Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Pero Lučin.
Beta-herpesviruses infect a large proportion of the human population and are associated with a variety of pathophysiological conditions. They are DNA viruses with a large genome that encodes a relatively large number of gene products for the construction of new viral progeny and the establishment of a complex series of interactions with infected cells.
- beta-herpesviruses
- beta-herpesvirus virions
- proteome
1. Host Cell Signatures within Virions
CMV envelopment, either the membrane wrapping around the virions or the virions budding into membranous organelles, must involve the incorporation of the host cell proteins into the virions. Thus, by analyzing virion composition, we can learn about the origin of the membrane domain used for envelopment and the biochemical requirements for the envelopment process (Figure 1C). However, because the virions are released from the cell together with extracellular vesicles (EVs), the analysis of the host cell signatures in the virions faces all the challenges that are strongly debated in the field of EVs. As discussed in a recent review [41], every cell generates various EVs using diverse mechanisms. Most EVs are developed directly at the PM or in the intracellular compartments, mainly MVBs and LEs, and are released from the cell through their fusion with the PM (Figure 21A). The heterogeneity of EVs is still not well understood and there is no consensus on their biogenesis and molecular composition [41]. EVs can also be released from the cell after autophagosomes have fused with MVB/LEs to form structures known as amphisomes, which deliver autophagocytosed cellular components to lysosomes for degradation but can also fuse with the PM and release the contents outside the cell (Figure 21A). This process, termed secretory autophagy, can contribute cellular contents to the secreted material in addition to increasing the heterogeneity of the extracellular material released. Thus, a major challenge in this field is the optimization of methods to isolate, separate, and characterize the different subpopulations of EVs.
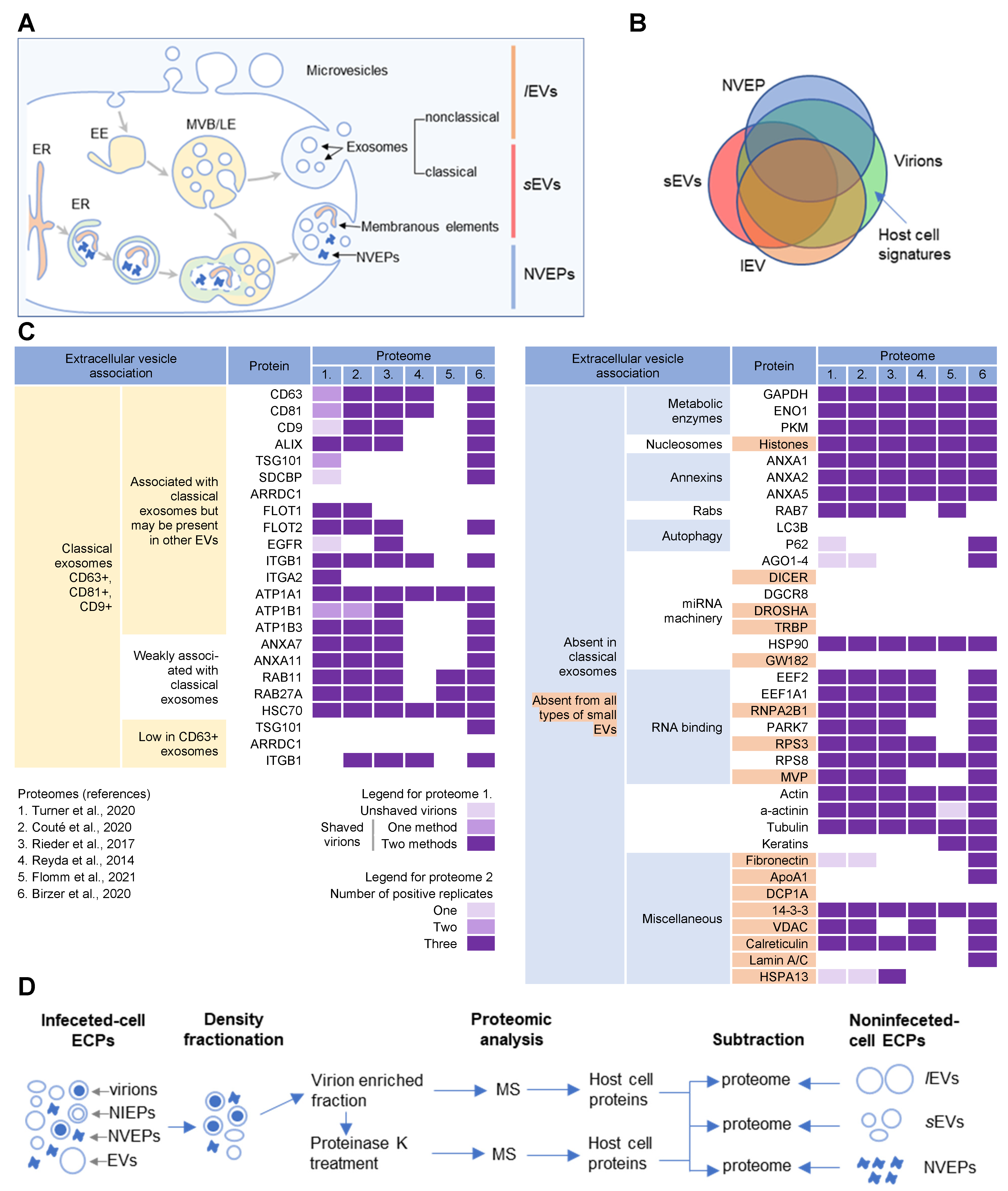
Figure 21. Extracellular nanoparticle content within preparations of beta-herpes virions. (A) Schematic representation of extracellular nanoparticle biogenesis via plasma membrane (PM), exosome, and amphisome-dependent pathways. Microvesicles and non-classical exosomes segregate as large (l) extracellular vesicles (lEVs), whereas classical exosomes segregate as small (s) extracellular vesicles (sEVs). Non-vesicular extracellular particles (NVEPs) and vesicular elements are released by amphisome-dependent and exosome-independent secretion [16,17,18]. (B) Schematic representation of the expected proteomic overlap between lEVs, sEVs, NVEPs, and extracellular virion preparations. (C) Reassessed nanoparticle content [16] identified in HCMV and HSV-1 extracellular virion proteomes. Proteins were identified in five proteomes of HCMV virion preparations (proteome 1–5) and one proteome of HSV-1 heavy particles (proteome 6). Proteome 1 [10] presents data for untreated HCMV virion preparations (unshaved) and the same preparation treated with proteinase K (“shaved” virions). Identification of proteins in shaved virions is marked with different color codes depending on whether they were detected by one or two mass spectrometric methods. Proteome 2 [11] displays data in different color codes depending on whether a protein was identified in one, two, or three biological replicates. Proteomes 3–6 (proteomes 3 [12], 4 [13], 5 [14], and 6 [15]) indicate whether a protein is present or not. (D) Workflow for identification of host cell signatures from proteomes of virion preparations. All proteomes [10,11,12,13,14,15] generated by the MS analysis of virion-enriched fractions after density-gradient separation contained many proteins that are present in EVs and NVEPs including one subjected to short proteinase K treatment [10]. Proteins identified in significant amounts in the proteomes of lEVs, sEVs, and NVEPs [16] are subtracted, which are defined as the lEV, sEV, and NVEP filters.
