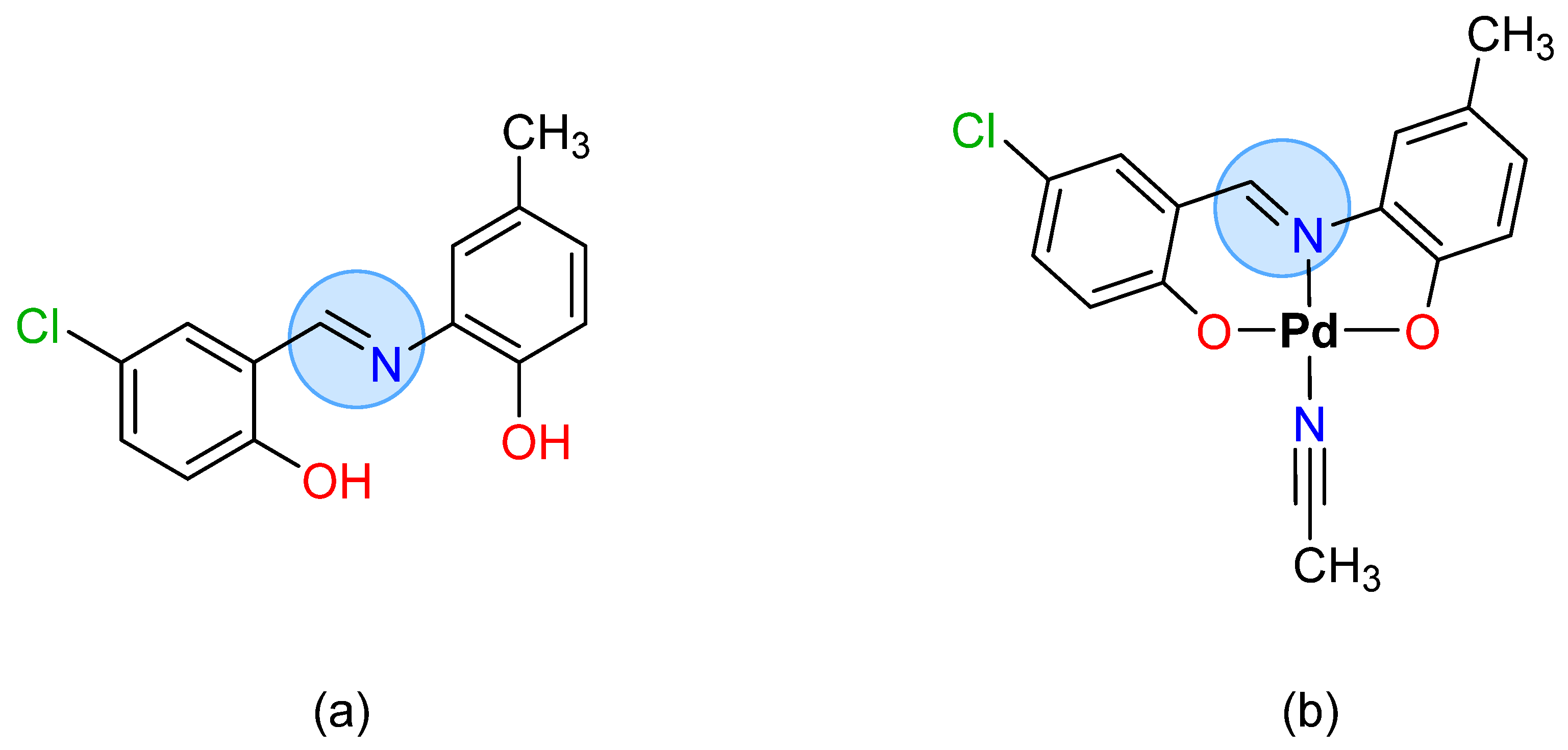
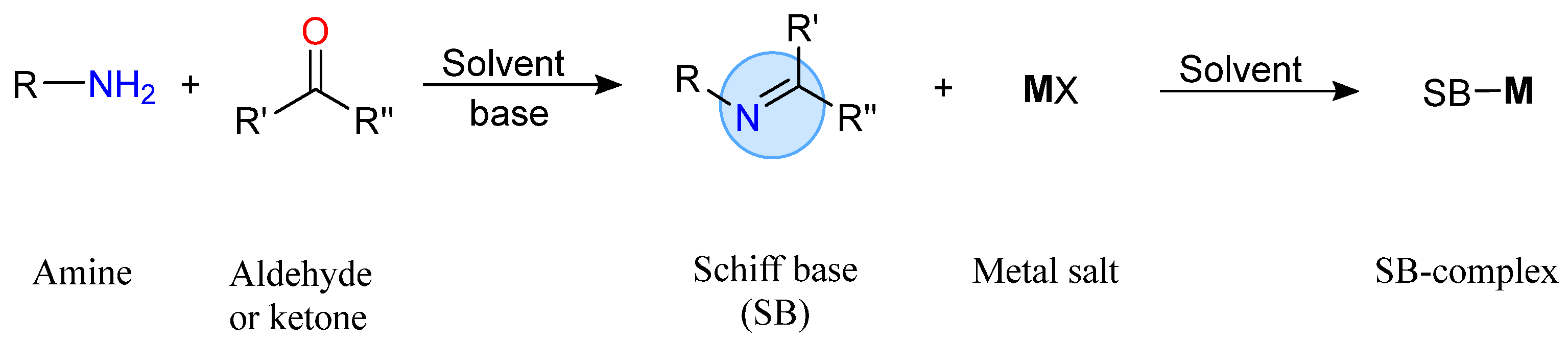
2. Antimicrobial Metallopharmaceuticals with Tridentate Schiff Bases
2.1. Main Group Elements
Medicinal inorganic chemistry is a growing field that has proven to be very effective in the treatment and diagnostic of many diseases [4]. Nowadays, transition metal complexes are the most known and most used compounds in the design of metallodrugs. However, main group elements have received extensive attention since the discovery of salvarsan, also known as arsphenamine or Ehrlich 606, a mixture of 3-amino-4-hydroxyphenyl-As(I) and As(V) compounds synthetized by Paul Ehrlich as an effective cure for syphilis. Salvarsan is acknowledged as the first pharmaceutic cure for a disease and opened the door to the research of new molecules that target specific cells to treat many infections [67]. Arsenic was widely used to treat parasite infections, skin diseases, and anaemia in the early 20th century. Livingstone reported the effective action of arsenic in trypanosomiasis, and subsequent reports showed the beneficial action of arsenic against the parasites in the blood stream, but also acknowledged that since the known forms of arsenic were toxic, its use could be lethal to the patient. Organic arsenicals were recognized as less toxic that their inorganic counterpart and were used to treat syphilis and sleeping sickness [68]. Atoxyl, melarsoprol, and melarsonyl are examples of chemotherapeutic drugs for the treatment of infectious diseases [68,69,70]. Furthermore, antimony-based chemotherapy drugs are the main treatment for Leishmaniosis [71]. The pentavalent antimonial compounds that had traditionally been used as treatment for Leishmaniosis (meglumine antimoniate and sodium stibogluconate) had important side effects, and over time the parasite became resistant, so the available drug was not safe enough nor effective [71,72].
