Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mateusz Sochacki and Version 2 by Camila Xu.
Sapindus mukorossi Gaertn., also called the washnut, is a tropical tree of the Sapindaceae family. The plant owes its name to its cleaning and washing properties used by the local population as a natural detergent. The most important ingredients of the plant are triterpenoid saponins contained in many parts of the plant, inducing fruits, galls, or roots. The tree also contains other valuable, biologically active compounds that are obtained by extraction methods. Raw or purified extract and isolated saponins are valuable plant products that can be used in the food, pharmaceutical, cosmetic, and chemical industries.
- Sapindus mukorossi
- washnut
- triterpenoid saponins
- natural surfactants
1. Introduction
Secondary plant metabolites are a rich source of many substances that manifest biological activity [1]. Contemporary economic development places particular emphasis on pro-ecological activities, including the preference for technological solutions based on natural, renewable material sources, especially using plant sources for this purpose [2][3][4][2,3,4]. Detergents are a key group of products of industrial importance, and they are intended for general use, with a strong impact on the environment [5]. Their surfactant application not only has to do with cleaning agents. Due to the amphiphilic nature of these compounds, which are responsible for adsorption, emulsion, washing, or foaming properties, they are also widely used in the industry [6][7][6,7]. Among other things, detergents are products or additives in the food, cosmetic, pharmaceutical, textile-leather, and metallurgical-petrochemical industries [8].
Saponins are natural, secondary plant metabolites with surfactant properties [9], synthesized by plants and some marine organisms [10]. In terms of chemical structure, they are classified as glycosides. The name saponins is derived from their soap-like properties [11], where the Latin word sapo means ‘soap’ [12]. In aqueous solutions, saponins reduce the surface tension of water and manifest foam-forming properties [13]. The detergent properties of saponins result from their amphiphilic structure [14], which consists of a hydrophobic skeleton known as aglycone (or genin) and hydrophilic sugar groups (glycone) [15][16][15,16]. The two glycoside-forming parts are the basis for the structural diversion of saponins in nature [11]. The glycone part consists of one or more sugar chains [17], which are then bonded with the aglycone via a glycosidic linkage [18]. The O-glycosidic bond separates the two structural parts of saponins [15], functioning as a border (Figure 1). Saponins are mainly classified on the basis of differences in aglycone structure or the number of sugar chains [19]. The basic classification based on the structure of the skeleton distinguishes two main groups: steroid and triterpenoid. Steroid glycoalkaloids are also sometimes included as saponins [10][11][10,11]. Steroidal aglycones typically consist of 27, while triterpenoid ones typically consist of 30 carbon units in the skeleton [20]. In addition to carbon variation, the structural diversity of the aglycone involves the different types and arrangements of substituents and further modifications in the backbone [21].
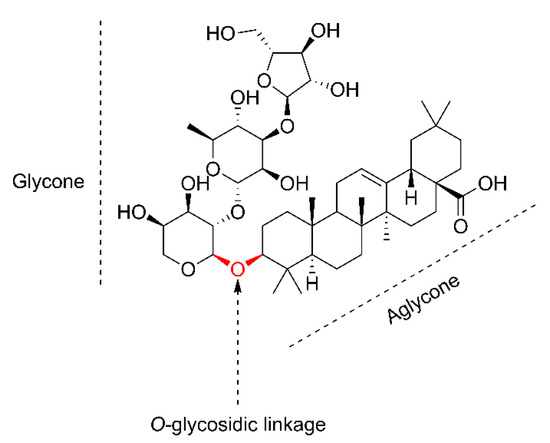
Figure 1.
Chemical structure description of oleanolic acid saponin present in the pulp of
S. mukorossi
Saponins are found among many families of vascular plants in the form of secondary metabolites [17]. This group also includes representatives of the Sapindaceae family, which synthesize triterpenoid-type saponins [23]. It consists of a number of species, including the Sapindus genus: Sapindus trifoliatus, Sapindus saponaria, Sapindus laurifolia, Sapindus oahuensis, and Sapindus mukorossi [24].