Several recent studies [16,17,18] have taken steps toward increasing the resolution of EVs’ purification and demonstrated that a large amount of secreted material is released from the cell by amphisomes as non-enveloped extracellular particles (NVEPs) that highly overlap in the content with large (l) EVs (lEVs) and small (s) EVs (sEVs) (Figure 21A,B). NVEPs [16] or exomeres [17,18] represent extracellular, non-membranous molecular assemblies released from cells after cytoplasmic components are engulfed in autophagosomes and fused with MVBs to form amphisomes. Most of the released proteins are the components of large cytoplasmic protein complexes, but components of the membranous organelles (ER, endosomes, and Golgi) can also be found.
2. Membrane-Trafficking Cargo Proteins
Cargo proteins are important markers that have been used over the years to learn about endosomal transport. They pass through different compartments within the membrane system and are usually retained in the intracellular localization with low exit rates. Cargo proteins are anchored in the membrane and therefore, when incorporated into the virions, represent the membrane fraction that becomes entrapped in the virions during envelopment, as well as indicate the transport pathway used by the virus for the envelopment process. The cargo proteins identified in the virion preparations were grouped according to the known transport pathways in the endosomal system (Figure 32).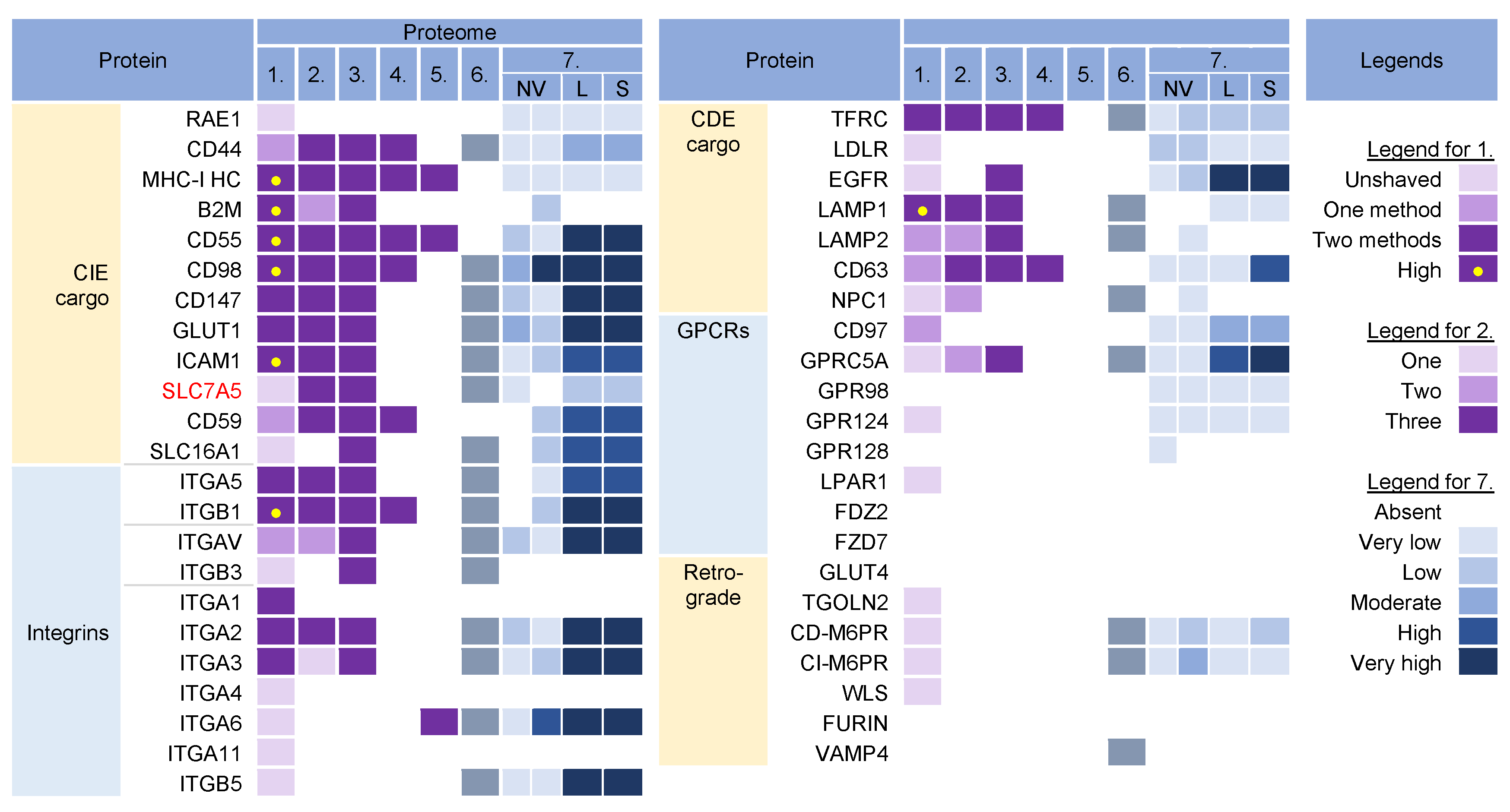
Figure 32. Identification of cargo proteins that travel the endosomal pathways in virion and extracellular nanoparticle preparations. Clathrin-independent endocytosis (CIE) [43,44,45,46] and clathrin-dependent endocytosis (CDE) [47,48,49,50,51,52] cargo proteins, integrins (both CIE and CDE proteins) [53], G protein-coupled receptors (GPCRs) [54], and cargo proteins of the retrograde EE-to-TGN pathway [48,55] were identified in five proteomes of HCMV virion preparations and one proteome of HSV-1 heavy particles as described in Figure 21. Proteome 7 [16] shows the abundance of a cargo protein in high-resolution density-gradient-purified non-vesicular (NV) samples of DKO-1 cells (left box) and Gli36 cells (right box), lEV (L), and sEV (S) samples of DKO-1 cells. A color code of very low (less than 2 × log2 abundance relative to average signal) was set up as a threshold [16]. Low means 2–3×, moderate 3–4×, high 4–5×, and very high ≥5×.
