Interaction of the ionizing radiation with the coolant (water) results in the generation of a variety of radiolysis products, with those being identified to include e−aq, H, OH, H2O2, HO2, HO2-, O2, O2-, O22-, O−, O, H2, OH−, H+ and possibly others. These species are electroactive (i.e., they can participate in charge transfer reactions at metal/solution interfaces), thereby impacting corrosion processes and may lead to enhanced damage and to failure of components in the reactor primary coolant circuits by generating an excessively high electrochemical corrosion potential (ECP). Therefore, to understand and predict the impact of corrosion on the structural materials in the IBED PHTS of the ITER, the concentrations of these species must be known. While methods are available for measuring the concentrations of some of the radiolysis products (e.g., O2, H2, H2O2) in the laboratory, few techniques are presently available for measuring the concentrations of the more energetic species, such as e−aq, OH, HO2, O−, O, O2-, O22- and O2−, all of which are present at very low concentrations, in a plant environment, although absorption spectroscopic methods are used with great success in the laboratory and might be adapted to the field. However, from an electrochemical/corrosion viewpoint, there is no pressing need for knowing the concentrations of many of these highly energetic species. This is because the contribution that any given species makes to determining the ECP and the corrosion rate is roughly proportional to its concentrations because the rate of transport to the surface and the partial current density are often mass transport controlled and hence are proportional to the concentration.
2. A Brief Primer on the Physics and Technology of ITER
One great advantage afforded by fusion as an energy source is demonstrated in
Table 1, which summarizes the mass conversions for five different energy production technologies. The conversion values are calculated using Einstein’s energy/mass equivalence formula:
ΔE=Δmc2, from the known energy yields, where
ΔE is the energy produced upon conversion of a rest mass of
Δm and
c is the velocity of light (3 × 10
8 m/s). One sees from these data that the nuclear technologies—fission of
235U
92 and the fusion of deuterium and tritium—are by far the most efficient in converting mass into energy, except for particle/antiparticle annihilation (e.g., e
−-p
+), for which the conversion efficiency is 100%. However, a cost-effective method for producing antiparticles (positrons) in sufficient amounts has yet to be devised to render this reaction to be of practical interest. It is also noted that of the technologies identified in
Table 1, all but fusion and particle/antiparticle annihilation are currently contributing electrical energy to the national grid.
Table 1.
Mass conversion factors for various energy production technologies.
A second great advantage of fusion power is the limited amount of waste that is produced per unit of energy produced. Thus, according to an ORNL pamphlet, a small US city of 100,000 homes over 1 month requires 330,000,000 MJ of energy. If powered by coal, 150 railroad cars of bituminous coal are required that would produce 31,135 metric tons of CO
2; 31 metric tons of CO; 67 metric tons of NO
x; 365 tons of SO
x; 411 metric tons of particulates; and 33 kg of formaldehyde, in addition to about 22 lbs (10 kg) of U and 57.6 lbs (26 kg) of Th. On the other hand, a fusion reactor would consume 5 kg of D (
2H
1) and T (
3H
1) and produce 4 kg of helium (
4He
2), for which a market already exists. Clearly, from an environmental impact viewpoint, fusion is the preferred technology. However, the production of high-energy neutrons necessarily means that neutron activation and transformation of some elements in the structural materials will occur. Accordingly, a radioactive disposal problem upon decommissioning will be faced, not unlike that in the decommissioning of a fission reactor.
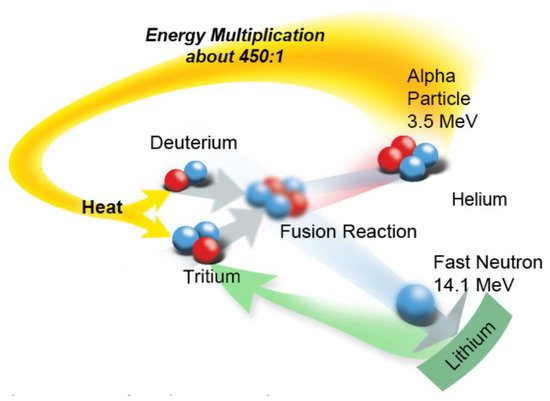
A schematic of the D–T fusion reaction, which has the lowest “ignition temperature” of alternative fission reactions listed in
Table 2, is presented in
Figure 21. It is seen that fusion is envisioned to occur via the fusion of the two nuclei to produce an unstable quasi-nucleus, comprising two protons and three neutrons. This entity then decomposes to produce a high-energy alpha particle (
4He
2, 3.5 MeV) and a high-energy neutron (
1n
0, 14.1 MeV) with a concomitant loss of mass, in addition to γ-photons of 15–25 MeV. For fusion to occur, the D and T nuclei must approach one another to within a few diameters of the nucleus (a few femtometers), where the strong nuclear force can overcome coulombic repulsion and quantum mechanical tunneling of nucleons from one nucleus to the other can occur. This can only occur at very high kinetic energies that are sufficiently high that positively charged nuclei can approach one another to the requisite distance for tunneling to occur; that requires very high temperatures, of the order of 20 keV or more than 300,000,000 K. The energy released during the fusion process theoretically represents a 450:1 multiplication over the energy required to heat hydrogen nuclei to the fusion point
[2][1].