Medicinal inorganic chemistry is a growing field that has proven to be very effective in the treatment and diagnostic of many diseases [13]. Nowadays, transition metal complexes are the most known and most used compounds in the design of metallodrugs. However, main group elements have received extensive attention since the discovery of salvarsan, also known as arsphenamine or Ehrlich 606, a mixture of 3-amino-4-hydroxyphenyl-As(I) and As(V) compounds synthetized by Paul Ehrlich as an effective cure for syphilis. Salvarsan is acknowledged as the first pharmaceutic cure for a disease and opened the door to the research of new molecules that target specific cells to treat many infections [14]. Arsenic was widely used to treat parasite infections, skin diseases, and anaemia in the early 20th century. Livingstone reported the effective action of arsenic in trypanosomiasis, and subsequent reports showed the beneficial action of arsenic against the parasites in the blood stream, but also acknowledged that since the known forms of arsenic were toxic, its use could be lethal to the patient. Organic arsenicals were recognized as less toxic that their inorganic counterpart and were used to treat syphilis and sleeping sickness [15]. Atoxyl, melarsoprol, and melarsonyl are examples of chemotherapeutic drugs for the treatment of infectious diseases [15][16][17]. Furthermore, antimony-based chemotherapy drugs are the main treatment for Leishmaniosis [18]. The pentavalent antimonial compounds that had traditionally been used as treatment for Leishmaniosis (meglumine antimoniate and sodium stibogluconate) had important side effects, and over time the parasite became resistant, so the available drug was not safe enough nor effective [18][19].
Research about the synergic effect of SBs with metals has been addressed mainly for transition metals, and the main group has received less attention, mostly due to the toxicological effects that these kinds of compounds have historically shown. However, in recent decades, the number of reports of medicinal research of SBs with the main group have been increasing. Tin is a main group metal that shares some characteristics with the transition metals. It can form complexes with several organic molecules bound through donor heteroatoms as oxygen, sulphur, or nitrogen, in a wide range of geometries.
SBs with Sn(IV) in vitro antimicrobial activities have been studied against Gram-positive bacteria, Gram-negative bacteria, and fungal strains. In many cases, these complexes showed better activity against Gram-positive strains, such as
S. aureus
and
B. subtilis
, than Gram-negative strains, such as
E. coli
and
P. aeruginosa [76,79,80,81]. This result can be explained by the chelation theory that sustains that chelation increases the lipophilic character of the complexes, allowing the permeation of the compound through the cell wall of the bacteria. Through chelation, the metal polarity is reduced by sharing its positive charge with the heteroatom in the ligand, originating an electron delocalization over the metallo-ring. As such, the biological activity of organotin(IV) complexes depend on the donor ligand, the geometry, and the coordination number over the tin atom [78,79].
[20][21][22][23]. This result can be explained by the chelation theory that sustains that chelation increases the lipophilic character of the complexes, allowing the permeation of the compound through the cell wall of the bacteria. Through chelation, the metal polarity is reduced by sharing its positive charge with the heteroatom in the ligand, originating an electron delocalization over the metallo-ring. As such, the biological activity of organotin(IV) complexes depend on the donor ligand, the geometry, and the coordination number over the tin atom [21][24].
Along with the chemistry of organotin(IV) complexes, the biological activities of hypercoordinated silicon compounds with SB ligands have also been studied [75,88]. Silicon compounds with tridentate SBs are scarce, with some early reports by Puri et al. looking to examine the physical and chemical properties of these compounds. In this report, a series of complexes, which exhibit a hexacoordinate Si(IV) atom and the ligand N,N′-diethylenetriamine-bis(salicylideneimine), are reported [89].
Along with the chemistry of organotin(IV) complexes, the biological activities of hypercoordinated silicon compounds with SB ligands have also been studied [25][26]. Silicon compounds with tridentate SBs are scarce, with some early reports by Puri et al. looking to examine the physical and chemical properties of these compounds. In this report, a series of complexes, which exhibit a hexacoordinate Si(IV) atom and the ligand N,N′-diethylenetriamine-bis(salicylideneimine), are reported [27].