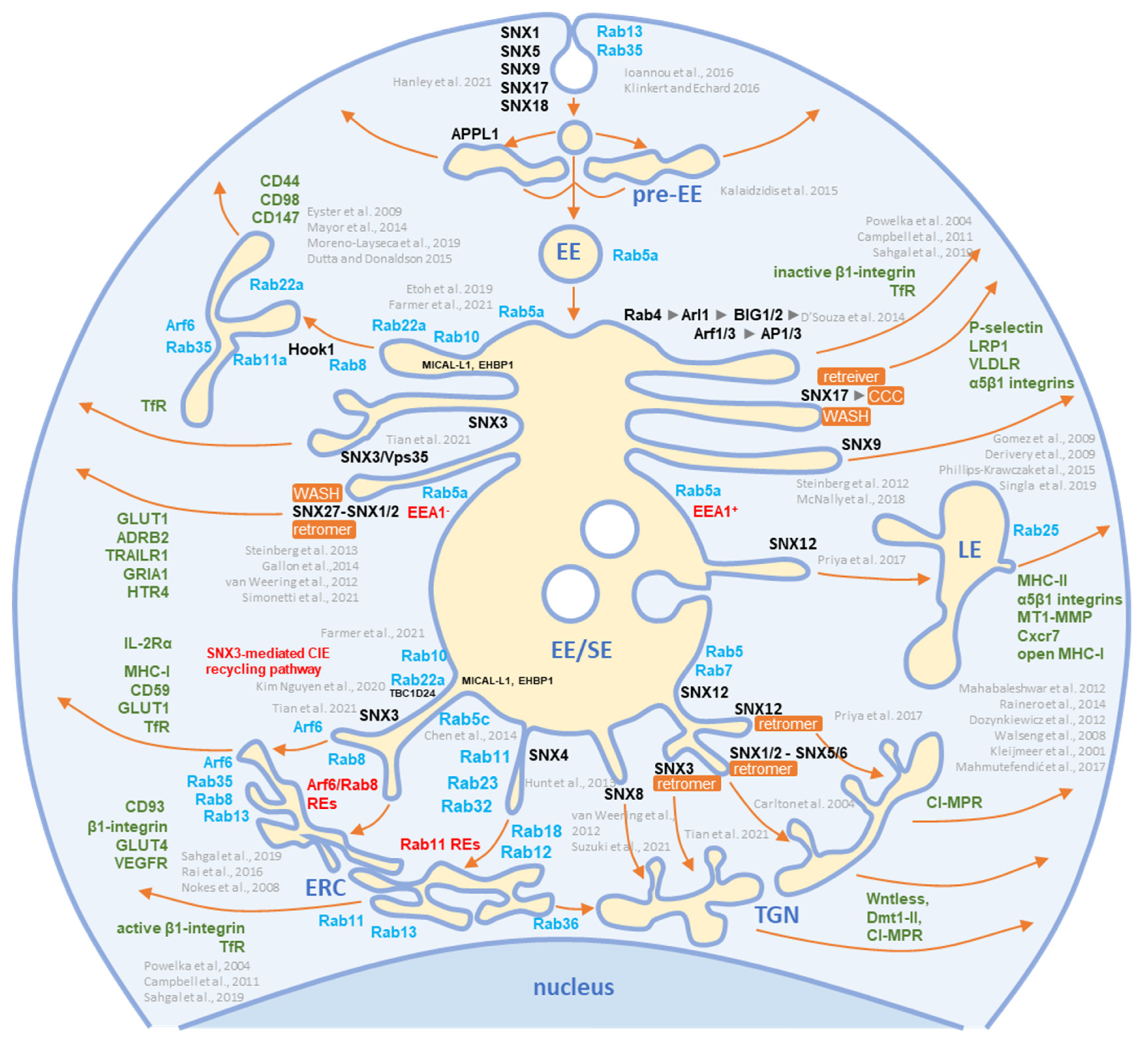
Figure 43. Schematic representation of known exit sites and recycling pathways from the early endosomal system. Cargo internalized by clathrin-dependent endocytosis (CDE) and clathrin-independent endocytosis (CIE) is collected in pre-early endosomes (pre-EEs) and either returned to the plasma membrane (PM) by rapid recycling or delivered to early endosomal (EEs) carriers. EEs undergo a series of fusion and maturation reactions that funnel cargo toward the cell center into enlarged early/sorting endosomes (EE/SE). The maturing EE/SEs sort transported cargo into tubular extensions forming a tubular endosomal network (TEN) that transports cargo directly to the plasma membrane (PM) for recycling, to pericentriolar clusters of tubular endosomes known as the endosomal recycling compartment (ERC) for indirect recycling to the PM, to the trans-Golgi network (TGN) for retrograde transport, or to late endosomes (LEs). EE/SEs also sort cargo by reverse budding into intraluminal vesicles (ILV). Upon completion of the sorting reactions, the remnants of EE /SEs enriched in ILVs become multivesicular endosomes (known as multivesicular bodies, MVBs), which undergo further LE maturation events and either fuse with lysosomes (degradation pathway) or release ILVs as exosomes (exosomal pathway). Cargo sorting into the tubular extension involves the complex and orchestrated recruitment of cellular proteins of the Rab and Arf families, their GEFs and GAPs, members of the sorting nexin (SNX) family, sorting complexes (i.e., retromer, retriever, CCC, and WASH), and other effector proteins required for endosomal tubulation and scission of transport carriers (e.g., dynamins, EHD proteins, AP complexes). The complex cascade of their recruitment is not yet fully elucidated, and the known locations in the EE/SE biogenesis are shown in the schematic. Rab and Arf family proteins are marked in blue, SNXs in black, and cargo molecules in green. Based on references [43,44,46,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94].
