Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Juan Carlos Hernandez Gonzalez and Version 1 by Juan Carlos Hernandez Gonzalez.
CLos coronaviruses are a large family of well- son una gran familia de patógenos bien established pathogens ofecidos de various hosts huéspedes, including domesticidos animals, wildes domésticos, animals, andes salvajes y humanos.
- lateral flow immunoassay
- biosensors
- COVID-19
1. Descripción general del SARS-CoV-2 Overview
CLos coronaviruses are a large family of well- son una gran familia de patógenos bien established pathogens ofecidos de various hosts huéspedes, including domesticidos animals, wildes domésticos, animals, andes salvajes y humanos [ 1 ] . Viruses that caused previous outbreaks iLos virus que causaron brotes anteriores en humanos, causing severeando enfermedades respiratory illness, lung injury, and death, areias graves, lesiones pulmonares y la muerte, son el SARS-CoV (severe acutcoronavirus del síndrome respiratory syndrome coronavirus) iio agudo severo) en 2003 andy el MERS-CoV (Middle Eastcoronavirus del síndrome respiratory syndrome coronavirus) iio de Oriente Medio) en 2012 [ 2 ] . Recent genome analysis with variouUn análisis genómico reciente con varias herramientas bioinformatics tools demonstrated thatáticas demostró que el SARS-CoV-2 has a highltiene un genoma muy similar genome as the Bat al del coronavirus and Bat y el dominio de unión al receptor-binding domain (RBD) of spike glyde la glicoprotein as Malayan pangolinína espiga como el coronavirus del pangolín malayo [ 3 ]. ThiEsta evidenceia indicates that the horseshoe bat is the natura que el murciélago de herradura es el reservoir, and primaryrio natural, y la evidence suggests that the Malayania principal sugiere que el pangolin is an ín malayo es un huésped intermediate hostrio [ 3 ] .
El SARS-CoV-2 is an enveloped virus with a es un virus envuelto con un ARN monocatenario de sentido positive-sense single-stranded RNA. The genome size of this pathogen varies from 29.8 kb to 29.o. El tamaño del genoma de este patógeno varía de 29,8 kb a 29,9 kb [ 4 ] . TheEl virus encodes at leastcodifica al menos 29 proteins. The ínas. Las proteínas estructural proteins are spikees son proteínas de espiga (S), membranea (M), envelope (E), andoltura (E) y nucleocapsidápside (NP) [ 5 ] . Las proteiínas [5].no The non-structural proteines (nsps) have functions required fortienen funciones necesarias para la replication andción y transcription in theción en el ciclo de vida del virus life cycle [ 6 ] . The viral particle size ranges from 80 to El tamaño de las partículas virales oscila entre 80 y 120 nm [ 7 ] .
TheEl mechanism of viralanismo de infection ición viral en humans is through droplets andos es a través de gotitas y aerosols, which can travel through the aires, que pueden viajar por el aire [ 8 ] . Infection occurs in cells thatLa infección ocurre en células que expressan ACE2 (angiotensin-enzima converting enzyme 2) anddora de angiotensina 2) y TMPRSS2 (transmembranproteasa de serine proteasea transmembrana 2) [ 9 ] . TheLa proteína S del coronavirus S protein binds to the se une a ACE2, the primaryel principal receptor fordel SARS-CoV-2 thatque mediates la entrada del virus entry into cells, and las células, y TMPRSS2 cleaves the Sescinde la protein (into S1 and S2 ína S (en las subunits) ofdades S1 y S2) del SARS-CoV-2 , lo que facilitating the fusion of la fusión del SARS-CoV-2 and cel CoV-2 y membrana celular membrane [ 9 ] [ 10 ] [ 11 ]. MorAdeover, it has been demonstrated that the endosomal cysteinemás, se ha demostrado que las cisteína proteases cathepsin B and cathepsin L can alsoas catepsina B y catepsina L endosomal también pueden contribute to thisir a este processo [ 10 ] [ 12 ] [ 13 ] . In theEn el tracto respiratory tractio, ACE2 andy TMPRSS2 arse expressed in thean en las células secretory and ciliated cells in the nose,as y ciliadas de la nariz, las células secretory andas y ciliated cells in thedas de las vías respiratorias conductive airways, in the type IIoras, en las células alveolar cells in the lung as well as in corneales de tipo II en los pulmones y en la conjunctiva in the eyetiva corneal del ojo [ 14 ] [ 15 ] [ 16 ] [ 17 ] .