Organotin(IV) complexes with 2-pyridineformamide thiosemicarbazone and its N(4)-methyl and N(4)-ethyl derivatives were evaluated against
C. albicans and
S. typhimurium, showing an activity for both the organic moiety alone, and for the complexes. In addition to the microbial activity, the complexes presented a high activity against malignant glioblastoma [99]. The interesting result of this report was the unusual activity of the 2-pyridineformamide-derived, which proved to be more active against , showing an activity for both the organic moiety alone, and for the complexes. In addition to the microbial activity, the complexes presented a high activity against malignant glioblastoma [28]. The interesting result of this report was the unusual activity of the 2-pyridineformamide-derived, which proved to be more active against S. typhimurium bacteria than against
C. albicans, in contradiction to other reports in the literature. The complex
20a had a higher activity against
S. typhimurium (MIC = 165 µM). All reported complexes had similar antifungal activities (MIC = 270–290 µM) and neither had a better action than the reference drugs chloramphenicol or nystatin. (MIC = 165 µM). All reported complexes had similar antifungal activities (MIC = 270–290 µM) and neither had a better action than the reference drugs chloramphenicol or nystatin.
2.2. Titanium Group
Group IV is the second group of transition metals in the periodic table. It contains four elements: titanium, zirconium, hafnium, and rutherfordium. Due to the small amounts produced and its short half-life, there are currently no uses for rutherfordium outside of basic scientific research, so there are no metal complexes. Hafnium easily absorbs neutrons and is used to control nuclear reactor rods and as an alloying agent with iron, titanium, niobium, and other metals. There are complexes reported but there are no biological evaluations [109]. Zirconium is a corrosion-resistant metal that is used in pumps, valves, and some types of surgical equipment. Zirconium has been used in some complexes of which few have biological evaluations [110].Group IV is the second group of transition metals in the periodic table. It contains four elements: titanium, zirconium, hafnium, and rutherfordium. Due to the small amounts produced and its short half-life, there are currently no uses for rutherfordium outside of basic scientific research, so there are no metal complexes. Hafnium easily absorbs neutrons and is used to control nuclear reactor rods and as an alloying agent with iron, titanium, niobium, and other metals. There are complexes reported but there are no biological evaluations [29]. Zirconium is a corrosion-resistant metal that is used in pumps, valves, and some types of surgical equipment. Zirconium has been used in some complexes of which few have biological evaluations [30].
The most widely used metal of this group for synthesis of SBs complexes is titanium, an extremely corrosion-resistant metal, widely distributed, and the ninth most abundant element in the earth’s crust. Several structures with this metal such as carboxaldehydes, hydroxyacetophenones, and aroylhydrazines present antibiotic properties. Titanium complexes have shown antibacterial, fungicidal, and antioxidant properties mainly in oxidation states of III and IV. Antibacterial and antifungal activities of Ti(III) complexes with SBs derived from furan-2-carboxaldehyde with L-histidine, L-tryptophan, L-valine, L-methionine, and L-glycine have been determined by single disc method against
B. subtilis,
E. coli,
A. fumigatus, and
A. niger using streptomycin as the control [111]. The results show that the activity of the complexes increases with respect to the metal used, in the order Cu(II) > Ni(II) > Ti(III). Interestingly, all the complexes showed moderate activities, whereas the ligands did not present any significant activity against the evaluated microorganisms. using streptomycin as the control [31]. The results show that the activity of the complexes increases with respect to the metal used, in the order Cu(II) > Ni(II) > Ti(III). Interestingly, all the complexes showed moderate activities, whereas the ligands did not present any significant activity against the evaluated microorganisms.