3. Rab GTPases
Rab proteins are small GTPases that are master regulators of membrane flux in eukaryotic cells [98]. Similar to other small GTPases, they are activated by guanine nucleotide exchange factors (GEFs) to bind GTP (GTP-bound form) and inactivated by GTPase-activating proteins (GAPs) that facilitate the conversion of GTP to GDP (GDP-bound form). Activated Rab proteins are prenylated and inserted into the membrane, and inactivated Rab proteins are extracted from the membranes by GDP dissociation inhibitor proteins (GDI) and stored in the cytosol. The cycles of activation, followed by insertion into membranes, and inactivation, followed by extraction, determine membrane identity. More than 60 Rab proteins, together with seven phosphoinositides, create the navigation tags that control the recruitment of the effector proteins to the membranes, regulating biogenesis, transport, tethering, and the fusion of the membrane organelles. Membrane flux can be viewed as a sequential wave of Rab recruitment and de-recruitment that directs membrane flux in different directions within the cell. Therefore, Rab signatures in CMV virions should shed light on the history of membranes used for envelopment and their biochemical properties in the context of the known functions of Rab proteins in the membrane system. Rab proteins are highly recruited to the AC area of CMV-infected cells [26], indicating a highly polymorphic membrane composition within the AC. This area is huge and may harbor thousands of membrane-like units in fibroblasts [26], as EM imaging showed that most membrane units are 50–200 nm in diameter [14,29,34,35,37,99]. Although several Rab proteins were expected in the virion preparations, the number of identified Rab proteins was surprisingly high, and at least 16 Rab proteins were detected in the virion preparations treated with proteinase K (Figure 54) Most of these Rab proteins were associated with the tubular domains of the REs, ERC, TGN, and ERGIC. Their presence in the virions would indicate that the virions are enveloped by a complex multidomain membrane structure. However, most of these Rab proteins were also enriched in lEVs, sEVs, and even NVEPs (Figure 54, proteome 7). Although their enrichment in vesicular and non-vesicular extracellular components does not preclude their incorporation into the virions, most of these Rab proteins cannot be considered a reliable signature within the virions. Among the 16 Rabs detected in the proteinase K-treated virion preparations, Rabs 12, 18, 32, and 23 were either not detected or were present in low amounts in lEVs, sEVs, and NVEPs (Figure 54, proteome 7). Three of them (Rabs 12, 18, and 23) were consistently reported in other proteomic studies of HCMV virions (Figure 54, proteomes 2–5). Thus, these Rabs have arisen as the likely signature that may reveal the identity of the envelopment membrane.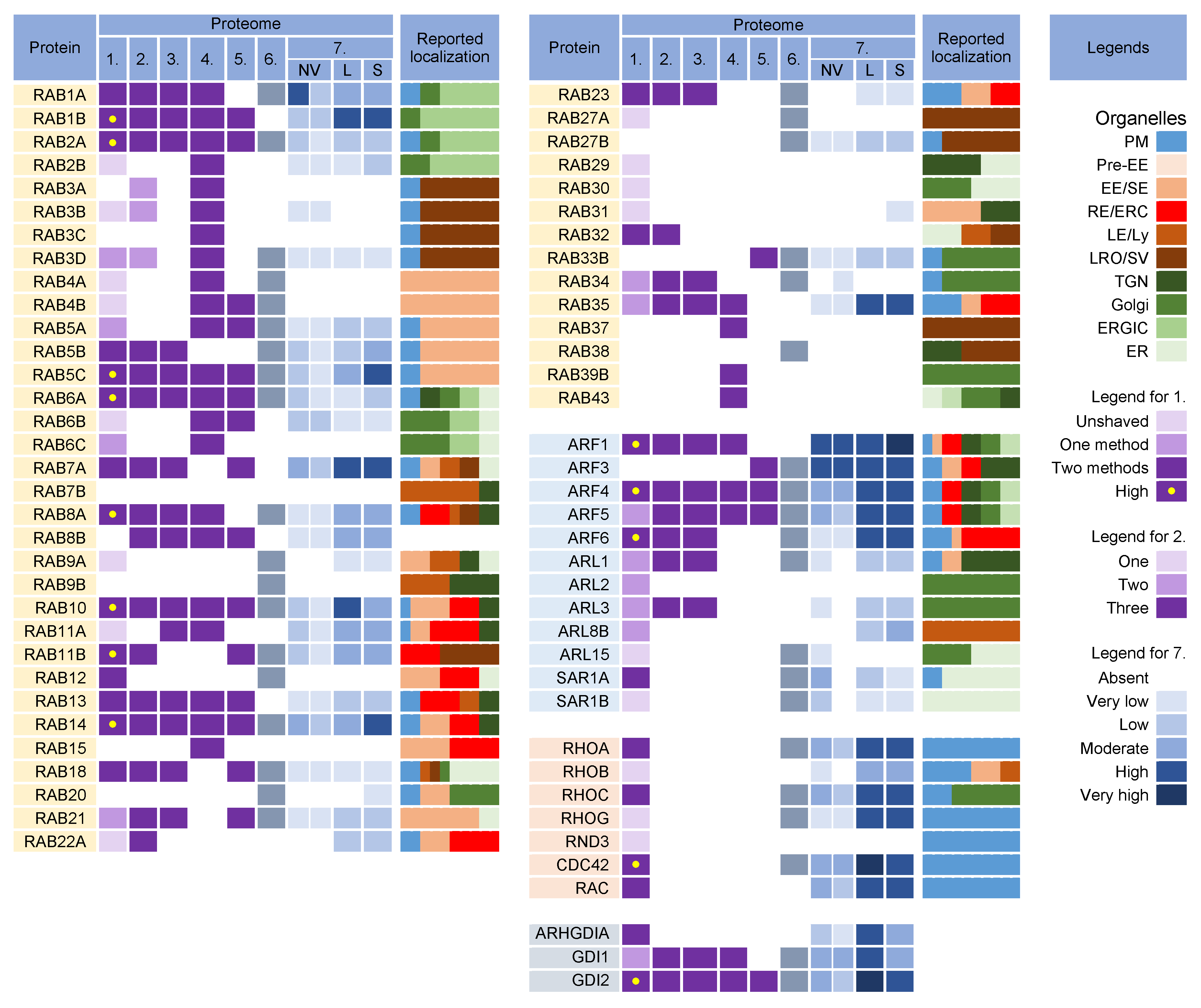
Figure 54. Membrane-trafficking-associated small GTPases in virion and extracellular nanoparticle preparations. Small GTPases were identified in proteomes of virion preparations (HCMV proteomes 1–5 and HSV-1 proteome 6), NVEPs (NV), lEVs (L), and sEVs (S) (proteome 7). The reported localization in membrane organelles of non-infected cells is shown by a color code to illustrate the preferred localization [49,59,60,69,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130]. Proteomes used for analysis and legends are described in Figure 32.
3.1. Rab12, Rab18, Rab23, and Rab32
Rab12 localizes to the EEs and ERC and controls the transport of specific cargo, such as TfR, from the ERC to lysosomes [125] and stimulates autophagy [131], autophagosome trafficking [132], and retrograde transport from the ERC to TGN [133]. It has been suggested that Rab12 facilitates the direct or indirect fusion of RE/ERC-derived compartments with lysosomes [125] and links autophagosomes to motor proteins to facilitate autophagosome trafficking [132]. Rab18 has been localized in the ER and endosomes, and its activity is associated with several processes including autophagy, secretion, and lipid droplet biogenesis (reviewed in [127]). However, its contribution to these processes has not been well characterized, and existing evidence shows that it plays an important role in tethering to the ER and autophagy. Together with Rab10, it regulates ER tubulation, with Rab10 required for ER tubule expansion and Rab18 contributing to the tethering and fusion [134]. The best-established interaction partner of Rab18 is