The etiological virus of the pandemic has continuously evolved, with many variants emerging worldwide. Variants are categorized as the variant of interest, variant of concern, and variant under monitoring [18]. There are five SARS-CoV-2 lineages designated as the variant of concern alpha, beta, gamma, delta, and omicron variants [19]. These variants increase transmissibility compared to the original virus and potentially increase disease severity [20].
2. Immune Response against SARS-CoV-2 in Brief
The SARS-CoV-2 infection involves diverse stages in the individual: start of infection, disease development, recovery, or systemic compromise. Each infection stage triggers and modulates innate and adaptative immune system mechanisms. Although SARS-CoV-2 is a virus that humanity is learning about, the immune response is equipped with mechanisms capable of dealing with this new threat. In the initial phase of SARS-CoV-2 infection, the individual presents a presymptomatic phase lasting up to 5 days, in which a high viral load is present [21]. In these early days of infection, antibodies may not have been produced. Therefore, innate immunity is the first activated. The innate immune response comprises soluble and cellular components that respond nonspecifically against the virus. The cellular compounds include dendritic cells (DC), monocytes, macrophages, neutrophils, natural killer (NK) cells, and other innate lymphoid cells (ILCs) [22]. Whereas soluble components include complement systems, soluble proteins, interferons, chemokines, and naturally occurring antibodies [23]. Immune response cells recognize pathogen-associated molecular patterns (PAMPs) of SARS-CoV-2 through pattern recognition receptors (PRRs) such as Toll-like receptors (TLR), RIG-I-like receptors (RLR), and melanoma differentiation-associated protein 5 (MDA5). The viral sensing triggers the activation of signaling pathways which induce the production of immune mediators to generate an antiviral state mainly mediated by type I (IFN-α/β) and type III (IFN-λ) interferons (IFNs) [24]. Reports have described that robust IFNs production during the early stage of infection is required to have a protective innate immune response against the virus [25]. On the contrary, an inadequate and slow response to type I and type III IFNs due to virus evasion mechanisms, host comorbidities, or genetic defects cause an exacerbated immune response. This inadequate response induces elevated levels of chemokines (CCL2, CCL8, CXCL2, CXCL8, CXCL9, and CXCL16), high expression of proinflammatory cytokines such as IL-6, IL-10, IL-1, and TNF, in addition to activation, and recruitment of immune cells [26][27]. The called “cytokine storm” leads to unbalanced levels of proinflammatory and antiviral mediators that remain the leading cause of ARDS and multi-organ failure [25][26][28].
On the other hand, the adaptive immune response is orchestrated by CD8+ T lymphocytes, TCD4+, and B lymphocytes, responsible for immunological memory. In response to SARS-CoV-2 infection, it has been shown that non-severe patients or patients with mild symptoms have a low viral load and may not have produced antibodies [29][30]. In contrast, antibodies have been detected by immunoassay tests and biosensors in patients with severe symptoms or cases [29][31]. Patients with a high viral load activate the humoral immune response in the first two weeks of infection [32]. The first seroconversion of antibodies is against protein N, followed by protein S of SARS-CoV-2 in patients with disease symptoms [33]. Immunoglobulins IgA and IgM begin to be detected within the first ten days of infection; however, both antibodies can cross-react with protein N, which is highly conserved among coronaviruses [34][35]. Moreover, high levels of IgG1 and IgG3 are expressed ten to fourteen days after infection in patients with disease symptoms [36][37]. Older adults and seriously ill individuals reach high specificity antibodies concentrations against SARS-CoV-2 S protein.
