1. Polymer–Metal Composite Materials for Dental Implant
Dental implant is one of the most common dental treatments to replace missing teeth. The primarily function of a dental implant is restoring the patient to a normal profile regardless of the status of the stomatognathic system
[1][2][130,131]. Similar to the orthopedic implants, conventional dental implant relies on titanium as implant materials to fulfill several requirements: (1) good fatigue strength and wear resistance to withstand cyclic occlusal load (axial bite force: 500 N–800 N)
[3][4][5][132,133,134], (2) good corrosion resistance to withstand different types of corrosions that take place in the oral cavity (galvanic, pitting, stress, and microbial corrosion)
[6][7][128,135], and (3) bioinert to body environment and good biocompatibility
[8][136]. In bulk scale, titanium alone is insufficient to fabricate dental implant for osseointegration (i.e., bone ingrowth into implant), presumably due to huge difference of elastic modulus (i.e., stiffness) between metal/metal alloy-based dental implant (titanium: 110 GPa, Zirconia: 210 GPa, Cobalt-Chromium: 180–210 GPa) and surrounding bond tissues (cortical bone: 13.8 GPa, spongy bone: 1.38 GPa)
[9][10][11][137,138,139], such difference in elastic modulus creates risk of mechanical overloading of bone (namely stress-shielding), leading to bone damage and bone resorption
[12][13][14][140,141,142]. In nanoscale, reported studies have shown that the immune reactions and bacterial infections may be the underlying mechanism of marginal bone lost and peri-implantitis
[15][16][17][18][19][20][21][22][23,143,144,145,146,147,148,149]; on the other hand, bone healing and bone remodeling after dental implant treatment is determined by osseointegration, peri-implant osteogenesis (i.e., distant osteogenesis and contact osteogenesis), and osteoclastogenesis
[9][23][24][25][26][27][28][137,150,151,152,153,154,155]. Incorporation of polymers into titanium dental implants via coating or polymer composite materials endow the dental implants with proper load transfer and distribution, enhanced bone healing, minimized immune reactions, and antimicrobial properties
[29][30][156,157].
1.1. Polymer–Metal Composite Dental Implant with Improved Load Transfer, Osseointegration, and Osteogenesis
Polyetheretherketone (PEEK) is FDA approved, a dominant member of Polyaryletherketone (PAEK) family which has been extensively used in dental implant due to their compatible elastic modulus (PEEK: 4 GPa, carbon-nanofibre-reinforced PEEK: 18 GPa)
[31][32][158,159], high flexural strength (140–170 MPa), radiolucent, highly biocompatible and bio-stable
[33][34][35][160,161,162]. Lee et al.
[36][163] has demonstrated that PEEK-coated titanium/zirconia dental implants exert lower levels of von-Mises stress to the bone in comparison with metal-based implants. The major limitations of PEEK-based dental implants are their limited osteoconductivity and lack of bioactivity which may trigger implantitis and implant failure
[37][164]. Inspired by the good osseointegration properties of titanium, metallic materials (metallic complex, metallic nanoparticles) may serve as a potential additives to endow PEEK bioactivity and make PEEK-based dental implant possible. As such there are different metallic materials being used to modify PEEK, such as titanium
[38][165], titanium dioxide
[39][40][41][42][166,167,168,169], and strontium based materials
[43][44][170,171], by either surface modification or melt-blending
[45][46][172,173]. Strontium based materials such as strontium ranelate coated PEEK can strengthen the osteoblast adhesion, increase the alkaline phosphatase activity, increase collagen secretion and ECM mineralization deposition
[43][170]. Titanium-coated PEEK has shown improved proliferation of osteoblast and higher percentage of bone-to-implant contact
[38][165]; while titanium oxide coating has also shown beneficial effects on the osseointegration
[39][40][41][42][47][48][166,167,168,169,174,175]. Bone remodeling comprise different levels of hierarchical structural changes at macro (e.g., cortical bone, spongy bone), micro (osteons, trabeculae), and nanoscale (collagen I and hydroxyapatite composited ceramic-interspersed collagen fibril)
[49][50][176,177]. However, the surface properties of PEEK do not favor osseointegration due to its inherent hydrophobicity and bioinert. It is well known that hydrophilic implants favor plasma proteins and cells adhesion, and one way to increase surface wettability is to control the surface roughness of materials
[51][178]. Metallic materials are useful tools to introduce micro and nanofeatures to implant surfaces, and endow new functionalities, such as rough surface (i.e., increase surface roughness), thereby promoting osteogenesis and osseointegration.