its GEF Rab3GAP [135], which also interacts with ER and the ERGIC protein ERGIC-53 [136], allowing its activation and binding to autophagosome membranes [137]. In addition to the ER, Rab18 is recruited to secretory granules [138], synaptic vesicles [139], and lysosomes [140] in neurons and neuroendocrine cells. Rab23 has been found in tubular endosomes [67], colocalized with Rab5, and internalized Tf in later stages of TfR trafficking in the early recycling compartment, but not in early stages of the EE pathway, LEs, and Golgi [105]. It also contributes to autophagosome development [141]. Rab32 is localized in the EE /REs, mitochondria, ER, and lysosomes [108,109]. Its activity is associated with SNX6/retromer trafficking, trafficking from the EE/REs to melanosomes, the recycling of VAMP7 from melanosomes to early/recycling endosomes, and the regulation of lysosome-related organelles [109]. It is also localized to the ER and supports autophagic membrane formation and autophagy under basal, nutrient-rich conditions [142]. Overall, all four Rab proteins that pass through the NVEP, lEV, and sEV filters are associated with membrane system tubulation, ERC function, and autophagy. Their presence in the virions suggests that envelopment may occur on the tubular membranes derived from the CIE recycling pathway, consistent with the identification of the CIE cargo proteins in the virions (Figure 32). It is likely that these membranes belong to the downstream segments of the CIE recycling pathway, as the virions did not contain appreciable amounts of Rabs 22a, 35, and 36 (Figure 54), three GTPases reported to act at a proximal stage of maturation of the CIE recycling pathway (Figure 43) [143]. Rabs 12, 23, and 32 are involved in the exit pathways from the recycling endosomes distinct from the Rab8a/b pathway [67,105,109,125]. However, their role in the endosomal recycling circuit is insufficiently studied, and it is not possible to determine the functional sequence between them or to assign the effector functions associated with their recruitment. Interestingly, all Rab proteins that pass the NVEP filter are associated with autophagosomes, suggesting that the functions acquired by their recruitment may be important for the maturation of autophagosomes, including the development of phagophores. Thus, it is possible that these functions are redundantly activated by the dysregulated recruitment of a “package” of Rab proteins in the downstream tubular compartments of the modified (rearranged) tubular compartments of the inner AC. These membranes can be expanded and used for envelopment.3.2. Rab Interactors and Effectors
Nine Rab proteins (Rabs 1a, 1b, 2a, 5c, 6a, 7a, 10, 11b, and 14) were consistently detected in all the CMV virion preparations (Figure 54, proteomes 1–5). However, these proteins did not pass the lEV, sEV, and NVEP filters (Figure 54, proteome 7), which does not allow conclusions about their incorporation into the virions. Given that these Rabs act as the master regulators of membrane flux and are highly recruited to the membranes of the AC [26], we also analyzed the presence of their interactors and effectors in the virion preparations. Rabs 1a, 1b, and 2a are key organizers of the intermediate compartment (IC) [102], a vesiculo-tubular cluster in the secretory pathway between the ER and cis-Golgi that forms several pericentrosomal and peripheral subcompartments located in close proximity to the highly curved tubular-vesicular membrane domains of the ER, known as ER Exit Sites (ERES) [102]. The pericentrosomal IC is often positioned as a linker compartment of the Golgi ribbon with spatiotemporal dynamics that overlap with the ERC [144,145]. This topology and tubular phenotype make the IC a suitable structure for CMV envelopment. The virion preparations are also rich in several components of COPI complexes (Figure S1), which are recruited to the IC by both Rab1 and Rab2 [102] but are also abundant in lEVs and NVEPs (Figure S1, proteome 7). Since almost all tethering proteins of IC and other effector proteins of Rab1 and Rab2 [102] are absent in the virions (Figure S1), there is no clue to suggest that IC-derived membranes are used for envelopment. Rab6a acts at the TGN, where it provides docking of endosome-derived vesicles [146]. It recruits the Golgin tethering factors and nucleates the tethering complexes required for the fusion of the arriving endosome-derived vesicles. Rab6 also regulates intra-Golgi trafficking and mediates retrograde transport from the