Due to the urgency of reducing thousands of people’s cases and deaths, scientists have developed several vaccines against COVID-19. Efforts are being made to apply vaccines with emergency use authorization to the world population. Vaccination elicits immune responses capable of potently neutralizing SARS-CoV-2. However, the available data show that most approved COVID-19 vaccines protect against severe disease but do not prevent the clinical manifestation of COVID-19 [38]. Instead, it has been demonstrated that new variants with mutations in the spike, the main target of neutralizing antibodies, can escape the neutralization of humoral immunity [39][40].
3. SARS-CoV-2 Detection
Molecular tests or biosensors are the tools for detecting SARS-CoV-2 nucleic acids/ antigens/antibodies against the virus (Figure 1). In the early part of the illness, viral particles and their subunits can be detected; beyond the first two weeks of illness onset, antibodies against the virus could be detected [41]. The SARS-CoV-2 infection stage is highly correlated to the diagnostic technique recommended for the pandemic. Early diagnosis of the disease and isolation of infected people is key to controlling the transmission of SARS-CoV-2 [42][43]. In the initial phase of SARS-CoV-2 infection, the individual presents a presymptomatic phase lasting up to 5 days, in which a high viral load is present [21]. During these early days of infection, antibodies may not be detected. Therefore, since the pandemic began, the diagnostic method has been based on detecting viral genes using the molecular PCR technique, the gold standard worldwide [44][45][46]. The pandemic has exceeded the ability to identify the virus in laboratories using molecular techniques; this has motivated the development of new technologies for the rapid detection of SARS-CoV-2 that are easy to perform compared to molecular tests in clinical laboratories. LFIA has been the unique device approved and available to use in mass worldwide. Biosensors with transducers are developing in SARS-CoV-2 diagnostic. However, most nanomaterials used in these biosensors present interferences with contaminants in human samples compared to performance under experimental conditions. It is important to emphasize that LFIAs have the unique properties of availability, accessibility, economy, and POC (including home use), these characteristics that are not shared by all biosensors with a transducer. In addition, biosensors with transducers require exclusive handling in laboratories certified under the Clinical Laboratory Improvement Amendments of 1998 [47][48]. The FDA have to date approved only one piezoelectric biosensor [47] (Figure 1).
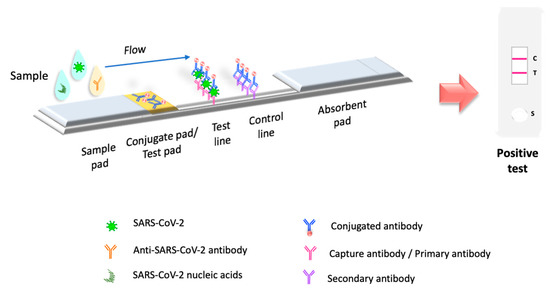
Figure 1. Principio de le of LFIA test. LFIA testa prueba LFIA. La prueba LFIA detects the target molecule on ana la molécula objetivo en una membrana absorbent membrane with antibodies aligned to form the test ande con anticuerpos alineados para formar las líneas de prueba y control lines. The sample is placed on a sample pad, then migrates to th. La muestra se coloca en una almohadilla de muestra, luego migra a la almohadilla de conjugate pad, which contains the immobilized conjugate, usually made of do, que contiene el conjugado inmovilizado, generalmente hecho de nanoparticles (colloidal gold,ículas (oro coloidal, látex colored orado o fluorescent latex,e, celulosa colored celluloseada) conjugated to antibodies or antigens. The sampledas con anticuerpos o antígenos. La muestra interacts with theúa con el conjugate, and bothdo y ambos migrate to the next section of the strip, where the biologicaln a la siguiente sección de la tira, donde se inmovilizan los components of the assayes biológicos del ensayo (proteinínas/antibodies/antigens) are immobilized. In this section, the analyte and cuerpos/antígenos). En esta sección, se capturan el analito y el conjugate are captured. Excess reagent passes through thado. El exceso de reactivo pasa a través de las líneas de capture lines and accumulates on thea y se acumula en la almohadilla absorbent pad. Thee. Los results arados se interpreted on than en la membrana de nitrocellulose membrane as the ulosa como la presence or absence of the test andia o ausencia de las líneas de prueba y control lines.