Elawardly et al.
[52][179] has demonstrated that both the ceramic-filled PEEK and the carbon fiber-reinforced PEEK discs shown significantly improved wettability after sandblasted treated with 50/110 microns of aluminum oxide particles, in compared with untreated group; such PEEK-based materials show good wettability when the surface average roughness (Ra) value was either <1.0 or >1.7 μm. Interestingly, in comparison with microscale surface roughness, implants with nanoscale surface roughness introduced have better osseointegration
[49][176]. Some reported studies have shown that introduction of titanium-based nanofeatures on implant surface, such as TiO
2 nanonodule
[53][180], TiO
2 nanotube
[54][181], and TiO
2 nanopores
[42][169] can further improve the osseointegration via several effects: (1) improved cell adhesion, cell spreading, and therefore, bone-implant integration, (2) improved ALP levels, and (3) promote the formation of hydroxyapatite (HA). The functions of nanoporous titanium implant surface has been recently shown that such nanofeature can promote osteogenesis by inhibiting the differentiation of macrophages into osteoclast (i.e., osteoclastogenesis) by blocking integrin mediated FAK phosphorylation and downstream MAPK pathway, and elicit a prohealing cytokines secretion profile
[41][168]. The titanium nanofeatures increase the hydrophilicity of PEEK dental implant, which has shown to facilitate immobilization and delivery of bone morphogenetic protein-2 (BMP-2), thereby significantly enhancing the osseoconductivity of PEEK implant
[40][167]. With the cytotoxicity concern on TiO
2 nanoparticle leaching from TiO
2 coating, Wu et al.
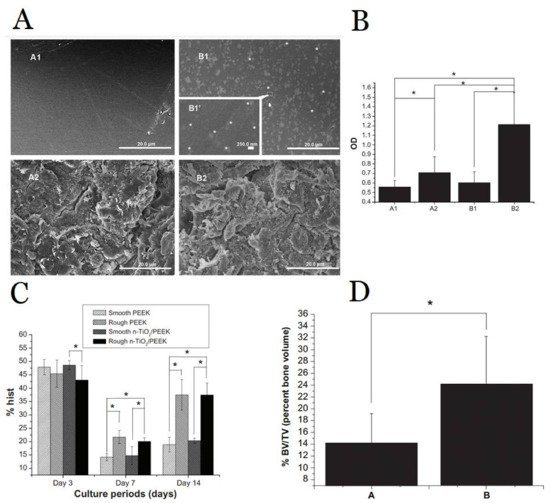
[40][167] has developed a titanium oxide nanoparticle/PEEK composite with rough surface that increase the growth rate of osteoblast and higher bone volume/tissue volume (
Figure 19). The interplay between the titanium and PEEK as a polymer–metal composite material has significantly improved the performance of dental implants in both nano scale and device scale. In nano scale, the titanium–based nanofeatures improve the bioactivity of the implant surface, thus improved osseointegration; in device scale, the PEEK-based implant becomes compatible with the stiffness of surrounding bone, which ensures proper load transfer upon occlusion force.
Figure 19. (
A) Scanning electron microscopy images of PEEK and TiO
2 nanoparticle/PEEK composite (n-TiO
2/PEEK) before and after blasted treatment (A1 smooth PEEK; A2 rough PEEK; B1 smooth n-TiO
2/PEEK; B2 rough n-TiO
2/PEEK; (
B) Cell attachment testing indicates significantly improved osteoblast cells adhesion in B2 rough n-TiO
2/PEEK than the other groups (A1, A2, B1); (
C) Flow cytometric analysis indicates rough implant surface promote cell proliferation (both PEEK and n-TiO
2/PEEK); (
D) Microcomputed tomography indicates significant increase in bone volume/tissue volume in n-TiO
2/PEEK than PEEK implant. Note: *
p < 0.05 Figure reprinted with permission from Ref.
