tyrosine Kinase respectively, which revoke PAX5′s ability to repress BLIMP-1 expression, thus enabling the progression of plasma cell development. On the other hand, a study conducted by Kovac et al., (2000) has demonstrated that Importin alpha-1 interacts with the nuclear localization signal on PAX5 to confer its nuclear localization and import, leading to greater PAX5 transactivation of downstream target genes[119] [194].
7. Discussion
It is well established that PAX5 products are important regulators of cell biology, notably in B-lineage commitment and maturation. It is also apparent that PAX5 is plagued not only by the high-profile genes it regulates but also, by its incredible vulnerability to genetic alterations leading to aberrant expression of PAX5 products. Given the reliance of crucial developmental program genes on PAX5 transactivity, perturbation of PAX5 expression and function at any level ultimately derails basic cellular processes, lending way to oncogenic manifestations. Moreover, given the requirements for coordinated and transitional PAX5 expression profiles during early (PAX5 activation) and late (PAX5 attenuation) phases of B-cell maturation, inadequate PAX5 activity leads to blockade of B-cell differentiation and uncontrolled proliferation of immature B-cells [7,62–64].
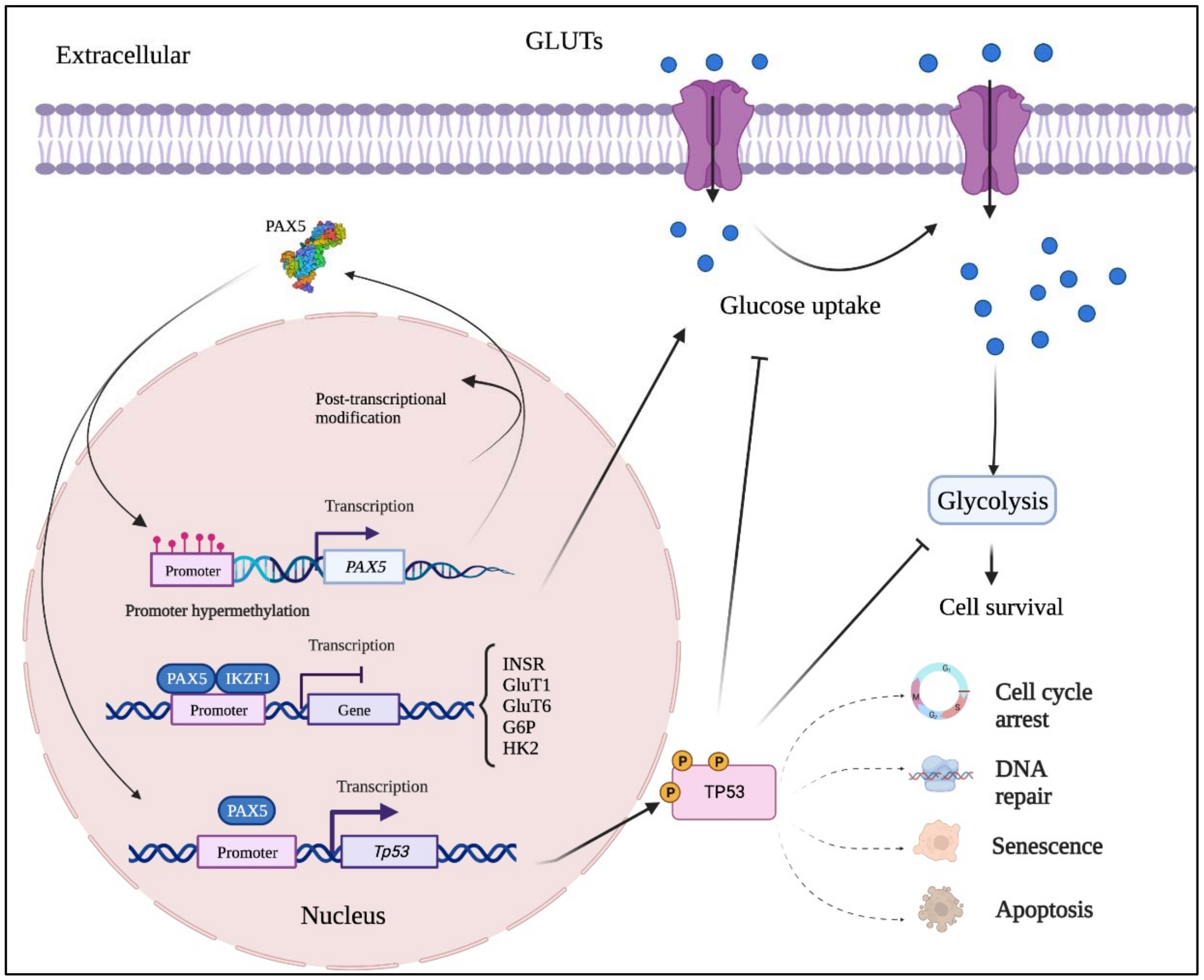

Figure 24. Mutation-independent mechanisms leading to aberrant PAX5 signaling and cell processes. Aside from PAX5 gene sequence alterations, deregulated PAX5 expression can also result from epigenetic events and post-transcription modifications. First, PAX5 gene promoter hypermethylation has been described in many cancers, notably when PAX5 behaves as a tumor suppressor. Post- transcriptional modifications (e.g., coding exon alternative splicing, 3′UTR shortening, and RNA circularization) also contribute to overall PAX5 expression and function. The net production of functional PAX5 transcription factors can thereafter collaborate with IKZF1 to regulate downstream metabolic genes to limit glucose uptake and energy supply required for oncogenic transformation. Adequate PAX5 function is also required to regulate Tp53 expression and avoid uncontrolled cancer phenotypes. Tp53 is also intimately linked to metabolic disfunction leading to cancer processes.