References
- Hasöksüz, M.; Kiliç, S.; Saraç, F. Coronaviruses and SARS-COV-2. Turk. J. Med. Sci. 2020, 50, 549–556.
- Gilbert, G.L. SARS, MERS and COVID-19—New Threats; Old Lessons. Int. J. Epidemiol. 2020, 49, 726–728.
- Kadam, S.B.; Sukhramani, G.S.; Bishnoi, P.; Pable, A.A.; Barvkar, V.T. SARS-CoV-2, the Pandemic Coronavirus: Molecular and Structural Insights. J. Basic Microbiol. 2021, 61, 180–202.
- Khailany, R.A.; Safdar, M.; Ozaslan, M. Genomic Characterization of a Novel SARS-CoV-2. Gene Rep. 2020, 19, 100682.
- Kim, D.; Lee, J.-Y.; Yang, J.-S.; Kim, J.W.; Kim, V.N.; Chang, H. The Architecture of SARS-CoV-2 Transcriptome. Cell 2020, 181, 914–921.e10.
- Bačenková, D.; Trebuňová, M.; Špakovská, T.; Schnitzer, M.; Bednarčíková, L.; Živčák, J. Comparison of Selected Characteristics of SARS-CoV-2, SARS-CoV, and HCoV-NL63. Appl. Sci. 2021, 11, 1497.
- Kirtipal, N.; Bharadwaj, S.; Kang, S.G. From SARS to SARS-CoV-2, Insights on Structure, Pathogenicity and Immunity Aspects of Pandemic Human Coronaviruses. Infect. Genet. Evol. 2020, 85, 104502.
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten Scientific Reasons in Support of Airborne Transmission of SARS-CoV-2. Lancet 2021, 397, 1603–1605.
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2021, 23, 3–20.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8.
- Papa, G.; Mallery, D.L.; Albecka, A.; Welch, L.G.; Cattin-Ortolá, J.; Luptak, J.; Paul, D.; McMahon, H.T.; Goodfellow, I.G.; Carter, A.; et al. Furin Cleavage of SARS-CoV-2 Spike Promotes but Is Not Essential for Infection and Cell-Cell Fusion. PLoS Pathog. 2021, 17, e1009246.
- Padmanabhan, P.; Desikan, R.; Dixit, N.M. Targeting TMPRSS2 and Cathepsin B/L Together May Be Synergistic against SARS-CoV-2 Infection. PLoS Comput. Biol. 2020, 16, e1008461.
- Prasad, K.; AlOmar, S.Y.; Almuqri, E.A.; Rudayni, H.A.; Kumar, V. Genomics-Guided Identification of Potential Modulators of SARS-CoV-2 Entry Proteases, TMPRSS2 and Cathepsins B/L. PLoS ONE 2021, 16, e0256141.
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 Entry Factors Are Highly Expressed in Nasal Epithelial Cells Together with Innate Immune Genes. Nat. Med. 2020, 26, 681–687.
- Lukassen, S.; Chua, R.L.; Trefzer, T.; Kahn, N.C.; Schneider, M.A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A.W.; et al. SARS-CoV-2 Receptor ACE2 and TMPRSS2 Are Primarily Expressed in Bronchial Transient Secretory Cells. EMBO J. 2020, 39, e105114.