Figure 21.
Schematic of the D–T fusion reaction. ©2018 US ITER.
Attempts to harness fusion power have been underway since the 1950s, during which two basic approaches have evolved. The first, toroidal magnetic confinement fusion (MCF), is a Russian invention in which a low-density D–T plasma is confined magnetically in a toroidal chamber and is heated to fusion temperatures using extremely-high-density current and high-energy neutral particle and/or ion injection. In the second approach, inertial confinement fusion (ICF), or laser implosion, a mixture of D–T is contained within a small pellet, which is pulse irradiated from all sides using high-energy lasers, with the resulting adiabatic compression raising the temperature to the fusion threshold. Thus, these two fusion devices attempt to emulate the processes that power the sun and the stars—viz. the fusion of deuterium (
2H
1) and tritium (
3H
1):
2H
1 +
3H
1 →
4He
2 (3.5 MeV) +
1n
0 (14.1 MeV), as noted above, and that occur in thermonuclear weapons, respectively. However, several other fusion reactions involving the isotopes of hydrogen, as summarized in
Table 2, are also possible. As noted above, the D–T reaction has the lowest “ignition temperature”, and hence this reaction is of the greatest interest in current fusion technology. It is also the reaction that results in the greatest conversion of mass into energy. Because this case uses tritium, which is not naturally occurring, tritium must be “bred” from some other nucleus, such as that described by the reaction
7Li
3(
1n
0,
4He
2)
3H
1. On the other hand, if the D–D reaction was chosen, D is of plentiful supply in nature, as about 140 ppm of this isotope exists in natural water and the technology for its extraction is well developed. However, it requires a higher ignition temperature, and the mass conversion factor is not as attractive as the D–T reaction.