cis-Golgi to ERGIC or ER via tubular carriers, known as the COPI-independent retrograde pathway [147]. The targeting and fusion of these retrograde carriers are mediated by active Rab18 at the ER membrane through interaction with ER-localized tethering factors [127,135,148]. Rab6a is also involved in the regulation of autophagy [149]. The trapping of Rab6 by enveloping the virions may suggest that envelopment also occurs at the Golgi-tract-derived membranes that form the inner (TGN) and outer (Golgi medial and cis- and trans-Golgi stacks) AC area of the CMV-infected cell [26,150]. However, none of the Golgin tethering factors and components of the COG and GARP tethering complexes (Table S1) and Rab6a interactors (Figure S2) were found in the virions, suggesting that Rab6-positive TGN-derived membranes do not contribute to envelopment. The exception is the presence of BICD2 (Figure S2), a known Rab6a effector at the Golgi–ERGIC–ER interface. Rab7a, a key component of the LE pathway, controls transport to LEs and lysosomes and lysosomal biogenesis, positioning, and functions [151]. It is also localized to the ER and modulates ER morphology by controlling ER homeostasis and ER stress [152]. Rab7 is also found on autophagosomes [141]. On endosomes and lysosomes, Rab7a interacts with several effectors, but none of these proteins were found in the virions (Figure S2), suggesting that the envelopment does not occur at the membranes of endosomal or lysosomal origin. Rab11b, a member of the Rab11 family, was highly enriched in the virion preparations. It recruits mainly to the membranes of the ERC and contributes to the control of endosomal recycling and the cell surface proteome [121]. It can also be localized in the EEs, TGN, and post-Golgi vesicles. Rab11b, like Rab11a, contributes to the control of the recycling of the CDE and CIE cargo proteins [43] but may act in different ways [153] and have different localization [154]. Rab11b can be recruited to peripheral lysosomes and regulate lysosome exocytosis [123]. However, none of the major regulatory components of lysosome exocytosis (Rab3a, Sec15, and GRAB) [123] were found in the virions. Rab10 has a variety of functions and subcellular localizations and is involved in various activities in the ERC, Golgi/ TGN and endosomes [155], ER tubulation [156], autophagosome biogenesis, and autophagic flux [157]. Thus, the capture of Rab10 to the enveloping virions may be associated with any tubular compartment, as Rab10 contributes to the initiation of tubulation. The multiple functions of Rab10 have been linked to the recruitment of numerous effectors (Figure S2), but none of these proteins have been found in the virions. Rab5c, similar to Rab5a and 5b isoforms, is an EE Rab essential for the cell survival and maintenance of EEs and LEs [158]. Rab5c can act semi-independently of the other Rab5 isoforms [159], can be recruited to the Arf6/integrin recycling pathway [160], and drives CD93 and active β1-integrin recycling [161]. Consistent with the capture of CIE cargo and several Rab proteins acting in the endosomal recycling pathway, Rab5c can also be captured by the enveloping virions in this pathway. In addition to the known effectors and interactors, an important sign for Rab protein recruitment may be the identification of specific GEF and GAP proteins, which should remain as a signature. However, none of the GEF and GAP proteins reviewed by Müler and Goody [130] were found in the virions (Figure S3).3.3. Why Were There So Many Rabs in the Virion Preparations?
The detection of nearly half of the Rab protein repertoire in the virion preparations may be related to their membrane-bound forms within heterogeneous EVs that copurify with the virions. Because of the physiology of the intracellular Rab cycling, Rab proteins could also be collected and detected in nonmembrane-bound forms either in EVs or NVEPs as adjacent cytosolic Rabs associated with chaperone proteins. The switch between the membrane and cytosol is an important mechanism for the regulation of Rabs [130,162]. After inactivation (conversion to a GDP-bound form), many Rabs are extracted from the membrane by GDI proteins and remain bound to the GDI near the membrane to await the next round of activation. Therefore, many Rab proteins detected in the virion preparations may be those associated with GDIs rather than membranes. Indeed, GDI2 was highly enriched in the virion preparations, as well as in lEVs, sEVs, and NVEPs (Figure 54).3.4. What Did We Learn from Rab Analysis?