[40][167] Copyright Dovepress.
1.2. Antimicrobial Polymer-Metal Composite Dental Implant
Periodontal disease is the main cause of tooth loss which is considered as a huge threat to oral health
[55][182]. The onset of periodontal disease is well known to be the collective outcome of bacterial infection and inflammatory responses
[56][183]. It is noteworthy that the periodontal tissue is continuously exposed to oral microbiota during mastication and respiration
[57][184]. In the healthy state, localized bacterial challenge and host immune response is balanced. However, the colonization of “keystone” pathogens (change of microbiota constituent and their total counts) increase the pathogenicity of local microbiota and over activate immune response
[58][59][185,186]. For example, the bacterial infection triggers the generation of specialized T
H17 cells, namely “bone-damaging T cells”, to fight against bacteria by concurrently initiating mucosal immune responses and inducing bone loss to inhibit infection
[60][187]. The dental implant confronts the same challenge after implantation when bacterial infection takes place, from both the implant-associated infection, daily mastication, and respiration which eventually lead to the peri-implantitis
[61][188]. Therefore, developing an antimicrobial dental implant can greatly reduce the major cause of periodontal disease by inhibiting bacterial growth and bacterial biofilm formation, thereby improving the oral health and the longevity of the dental implant.
Typical dental implant contaminations involve the polymicrobial biofilm formation, which can be suppressed using antibiotics. Therefore, antibiotics coated/encapsulated dental implants have been demonstrated as one of the major approaches to fight against bacterial infection
[62][189]. Polymer-based coatings are advantageous for antibiotics delivery due to its high antibiotics upload capacity, controlled release of therapeutic concentration of antibiotics in proximity to infected area, biocompatible, and biodegradable; many antibiotic-encapsulated polymer/biopolymer coatings have been developed in the last two decades
[62][63][189,190]. The early stage of polymer-coating development employed polymers such as poly(D,L-lactide)
[64][65][191,192], poly-L-lactide
[66][193], due to their excellent biodegradability. Recently, several new coating strategies have been developed with mild processing conditions (low temperature, organic solvent free, radiation free) used in titanium implants, such as polydopamine coating, surface controlled free radical polymerization, azide-alkyne click reaction, salinization, polyelectrolytes deposition, electrophoretic deposition (EPD), and dynamic covalent chemistry, which allow more bioactive polymers employed as an antibiotics cargo with improved antimicrobial outcomes
[67][68][69][70][71][72][194,195,196,197,198,199]. Namely, chitosan can improve drug residence time at mucosal surface
[73][200]; polymethacrylate grafted with functionalized poly(ethylene glycol) (PEG) improve conjugation of antibiotics thereby increase antibiotics loading
[67][194]; PEG dimethacrylate hydrogel functionalized with oligonucleotide enhanced stability to human serum
[68][195]; and PEG-poly(propylene sulfide) enable oxidation responsive (i.e., hyperinflammatory environment) release of antibiotics
[74][75][201,202]. Interestingly, studies have shown that antibiotics-loaded with nanostructured titanium implants (pillar-type and pocket-type nanostructure) have better anti-biofilm performance
[76][77][203,204]. However, systematic study on the effect of nanofeatures on the polymeric coated titanium dental implants has remained unexplored.
One of the major concerns of antibiotic release dental implants is the associated risks of bacterial resistance
[78][205], and therefore, various antimicrobial materials have developed to serve as an alternative to antibiotics. Chitosan-based coating is a promising antimicrobial biopolymer for dental implants, as chitosan elicits excellent antimicrobial properties towards both Gram-positive and Gram-negative bacteria via disruption of their membrane functions
[79][206]. Other attributes of chitosan such as anti-inflammatory properties, non-toxic, and biocompatible render chitosan-based dental implants worthy for clinical studies
[80][207]. Chitosan combined with other bioactive materials like hydroxyapatite and graphene have been demonstrated in promoting osteoblast proliferation and inhibiting microbial growth
[81][82][208,209]. Antimicrobial polypeptides (AMPs) is another class of antimicrobial materials with amphipathic properties and notable anti-adhesive properties
[83][210]. Such extra anti-adhesive properties render AMP-coated dental implants extra protection against the formation of biofilm and subsequent implant contamination
[84][85][86][87][211,212,213,214]. It is noteworthy that different antimicrobial dental implants using antibiotics or AMPs generally suffered from gradual depletion of the antimicrobial properties, presumably due to lack of renewable properties. Recently, Wu et al.