In 1957, Lawson
[6][2] derived the conditions that must be attained in the plasma for “breakeven” fusion (i.e., energy produced by fusion exceeding the energy input) as the “triple product”:
where
n is the plasma density (#/m
3),
T is the temperature (keV), and
τE is the confinement time. The two approaches to controlled thermonuclear fusion differ in how these parameters are combined to achieve the Lawson energy balance. Thus, MCF aims at moderate
n and
T and a high
τE to achieve the balance, while ICF makes use of high
n and
T combined with short
τE to achieve the required breakeven condition. Furthermore, MCF seeks to produce a controlled, sustained “burn,” while ICF seeks to produce repetitive, miniature thermonuclear explosions. To date, MCF has come closest to attaining its stated goal.
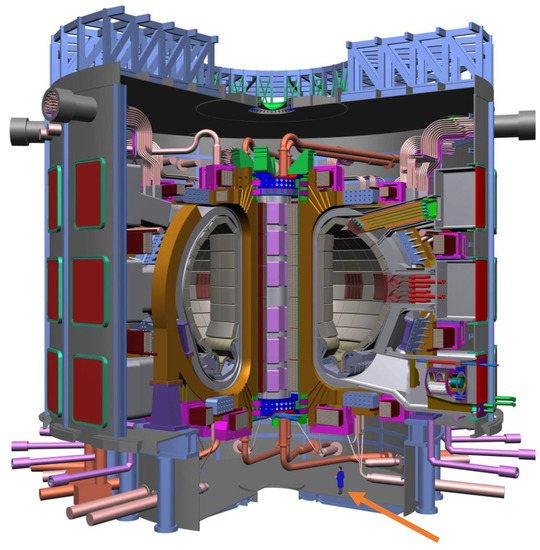
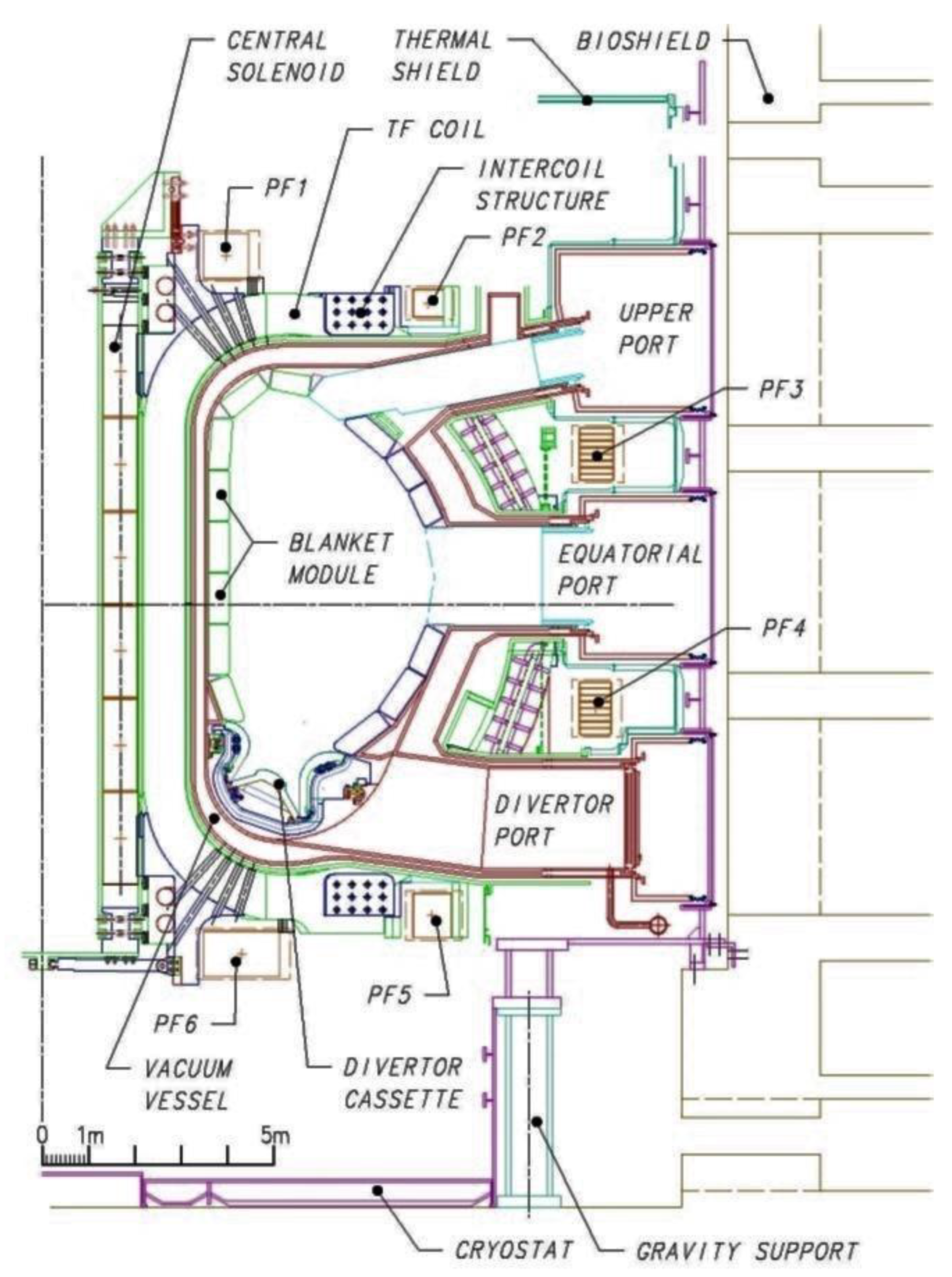
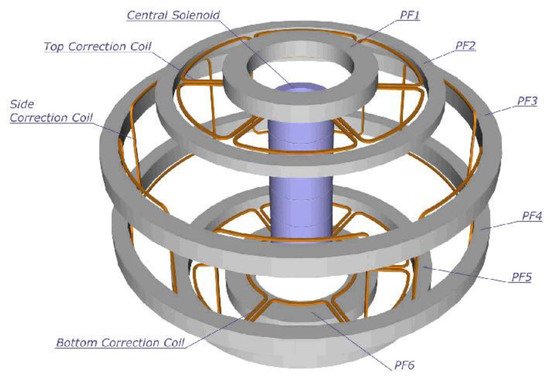
ITER is an example of the first approach (MCF) and is the logical extension of JET (Joint European Torus), which is operated in Oxford, England, UK. JET achieved a
Q-value (i.e., the energy produced from fusion/input energy to heat the plasma) of 0.67 (16 MW out for 24 MW in), but ITER is expected to yield
Q ≥ 10 (500 MW out for 50 MW in) over a sustained “burn” of about 550 s followed by a dwell period of 1250 s, to yield a repetition period of 1800 s (
Table 3). Thus, ITER is a confined plasma device comprising a toroidal vacuum chamber and 5.3 T, Nb
3Sn superconducting magnets that are designed to confine the hot plasma away from the walls.
Table 3. ITER Tokamak operating parameters (from ITER Technical Basis [4]). ITER Tokamak operating parameters (from ITER Technical Basis [3]).