Mononuclear complexes of Ti(III), Cr(III), Mn(III), and Fe(III) with tridentate hydrazone ligands 2-hydroxy-5-chloroacetophenonebenzoylhydrazone and 2-hydroxy-3,5-dichloroacetophenone-4-nitrobenzoylhydrazone were screened for their antimicrobial activity on a nutrient agar medium. In all cases, the complexes showed greater inhibition compared to the free ligands [113]. The Ti(III) complexes, as well as the other complexes, showed a moderate activity against the bacterial strains Mononuclear complexes of Ti(III), Cr(III), Mn(III), and Fe(III) with tridentate hydrazone ligands 2-hydroxy-5-chloroacetophenonebenzoylhydrazone and 2-hydroxy-3,5-dichloroacetophenone-4-nitrobenzoylhydrazone were screened for their antimicrobial activity on a nutrient agar medium. In all cases, the complexes showed greater inhibition compared to the free ligands [32]. The Ti(III) complexes, as well as the other complexes, showed a moderate activity against the bacterial strains E. coli,
S. abony,
P. aeruginosa,
S. aureus, and
B. subtilis, as well as against the fungal strains
A. niger and
C. albicans, with complex being the most active against
A. niger (inhibition zone = 16.50 mm) and for the bacterial strains
E. coli and
S. aboni (inhibition zone = 13.50 and 13.00 mm, respectively). It is suggested that the antibacterial activity is related to the structure of the C=N bond that reduces the polarity of the metal atom, as chelation favours the lipophicity of the metal and increases the permeability of the cell membrane.
2.3. Vanadium Group
Group V of the periodic table contains vanadium, niobium, tantalum, and dubnium. There are complexes with SBs that contain niobium but do not report biological activity [116]. Small amounts of dubnium (Db) are produced and its half-life is short; therefore, there are currently no uses for dubnium outside of basic scientific research, so no complexes are reported. Tantalum is a transition metal with properties very similar to niobium and a very low abundance in the Earth’s crust (0.7 parts per million), which could explain why it is not used to produce complexes.Group V of the periodic table contains vanadium, niobium, tantalum, and dubnium. There are complexes with SBs that contain niobium but do not report biological activity [33]. Small amounts of dubnium (Db) are produced and its half-life is short; therefore, there are currently no uses for dubnium outside of basic scientific research, so no complexes are reported. Tantalum is a transition metal with properties very similar to niobium and a very low abundance in the Earth’s crust (0.7 parts per million), which could explain why it is not used to produce complexes.
Vanadium is a ubiquitous metal and exists in +2, +3, +4, and +5 oxidation states, most commonly in tetravalent and pentavalent forms, which can form several compounds and act as an anion or cation. It is present in trace amounts in plant and animal tissues such as bones, kidneys, liver, spleen, and in less quantity in the brain. In cells, vanadium can be found in the pentavalent form in the nucleus, mitochondria, and cytosol. Intracellularly, it is reduced to vanadyl (VO(IV); VO(II); V(IV)) affecting cellular metabolism. Vanadium forms compounds mainly in +3, +4, and +5 oxidation states, and different structures have previously been evaluated and reviewed [33,117,118]. Structures such as aminoantipyrines, antipyrines, and pyridinecarboxaldehyde, among others, have been evaluated mainly for their antibacterial and antidiabetic activity with good results. P. K. Panchal et al. presented the synthesis of oxovanadium(IV) complexes with SBs: salicylidene-Vanadium is a ubiquitous metal and exists in +2, +3, +4, and +5 oxidation states, most commonly in tetravalent and pentavalent forms, which can form several compounds and act as an anion or cation. It is present in trace amounts in plant and animal tissues such as bones, kidneys, liver, spleen, and in less quantity in the brain. In cells, vanadium can be found in the pentavalent form in the nucleus, mitochondria, and cytosol. Intracellularly, it is reduced to vanadyl (VO(IV); VO(II); V(IV)) affecting cellular metabolism. Vanadium forms compounds mainly in +3, +4, and +5 oxidation states [3][34][35]. Structures such as aminoantipyrines, antipyrines, and pyridinecarboxaldehyde, among others, have been evaluated mainly for their antibacterial and antidiabetic activity with good results. P. K. Panchal et al. presented the synthesis of oxovanadium(IV) complexes with SBs: salicylidene- o-aminothiophenol, bis(benzylidene)ethylenediamine, bis(acetophenone)ethylenediamine, 2,2′-bipyridylamine, bis(benzylidene)-1,8-diaminonaphthalene, thiophene-
o-carboxaldeneaniline, and thiophene-
o-carboxaldene-
p-anisidine. The bactericidal activity of these compounds was evaluated against
S. typhi,
E. coli, and
S. mercescens using the disc diffusion method, finding that the VO(IV) complexes were more active (40–60% inhibition) than the free ligands (10–25% inhibition), but not as effective as the tetracycline control drug (70–100% inhibition). The explanation for the synergistic effect with the presence of oxovanadium(IV) was based on the Overtone concept and the Tweedy chelation theory, where the liposolubility of the molecule is improved, while the polarity of the metal ion is reduced [117]. using the disc diffusion method, finding that the VO(IV) complexes were more active (40–60% inhibition) than the free ligands (10–25% inhibition), but not as effective as the tetracycline control drug (70–100% inhibition). The explanation for the synergistic effect with the presence of oxovanadium(IV) was based on the Overtone concept and the Tweedy chelation theory, where the liposolubility of the molecule is improved, while the polarity of the metal ion is reduced [34].