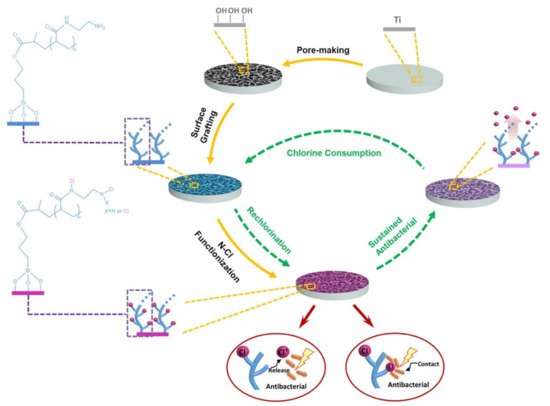
[88][215] have developed a long-lasting antibacterial porous polymeric coatings with self-renewal properties using N-halamine polymer (
Figure 210), which is prepared from surface pore-making of titanium implant, followed by surface grafting of polyacrylic acid (PAA), and reacted with ethylenediamine and sodium hypochlorite to give the chlorinated N-halamine surface (Ti-PAA-NCl). The amide N-halamine group possesses moderate transfer rate of oxidative Cl+, which is able to kill bacteria through both contact killing and release killing. Ti-PAA-NCl retains its antibacterial properties (68% against
P. gingivalis) after 20th cyclic antibacterial test and after stored in PBS for 4 weeks (89%) and 12 weeks (79%), which is durable enough to cover the period of osseointegration (at least 4 weeks after implantation, 3 months to complete)
[89][216]. Notably, Ti-PAA-NCl can replenish its antibacterial property by simply peri-implant irrigation.
Figure 210. Preparation, mechanism of antibacterial property, and regeneration of antibacterial property of the porous N-halamine polymeric coated titanium. Antibacterial property replenished by simply peri-implant irrigation using 5% NaOCl solution for 15 min. Figure reprinted with permission from Ref.
[88][215] Copyright 2021 Springer Nature.
1.3. Polymer–Metal Composite Dental Implant with Minimized Immune Reactions
One of the consequences of dental bacterial infection is the onset of immune responses that eventually disrupt bone remodeling and cause bone loss
[58][59][185,186]. However, induced immune responses in the stomatognathic system cannot be completely prevented by simply incorporating antimicrobial functions to the dental implant. Nano-micro size wear particles can be generated during implant fabrication, implantoplasty, and cyclic occlusal force (i.e., biting)
[90][91][217,218]. The impacts of different types of wear debris generated by dental implants have been discussed in detail:
[92][93][99,100]. In brief, cells in the oral cavity generally elicit adverse effects when in contact with, or upon the uptake of the nano-micro size wear particles, including impaired osteoblast adhesion, proliferation, osteogenic differentiation and mineralization, elevated RANKL/OPG ratio in osteoblast, and secretion of proinflammatory cytokines from both osteoblast and activated macrophage, and collectively lead to osteolysis and peri-implantitis
[94][95][219,220].
Dental implants mainly contain three compartments: the screw (insertion to the alveolar bone), the abutment (connector between the dental crown and implanted screw), and the dental crown (in contact with oral cavity and natural teeth). It is noteworthy that wear debris can be generated upon the loading of the abutment to the screw
[96][221], which can be prevented using a protective film. Łępicka et al.