- Zhou, L.; Xu, Z.; Castiglione, G.M.; Soiberman, U.S.; Eberhart, C.G.; Duh, E.J. ACE2 and TMPRSS2 Are Expressed on the Human Ocular Surface, Suggesting Susceptibility to SARS-CoV-2 Infection. Ocul. Surf. 2020, 18, 537–544.
- Fodoulian, L.; Tuberosa, J.; Rossier, D.; Boillat, M.; Kan, C.; Pauli, V.; Egervari, K.; Lobrinus, J.A.; Landis, B.N.; Carleton, A.; et al. SARS-CoV-2 Receptors and Entry Genes Are Expressed in the Human Olfactory Neuroepithelium and Brain. iScience 2020, 23, 101839.
- World Health Organization. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 23 May 2022).
- Khandia, R.; Singhal, S.; Alqahtani, T.; Kamal, M.A.; El-Shall, N.A.; Nainu, F.; Desingu, P.A.; Dhama, K. Emergence of SARS-CoV-2 Omicron (B.1.1.529) Variant, Salient Features, High Global Health Concerns and Strategies to Counter It amid Ongoing COVID-19 Pandemic. Environ. Res. 2022, 209, 112816.
- Choi, J.Y.; Smith, D.M. SARS-CoV-2 Variants of Concern. Yonsei Med. J. 2021, 62, 961–968.
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission from People Without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057.
- Diamond, M.S.; Kanneganti, T.-D. Innate Immunity: The First Line of Defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176.
- Boechat, J.L.; Chora, I.; Morais, A.; Delgado, L. The Immune Response to SARS-CoV-2 and COVID-19 Immunopathology—Current Perspectives. Pulmonology 2021, 27, 423–437.
- Thorne, L.G.; Reuschl, A.-K.; Zuliani-Alvarez, L.; Whelan, M.V.X.; Turner, J.; Noursadeghi, M.; Jolly, C.; Towers, G.J. SARS-CoV-2 Sensing by RIG-I and MDA5 Links Epithelial Infection to Macrophage Inflammation. EMBO J. 2021, 40, e107826.
- Severa, M.; Diotti, R.A.; Etna, M.P.; Rizzo, F.; Fiore, S.; Ricci, D.; Iannetta, M.; Sinigaglia, A.; Lodi, A.; Mancini, N.; et al. Differential Plasmacytoid Dendritic Cell Phenotype and Type I Interferon Response in Asymptomatic and Severe COVID-19 Infection. PLOS Pathog. 2021, 17, e1009878.
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9.
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine Elevation in Severe and Critical COVID-19: A Rapid Systematic Review, Meta-Analysis, and Comparison with Other Inflammatory Syndromes. Lancet Respir. Med. 2020, 8, 1233–1244.
- García-Pérez, B.E.; González-Rojas, J.A.; Salazar, M.I.; Torres-Torres, C.; Castrejón-Jiménez, N.S. Taming the Autophagy as a Strategy for Treating COVID-19. Cells 2020, 9, 2679.
- Casadevall, A.; Joyner, M.J.; Pirofski, L.-A. SARS-CoV-2 Viral Load and Antibody Responses: The Case for Convalescent Plasma Therapy. J. Clin. Invest. 2020, 130, 5112–5114.
- Wang, H.; Yuan, Y.; Xiao, M.; Chen, L.; Zhao, Y.; Zhang, H.; Long, P.; Zhou, Y.; Xu, X.; Lei, Y.; et al. Dynamics of the SARS-CoV-2 Antibody Response up to 10 Months after Infection. Cell Mol. Immunol. 2021, 18, 1832–1834.
- Xu, L.; Li, D.; Ramadan, S.; Li, Y.; Klein, N. Facile Biosensors for Rapid Detection of COVID-19. Biosens. Bioelectron. 2020, 170, 112673.