Analysis of the Rab proteins in the virions could not answer the question of the origin and identity of the envelopment organelle. Most of the identified Rab proteins passing the lEV, sEV, and NVEP filters (Rabs 12, 23, and 32) are associated with tubular REs harboring the CIE recycling cargo, consistent with the identification of CIE recycling cargo proteins in the virions. These Rabs suggest that the envelopment organelle develops from membranes derived from REs, consistent with the abundance of RE domains within the inner AC [20,26,163]. However, the identification of other Rab proteins that do not pass the lEV, sEV, and NVEP filters should not be neglected. Most of these Rabs are also associated with REs (Rabs 5c, 10, 11b, and 14) but some of them are also associated with tubulation at the ER and ERGIC (Rabs 6a, 7a, 10, and 18) and might suggest that these tubular domains are used for the establishment of the envelopment organelle. The bulk of the ER and ERGIC membranes are dislocated outside the inner AC [19,20,22,26], but there is insufficient information on the presence of ER- and ERGIC-derived membranes in the inner AC. The identified Rab proteins do not rule out a contribution from the TGN tubular domains and weakly support the possibility that the envelopment organelle could derive from the vacuolar EE domains abundant in the inner AC [19,23,26,28,163,164] or from LE domains, which are extruded from the inner AC [19,22,26,163]. All of the Rabs found in the virions are associated with autophagosome biogenesis and maturation, suggesting that autophagosomal membranes can be used for envelopment. However, as discussed later, none of the key autophagic factors were identified in the virions.4. Arf GTPases
The Arf superfamily includes 6 Arfs, 22 Arls, and 2 Sars that control a wide range of cellular functions [114]. All members of the Arf subfamily were found in the virion preparations (Figure 54, proteomes 1–6). However, Arfs 1, 3, 4, and 5 were substantially present in NVEPs, lEVs, and sEVs, whereas Arf6 was highly enriched in lEVs and sEVs (Figure 54, proteome 7). Only five members of the Arl subfamily were detected in the viral proteomes (Figure 54, proteomes 1–6) but none of them were detected in convincing amounts after proteinase K treatment (Figure 54, proteome 1). Both members of the Sar subfamily were detected in the HCMV preparations and Sar1a was present in convincing amounts (Figure 54, proteome 1) but did not pass the NVEP and lEV filters (Figure 54, proteome 7). Unfortunately, the incorporation of Arf proteins into the virions is not a suitable signature. All Arf proteins can be activated at different sites within the membrane system (Figure 54, [113,114,115,118]) and their recruitment into the virions can indicate any membrane structure within the system. In addition, Arf proteins are highly entrapped in EVs and NVEPs, which may complicate their detection in the virions. Nonetheless, Arf proteins appear to be of prominent importance in the biogenesis of the AC and possibly in the secondary envelopment of beta-herpesviruses and virion egress. All Arf proteins have been shown to be highly recruited to membranes within the AC of MCMV-infected cells: Arf3 at the outer and all Arfs at the inner AC membranes [26,165]. Class I (Arfs 1 and 3) and class II (Arfs 4 and 5) Arfs are also overexpressed in the early phase of MCMV infection and remain elevated in the later stages during virion assembly [165]. The suppression of Arf1, Arf3, Arf4, or Arf6 functions by siRNA prevented the establishment of the pre-AC in MCMV-infected cells [165], suggesting their significant contribution to membrane organelle remodeling. However, the suppression of Arf1 and Arf6 also inhibited the establishment of infection [165], suggesting that these GTPases are also involved in the earliest stages of infection. Several studies on HCMV- [30,164,166] and MCMV- [25,26,27,97,165] infected cells have shown that the CIE and CDE cargo are retained inside the AC and their recycling is inhibited. These observations support the identification of the CDE and CIE cargo proteins within the virions Figure 32. The inhibition of the cargo exit from the endosomes of CMV-infected cells has been associated with the alteration of Arf6 function and may provide the basis for the envelopment organelle formation. Immunofluorescence studies of HCMV [30] and MCMV [25,26,27,165] showed excessive recruitment of Arf6 to the membrane units within the inner AC, beginning early in infection. This region of the AC is the site of the secondary envelopment of CMV and is composed mainly of EE -, ERC-, and TGN-derived elements. The HCMV study [30] demonstrated that the CIE cargo is stacked in enlarged Arf6 endosomes that retain Arf6 in the