[97][222] developed an anti-wear abutment screw using polysiloxane-TiO
2 nanoparticle composite film, which serves as a “locking coating” on the titanium abutment and screw. Compared to conventional protective films such as pure GPTMS/TEOS matrix, the nanoindentation results indicated that the polysiloxane film with TiO
2 NP in the polymeric backbone significantly reduced the hardness and elastic modulus, thereby rendering the film with anti-wear property
[97][222]. Moreover, the wear resistance of Ti-6Al-4V alloy has been shown to be improved with a simple coating of PEEK due to its high wear resistance
[98][223], which can be a generic approach to improve the wear resistance of dental implants. In the case of the dental crown, conventional dental crown materials include a resin-based composite, composed of organic fillers (e.g., zirconia), and organic monomers (e.g., triethylene glycol dimethacrylate (TEGDMA), bisphenol A-glycidyl methacrylate (Bis-GMA)). Early resin composites suffered from a high wear rate, presumably due to the larger filler particles. Recently, micro/nano-hybrid composites have been employed and the wear resistance significantly improved
[99][224]. In contrast, the wear behavior of composite resins using metallic nanofillers such as TiO
2 NPs can be adjusted by the type of polymers and contact conditions. TiO
2 NPs can only be beneficial when they can be blended with other wear debris to form a strengthened transfer film (generated when sliding two materials together), thereby providing the adequate support required for other dislodged TiO
2 NPs to elicit a rolling effect, and thus anti-wear property
[100][225]. Hence, it is important to firstly investigate the interactions between different tribo-fillers with well-designed contact conditions to mimic real clinical settings (biotribology model for oral cavity), and to design high wear resistant polymer metal composite materials for dental crowns.
2. Polymer–Metal Composite Materials for Cardiovascular Stent
Myocardial infarction (MI) is regarded as one of the most lethal cardiovascular diseases worldwide, presumably due to the narrowing of artery vessels, namely atherosclerosis
[101][102][226,227]. Percutaneous coronary intervention (PCI) was first pioneered by Andreas Grüntzig in 1977 and became one of the most commonly used angioplasty surgeries used to treat atherosclerosis, with the help of the stainless bare-metal stent (BMS) to prevent artery contraction and therefore alleviate ischemia
[103][104][228,229]. However, the PCI-related arterial wall injury triggers neointimal hyperplasia, which leads to the lethal restenosis within the first 12 months after the BA treatment (30~50%)
[105][230]. The dual-antiplatelet therapy (DAPT) was developed to inhibit platelet activation and vascular smooth muscle cell hyperproliferation, thereby preventing early stent-restenosis
[106][107][231,232]. Nevertheless, a considerable group of patients still suffered from early stent-restenosis (20–30%)
[108][109][233,234]. The effective inhibition of restenosis require proximal drug delivery to the iatrogenic injured vessel. A metallic drug eluting stent was developed, but with a limited amount of drugs, and the stent failed to release the drug molecules in a suitable time frame
[110][235]. Polymer-based drug eluting stents, namely bioresorbable vascular stents (BVS), have recently been developed as a new class of cardiovascular stent, which aims to improve the atherosclerotic vessel healing by gradual bioresorption, thereby effacing the foreign implant over time
[111][236]. However, the vessel healing using BVS could not meet the expected advantages and patients treated with BVS suffering from a higher risk of late stent thrombosis (LST), very late stent thrombosis (VLST), target lesion failure (TLF), and cardiac death, presumably due to the inherent properties of polymeric stent, such as limit stent expansion and weaker radial strength when compared with the metallic stent
[112][113][114][115][237,238,239,240]. The BVS has to be made with thicker and wider strut to provide appropriate mechanical support, which disrupts the laminar blood flow, thus promoting platelet activation and subsequent thrombosis
[116][241]. To date, the FDA approved cardiovascular stents are polymer–metal composite-based stents, mainly from two major classes: (1) first-generation drug-eluting stent using durable polymers, and (2) second-generation drug-eluting stent using biodegradable polymers.