2.4. Chromium Group
Group VI contains the elements of molybdenum, tungsten, and chromium. There are several complexes containing molybdenum and tungsten but no biological evaluations of them have been available so far [126,127,128]. Molybdenum is an essential trace element that is present in the human body in the liver, kidney, adrenal glands, and bone, and is required for the function of many enzymes. Tungsten is naturally found in rocks and minerals, always combined with other chemicals, and it is used as a catalyst to speed up chemical reactions and several products, such as X-ray tubes, light bulbs, high-speed tools, and others. There are other complexes with SBs but the biological activity is not evaluated [129,130]. Chromium is the most used and biologically evaluated element of this group. It is a natural element in biologic systems, such as animals and plants, predominantly in oxidation states: trivalent chromium, Cr(III), or hexavalent chromium, Cr (VI). Cr(III) is an essential nutrient to normal glucose, proteins, and fats metabolism. The body has systems for reducing chromium(VI) to chromium(III). A chromium(VI) detoxification leads to an increase in the chromium(III) levels. Humans are generally exposed to chromium(III) by eating food, drinking water, and inhaling air that contains the chemical. Some structures with SBs of metformin, aminopyridines, and aminophenols have been obtained and several biological activities have been evaluated, such as the antidiabetic activity [131], but principally the antimicrobial activity against bacterial and fungal species [132]. The biological activity of a complex obtained by the reaction of Cr(NOGroup VI contains the elements of molybdenum, tungsten, and chromium. There are several complexes containing molybdenum and tungsten but no biological evaluations of them have been available so far [36][37][38]. Molybdenum is an essential trace element that is present in the human body in the liver, kidney, adrenal glands, and bone, and is required for the function of many enzymes. Tungsten is naturally found in rocks and minerals, always combined with other chemicals, and it is used as a catalyst to speed up chemical reactions and several products, such as X-ray tubes, light bulbs, high-speed tools, and others. There are other complexes with SBs but the biological activity is not evaluated [39][40]. Chromium is the most used and biologically evaluated element of this group. It is a natural element in biologic systems, such as animals and plants, predominantly in oxidation states: trivalent chromium, Cr(III), or hexavalent chromium, Cr (VI). Cr(III) is an essential nutrient to normal glucose, proteins, and fats metabolism. The body has systems for reducing chromium(VI) to chromium(III). A chromium(VI) detoxification leads to an increase in the chromium(III) levels. Humans are generally exposed to chromium(III) by eating food, drinking water, and inhaling air that contains the chemical. Some structures with SBs of metformin, aminopyridines, and aminophenols have been obtained and several biological activities have been evaluated, such as the antidiabetic activity [41], but principally the antimicrobial activity against bacterial and fungal species [42]. The biological activity of a complex obtained by the reaction of Cr(NO 3)
3∙6H
2O with the SB named (Pyrimidin-2-yliminomethyl)-naphthalen-2-ol was reported by A.M. Abu-Dief et al. testing the antimicrobial activity using the well diffusion method. The results obtained at 10 µg/mL were moderate (inhibition zone = 14.0 mm for