- Yongchen, Z.; Shen, H.; Wang, X.; Shi, X.; Li, Y.; Yan, J.; Chen, Y.; Gu, B. Different Longitudinal Patterns of Nucleic Acid and Serology Testing Results Based on Disease Severity of COVID-19 Patients. Emerg. Microbes Infect. 2020, 9, 833–836.
- Herroelen, P.H.; Martens, G.A.; De Smet, D.; Swaerts, K.; Decavele, A.-S. Humoral Immune Response to SARS-CoV-2: Comparative Clinical Performance of Seven Commercial Serology Tests. Am. J. Clin. Pathol. 2020, 154, 610–619.
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020, 26, 1478–1488.
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody Responses to SARS-CoV-2 in Patients with COVID-19. Nat. Med. 2020, 26, 845–848.
- Loos, C.; Atyeo, C.; Fischinger, S.; Burke, J.; Slein, M.D.; Streeck, H.; Lauffenburger, D.; Ryan, E.T.; Charles, R.C.; Alter, G. Evolution of Early SARS-CoV-2 and Cross-Coronavirus Immunity. mSphere 2020, 5, e00622-20.
- Luo, H.; Jia, T.; Chen, J.; Zeng, S.; Qiu, Z.; Wu, S.; Li, X.; Lei, Y.; Wang, X.; Wu, W.; et al. The Characterization of Disease Severity Associated IgG Subclasses Response in COVID-19 Patients. Front. Immunol. 2021, 12, 632814.
- Kyei-Barffour, I.; Addo, S.A.; Aninagyei, E.; Ghartey-Kwansah, G.; Acheampong, D.O. Sterilizing Immunity against COVID-19: Developing Helper T Cells I and II Activating Vaccines Is Imperative. Biomed. Pharmacother. 2021, 144, 112282.
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 Variants Escape Neutralization by Vaccine-Induced Humoral Immunity. Cell 2021, 184, 2372–2383.e9.
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced Sensitivity of SARS-CoV-2 Variant Delta to Antibody Neutralization. Nature 2021, 596, 276–280.
- Sethuraman, N.; Jeremiah, S.S.; Ryo, A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020, 323, 2249–2251.
- Caliendo, A.M.; Gilbert, D.N.; Ginocchio, C.C.; Hanson, K.E.; May, L.; Quinn, T.C.; Tenover, F.C.; Alland, D.; Blaschke, A.J.; Bonomo, R.A.; et al. Better Tests, Better Care: Improved Diagnostics for Infectious Diseases. Clin. Infect. Dis. 2013, 57, S139–S170.
- Kevadiya, B.D.; Machhi, J.; Herskovitz, J.; Oleynikov, M.D.; Blomberg, W.R.; Bajwa, N.; Soni, D.; Das, S.; Hasan, M.; Patel, M.; et al. Diagnostics for SARS-CoV-2 Infections. Nat. Mater. 2021, 20, 593–605.
- World Health Organization. Laboratory Testing for 2019 Novel Coronavirus (2019-NCoV) in Suspected Human Cases. Available online: https://www.who.int/publications-detail-redirect/10665-331501 (accessed on 23 May 2022).
- Ravi, N.; Cortade, D.L.; Ng, E.; Wang, S.X. Diagnostics for SARS-CoV-2 Detection: A Comprehensive Review of the FDA-EUA COVID-19 Testing Landscape. Biosens. Bioelectron. 2020, 165, 112454.
- Corman, V.M.; Drosten, C. Authors’ Response: SARS-CoV-2 Detection by Real-Time RT-PCR. Eurosurveillance 2020, 25, 2001035.
- Food and Drug Administration. In Vitro Diagnostics EUAs—Antigen Diagnostic Tests for SARS-CoV-2; FDA: Washington, DC, USA, 2022.
- CDC. Clinical Laboratory Improvement Amendments (CLIA). Available online: https://www.cdc.gov/clia/index.html (accessed on 27 August 2022).
More