2.1. Durable Polymer Metal Stent: First-Generation Drug Eluting Stent
The first major breakthrough of the PCI technology occured with the invention of the 1G-DES, a polymer-coated metal stent with antiproliferative drugs
[117][27]. Current FDA approved 1G-DES released either an antiproliferative drug paclitaxel or an immunosuppressive drug sirolimus from a layer of polymer coating
[118][242]. The FDA approved 1G-DES are coated with durable polymers such as poly(styrene-block-isobutylene-block-styrene) (SIBS), Poly(ethylene-co-vinyl acetate) (PEVA), and Poly(
n-butyl methacrylate) (PBMA)
[118][242]. The polymer coatings offer new functions to the cardiovascular stent: (1) serve as a reservoir to endow temporally controlled drug release from the polymer metal stent, to inhibit blood clotting, and to limit the overgrowth of tissues that triggered by PCI-related arterial wall injury; (2) provide protection to metallic stent from corrosion to maintain radical strength of the stent and to prevent stent fracture
[119][243]. Moreover, 1G-DES creates much less burden for the patient in terms of in-stent restenosis, but with the cost of another problem, the development of LST, (between one month and one year after implantation) and VLST, (>1 year after implantation)
[120][121][244,245]. The generally accepted underlying mechanism of such increased LST and VLST risk is due to the antiproliferative drugs in the 1G-DES delaying the re-endothelialization of blood vessels
[120][121][244,245]. Hence, 1G-DES has been shown to present a considerably higher risk of developing VLST in comparison with BMS (adjusted risk ratio (RR) 1.87 (1.47–2.25))
[122][246]. Other studies have shown that the permanent polymer coatings (such as PEVA and PBMA) in 1G-DES contribute to impaired arterial healing
[123][247]. These findings support that the permanent polymer coatings in 1G-DES hamper normal re-endothelialization. Moreover, the permanent polymer coating in 1G-DES is inevitably creating higher risk factors, such as accelerated neoatherosclerosis (NA) plaque growth and hypersensitivity reactions that will eventually develop into ST
[123][124][125][247,248,249]. Indeed, the healing of atherosclerotic vessels into normal blood vessels requires long-term monitoring. At the early stage after the stent implantation, the drug-eluting should exert an antiproliferative effect in order to prevent restenosis, and the patients should receive the DAPT therapy for at least 12 months to prevent early thrombosis
[126][127][250,251]. Proper blood vessel re-endothelialization after the PCI is pivotal to prevent LST and VLST.
2.2. Biodegradable Polymer Metal Stent: Second-Generation Drug Eluting Stent
With the lesson learnt from the 1G-DES, the second-generation drug eluting stent (2G-DES) aims to select the more biocompatible, biodegradable polymer to replace durable polymer as new coatings for drug eluting stents. The 2G-DES with a biodegradable coating (e.g., CoCr-Everolimus with poly-L-lactide (PLLA) coating, CoCr-EES) mitigates polymer-associated chronic inflammation and hypersensitive reactions, thereby providing superior protection from LST and VLST (CoCr-EES vs. PES, a 1G-DES or vs. BMS, at median follow-up of 3.8 years)
[105][128][230,252]. The 2G-DES also showed better clinical outcome than 1G-DES in terms of disease complications and mortality, such as reduced repeat revascularization in ST-segment elevation myocardial infarction (STEMI) patients
[129][253]; lower rates in major adverse cardiovascular events (MACE), cardiac death, recurrent MI, as well as target or non-target lesion revascularization (TLR/non-TLR) in non-ST-segment elevation myocardial infarction (NSTEMI) patients
[130][131][254,255]. This evidence places 2G-DESs as the current gold standard and benchmark comparator to ongoing trials
[105][128][230,252].
2.3. The Advance of Cardiovascular Stent: Polymer–Metal Stents Engineered with Macroscopic and Microscopic Features
Recent advancements in stent technology have greatly reduced the risk of restenosis, stent thrombosis, and other clinical complications. The stent thrombosis remains a difficult task to solve due to its high mortality (45%) and high recurrence rate (15–20% at five years)
[132][133][256,257]. There is a myriad of factors associated with the occurrence of thrombosis, such as the profile of patient, progress of lesion, and execution of the procedure, that have already been discussed in detail in other published reviews
[134][135][136][258,259,260].