GDP-bound form. Arf6 acts mainly on tubular endosomes that mediate endosomal recycling [167] and contributes to the regulation of autophagy [167,168,169]. These tubular endosomes, known as the tubular endosomal network (TEN) [67,68,170], recycle a subset of CIE cargo proteins to the PM (i.e., CD44, CD98, and CD147) [43,44]. The entire process requires the sequential and orchestrated activation of Rab5, Rab10, Rab22a, Rab35, and Rab11, culminating in the activation of Arf6 (ARF6:GTP) and its hydrolysis to Arf6:GDP before the recycling carriers are released toward the PM [43,44,66]. Another subset of cargo proteins (i.e., MHCI, CD55, and CD59) moves downstream in the EE tract, accumulates in EEA1-positive vacuolar endosomes, and is sorted into the tubular extension to be packaged into transport carriers [44,66,171]. Some of these carriers are returned to the PM via the TEN, whereas others are transported to the juxtanuclear cluster of tubular endosomes that form the ERC [44,46]. Transport to the ERC requires Rab11 activity and the CIE cargo proteins are sorted into Rab8/Arf6/Rab35 intermediates [172]. At the Rab8/Arf6/Rab35 endosomes, Rab35 can recruit multiple Rabs (i.e., Rabs 8, 13, 36) that move on in different directions [111]. Rab8 carriers with recruited Arf6 turn off Rab35 [111,173] and migrate to the PM to ensure the recycling of the CIE cargo from the ERC [111]. It is unclear to what extent the branching can occur at this stage. It is known that Rab36 is required for the delivery of carriers with cargo to the TGN [111], but also that Rab12 [125], Rab23 [67,105], and Rab32 [109] may be recruited and can contribute to different trafficking routes. Thus, multiple recycling paths may be associated with the Arf6-dependent recycling route, and there is still a long way to go to fully understand how the recycling cargo is sorted at this point [70]. In the end, Arf6:GTP hydrolysis is required for recycling and if Arf6 is not inactivated, the endosomal carriers do not reach the point of further progression, the cargo is not recycled, and the endosomal compartment enlarges and retains the endocytic cargo [43,44], as occurs in CMV-infected cells.5. Rho Family GTPases
Some virion preparations were also enriched in RhoA, Rac1, and Cdc42 (Figure 54), the three canonical members of the Rho family GTPases, which consists of 22 genes encoding at least 25 proteins [126]. The Rho family is involved in all cellular processes that depend on the organization of the cytoskeleton and plays a role in the regulation of vesicle transport and endocytosis. However, they were also highly enriched in lEVs, sEVs, and NVEPs (Figure 54, proteome 7) and, therefore, cannot be identified as host cell signatures.6. Adaptor Protein Complexes for Cargo Sorting
The formation of coated vesicles for transport between the membrane compartments is orchestrated by heterotetrameric adaptor protein complexes (AP) [174,175]. They recruit cargo proteins by binding to their cytoplasmic tails and facilitate the formation of clathrin-coated vesicles on membranes. They are activated by cargo and clathrin recruitment and by membrane-associated proteins such as Arf1 and FCH domain-only (FCHo) proteins [176]. Adaptor protein 1 (AP1) complexes can be activated at the TGN, EE, LE, and RE/ERC and can mediate the transport of cargo between the TGN and LE in both directions, from the TGN to the PM, and from the ERC/RE to the PM (recycling). AP2 complexes are associated with endocytic activities at the PM, AP3 likely mediates transport from the TGN to LEs and from LEs to lysosomes, AP4 is involved in transport from the TGN to EEs, and AP5 complexes regulate transport from LEs [174]. Almost all components of AP1 and AP2 complexes were found in the virion preparations (Figure 65, proteomes 1–6) and AP2 components were retained after the proteinase K treatment (Figure 65A, proteome 1). However, the components of AP1 and AP2 complexes were highly enriched in lEVs, sEVs, and NVEPs (Figure 65A, proteome 7). Similarly, clathrin heavy chains and dynamin 2 were highly enriched in most of the virion preparations [10,11,12] but also in lEVs, sEVs, and NVEPs [16] (data not shown). Nevertheless, the AP complexes, clathrin, and dynamin may play an important role in CMV assembly as they are enriched at the membranes of the inner AC of MCMV- [24,26] and HCMV- [29,163,177] infected cells.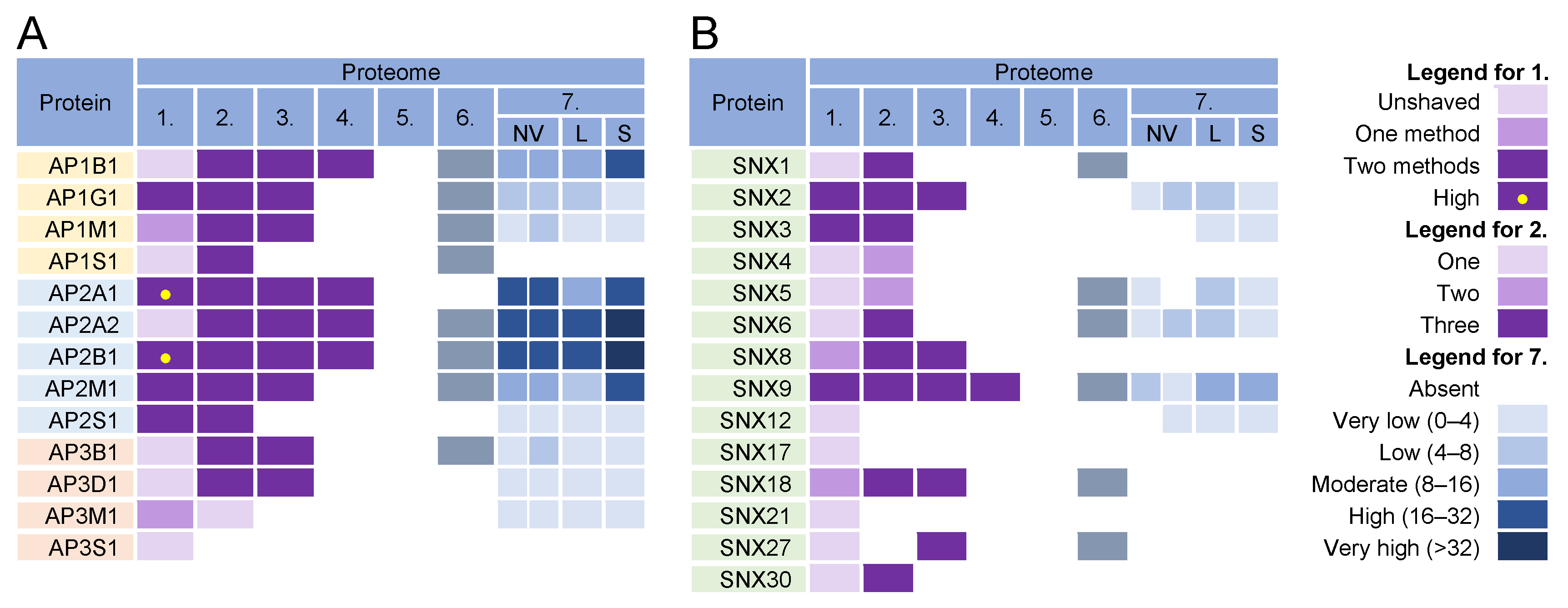
Figure 65. Identification of (A) adaptor protein (AP) complexes and (B) sorting nexins (SNX) in virions and NVEPs in extracellular vesicle preparations. The proteins are identified in five proteomes of HCMV virion preparations (proteome 1–5), proteomes of HSV-1 heavy particles (proteome 6), and NVEPs (proteome 7), as described in Figure 32.