Compared to other implant biomaterials (e.g., dental implant, pacemaker, and joint replacement), the cardiovascular stent is placed in a bent vessel with continuous blood flow. The cardiovascular stent has to maintain structural integrity to prevent narrowing of the artery, and concurrently the stent should not exert too much circumferential stress on the artery that may lead to unnecessary trauma, subsequently leading to disease complications
[137][138][139][261,262,263]. Therefore, the cardiovascular design requires the fine tuning of some extra macroscopic properties of the material, such as radial strength, elastic strength, and Poisson’s ratio
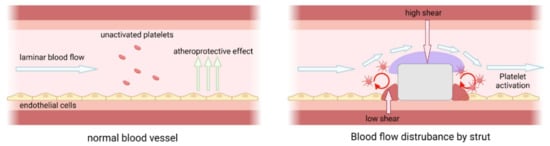
[140][141][142][264,265,266]. On the other hand, the haemodynamic nature of the blood vessel has been well known to be pivotal to proper vessel healing and tied to the occurrence of thrombosis. Laminar blood flow with high shear stress to artery is crucial to trigger atheroprotective effects that promote the survival of endothelial cells. Blood flow disturbances, such as turbulent flow/circulating flow, conversely, trigger both platelet activation and prevent endothelialization (
Figure 311)
[143][144][145][267,268,269].
Figure 311. (left) The atheroprotective effect of laminar blood flow and (right) blood flow disturbance by a thick strut. Laminar blood flow creates high shear stress to artery and trigger the release of anticoagulant and antithrombotic molecules (e.g., NO, PGI2, TFPI, tPA, thrombomodulin) by endothelial cell, migration of leukocytes and monocytes, and proliferation of smooth muscle cells that collectively promote the survival of endothelial cells. Blood flow disturbance such as turbulent flow/circulating flow, conversely, trigger both platelet activation and prevent endothelialization.
The current design of cardiovascular stent aims to minimize turbulent flow via the development of different geometric stent models with minimal stent thickness (i.e., minimum polymer and metal) to avoid flow disturbance
[146][147][270,271], without compromising their protective effects (e.g., optimal drug release kinetics, and stent radial strength). The effects of polymer–metal stent macroscopic properties on the performance of cardiovascular stents and clinical performance are summarized in
Table 1. Stent geometry (i.e., strut distance, strut width, stent shape) is one of the major parameters that affect the haemodynamic nature of arteries as discussed in other reviews
[148][149][150][151][272,273,274,275]. On the other hand, thin stents are well known to minimize blood flow disturbance more than thick stents, thus benefiting their clinical performances (e.g., reduced the incidence of stent malapposition and evaginations)
[147][152][271,276]. Therefore, the development of new alloys with better mechanical strength and radial strength
[153][277], and thus new polymers with better drug loading and optimized drug release profile
[154][278], can collectively contribute to the development of thinner stents.
2.4. Key Microscopic Features of Polymer—Metal Stent
Endothelialization (i.e., adhesion of endothelial cells) is a crucial step for arterial healing after the inevitable artery injury caused by PCI. Studies have indicated the critical role of the endothelium (i.e., layer of vascular endothelial cells) in preventing disease complications, such as vascular thrombosis, intimal hyperplasia, LST, and VLST
[120][121][161][244,245,285]. A healthy endothelium is considered as an anticoagulative phenotype that secretes a high level of vasodilators (e.g., nitric oxide (NO), prostacyclin (PGI2)), and prevents the exposure of elastic lamina and SMCs that can release a pro-coagulant thromboplastin into the bloodstream. Incomplete endothelialization or dysfunctional endothelium leads to a biological cascade that promotes SMCs proliferation and platelet activation, and thus blood coagulation, thereby causing thrombosis
[162][163][164][165][286,287,288,289]. Therefore, it is of importance to promote early re-endothelialization after implantation of cardiovascular stents to prevent PCI associated complications. The advance of nanotechnology in biomaterials provides new tools for cell adhesion. Rapid re-endothelialization can be achieved via different nanotechnologies, such as the use of core-shell nano/micro particles as a multi-drug/bioactive molecules delivery system
[166][167][290,291], creating different nanostructured stent surfaces (e.g., change of surface topography)
[168][169][292,293], and by virtue of bioactive ligand-mediated cell adhesion
[170][294].
One way to achieve early re-endothelialization is the implantation of a stent with drug and bioactive molecules that release in proximity to wounded arteries. Wang et al.
[171][295] reported a hydrophobic core/hydrophilic shell nano/micro particles that was then coated as a drug eluting stent coating. The PLGA solution contains the antiproliferative drug and serves as the precursor of core coating, whereas the chitosan solution contains the platelet glycoprotein IIb/IIIa receptor monoclonal antibody SZ-21 for shell coating. These two solutions were injected from the coaxial nozzle and form the dual core/shell drug particles. Such a coating can inhibit platelet adhesion and activation, as well as SMCs proliferation and migration in vitro. In vivo data (porcine coronary artery model) have indicated that the SZ-21/DTX drug-loaded hydrophobic core/hydrophilic shell particle coating stents promotes re-endothelialization and inhibit neointimal hyperplasia.
Another way to achieve rapid re-endothelialization is to modulate artery microenvironment at the molecular level. Nitric oxide (NO) releasing materials have also been incorporated into a part of the polymer coating to elicit anti-thrombosis/restenosis functions, presumably due to their crucial role in vasodilation
[172][173][296,297]. Pioneer research on NO-releasing/generating coatings involved either an NO supplier or catalyzer (e.g., N-diazeniumdiolate or ascorbic acid) that releases the therapeutic dosage of NO to restore endothelial cell functions
[174][175][176][298,299,300]. On the other hand, there are various biomolecules/bioactive ligands that can promote the growth of endothelial cells, including heparin, hyaluronic acid, chondroitin sulfate, fucoidan, and gallic acid
[177][178][179][180][181][301,302,303,304,305]. Combining the NO releasing coating with bioactive ligands can thereby promote re-endothelialization via biomimetic microenvironment. Zhao et al.
[182][306] reported a stepwise copper-catechol-amine (MCA) surface coating approach on vascular stent. The amine groups of MCA were conjugated with heparin, and the CuII-DA/HD networks elicited a glutathione peroxidase (GPx) like activity similar to CuII, which triggered the decomposition of blood stream S-nitrosothiols (RSNO) into NO. The heparin moiety and NO release exhibited synergistic effects in terms of rapid re-endothelialization, enhanced antithrombogenicity, SMCs suppression, inhibition of intimal hyperplasia, and in-stent restenosis in the adult New Zealand white rabbit model. Wu et al.
[183][307] reported a NO eluting hydrogel coated vascular stent that can suppress neointimal hyperplasia by virtue of GPx mimetic organoselenium generated NO. The hydrogel is composed of alginate and gelatin that are analogous to hyaluronic acid and collagen in the ECM. The hydrogel composition was optimized so that it is mechanically strong enough to withstand balloon angioplasty. The alginate backbone was modified with selenocysteine eliciting a GPx-3 like catalytic properties that were able to degrade RSNO into NO. The hydrogel coated vascular stent had shown the persistent suppression of neointimal hyperplasia in both the New Zealand white rabbit model and Bama miniature pig model.
Surface topography/nanotopography is an emerging technology to regulate cellular behaviors
[184][185][308,309]. A change of surface topography can influence protein adhesion and even endow stent adhesive properties to a specific cell type. The design of surface topography primarily depends on the cell morphology and cell alignment of interest. In the case of endothelial cells, which possess an elongated morphology, studies have shown that substrates with a parallel grooved surface promote the migration of endothelial cells in comparison with the smooth counterpart
[186][310]. Such groove patterns have shown the selective adhesion of endothelial cells and concurrent inhibition of SMCs adhesion
[187][311]. The surface of the stent can also be modified by the addition of a nanostructure, such as TiO
2 nanotubes. Desai et al.
[188][189][312,313] reported that the nanotubular titanium oxide surface promotes endothelial cell adhesion while inhibiting SMCs adhesion. Iglič et al.
[190][314] reported the beneficial effects of an electrochemical oxidized TiO
2 nanotube array. This oxidized TiO
2 nanotube array (15 nm in diameter) elicited selective endothelial cell adhesion over the SMCs, and concurrently inhibited platelet adhesion, which is instrumental for antithrombotic metal stents.