2. Plant Description
Sapindus mukorossi Gaertn., also called the Chinese soapberry, soapnut, reetha, or washnut, is part of the Sapindaceae family [24]. The plant is a deciduous tree found in the tropical and sub-tropical regions of Asia [25], native to China, and cultivated in Japan, India, Bengal, and Pakistan [26]. The tree is a widely cultivated species due to its many applications [27].2.1. Plant Morphology
The tree occupies the upper reaches of the Indo-Gangetic plains, Shivaliks, and sub-Himalayan areas at altitudes of 200–1500 m. In most cases, one can find a tree growing naturally in North India. The plant can reach from 12 to 15 m in height, occasionally reaching up to 20 m and 1.8 m in girth. The trunk is covered with bark of dark-pale yellow, fairly smooth, with numerous vertical line lenticels and fissures exfoliating in irregular wood scales. The tree is covered with leaves (30–50 cm in length), alternate and paripinnate, consisting of 5–10 pairs of leaflets of lanceolate shape, alternate and opposite. Each of the leaflets has a length of 2.5–5 cm. Leaves develop from March or April. At the end of December, they turn yellow and are shed for the period from December to January. For about 2 months (until March), the tree is leafless, then overgrows again. Inflorescences consist of terminal panicles about 30 cm long, with pubescent branches. Numerous greenish-white polygamous flowers, mostly bisexual with five sepals, reach 5 mm across. Flower panicles appear in April with white or purple color. The tree bears fruit in May and matures in June–July. In October and November, ripe fruits change color from yellow-orange to dark brown. The fruits have a spherical shape with one, rarely with two drupels, 1.8–2.5 cm across. Spherical, black seeds reach diameters of 0.8–1.3 cm and are present loosely in the dry fruit [24].2.2. Traditional Plant Applications
Plants of the Sapindus genus were utilized by the indigenous people and are now perceived as valuable plant raw materials. Many plant parts of Sapindus species are regarded as therapeutic resources, including fruits, bark, roots, seeds, and leaves. These plants are also a source of natural detergents, which have been used to wash silk and wool. Indian jewelers used fruits as a cleaner for precious metal ornaments and to wash out the cardamom. Sapindus trees can also be used for phytoremediation, land reclamation, and afforestation [24]. As mentioned, fruits are a valuable resource for the washnut tree [28]. Traditionally in Japan, S. mukorossi pericarps are called enmei-hi, which means ‘life-prolonging pericarp’, and in China, wu-huan-zi, as ‘non-illness fruit’ [29]. In natural medicine, they are used to treat eczema, pimples, psoriasis, epilepsy, chlorosis, migraine, and due to the presence of saponin, also to remove lice from the scalp [27]. Moreover, ground seeds of the soapnut are used to treat problems with dentition, arthritis, colds, nausea, and constipation [26]. In Ayurvedic medicine, seeds were used to remove tan and skin wrinkles [30]. The leaves are used in baths to relieve joint pain and the roots for the treatment of gout and rheumatism [31]. Plants of the genus Sapindus are often used for similar purposes. The availability of the species S. trifoliatus, S. Saponaria, and S. mukorossi has contributed to their wide medical use [24].3. Plant Phytoconstituents
The interest in the Sapindus species is due to the presence of different saponins in many parts of the plant. Sapindus plants also contain many different types of active substances [24][30][24,30]. It is assumed that this is due, as in the case of secondary metabolites, to the function they perform in the plant, including mainly ensuring its survival [32]. Considering the elements of the plant, various phytoactive compounds can be distinguished in the washnut tree. The methanolic extract of S. mukorossi leaves contains many bioactive compounds, including alkaloids, flavonoids, phenols, carbohydrates, terpenoids, and saponins [33]. The stems also include flavonoid, phenolic, and polysaccharide constituents [31][34][31,34]. A large amount of saponins, amounting to about 10.1–11.5% of the fruit, are present in the pericarp (Figure 2), where this value increases to 56.5% in the drupe [27]. The fruit also contains about 10% sugars, mucilage [35], and sesquiterpene oligoglycosides [36]. Kernel mass consists of 40% oil, which is a mixture of medium-chain monounsaturated and polyunsaturated fatty acids, mostly of oleic and linoleic acid, respectively [28], along with triglycerides [37]. Roots, flowers, and galls are also a source of triterpenoid saponins [38][39][40][41][42][43][44][38,39,40,41,42,43,44]. The plant is grown for its fruit, the pericarp of which is used as a natural soap. Other parts of the plant are also used for many other purposes [26]. Among the triterpenoid saponins that occur in the plant, three types are most common. Oleanane, dammarane, and tirucallane-type saponins occur in roots, flowers, fruits, pericarps, and galls [38][39][40][41][42][43][44][45][46][47][48][38,39,40,41,42,43,44,45,46,47,48], and the recently discovered lupane-type present in the pulp of the plant [22]. The aforementioned structural diversion of aglycone (Figure 3) and glycone part is present in S. mukorossi.
Figure 2.
Commercially available, dry washnut pericarps.
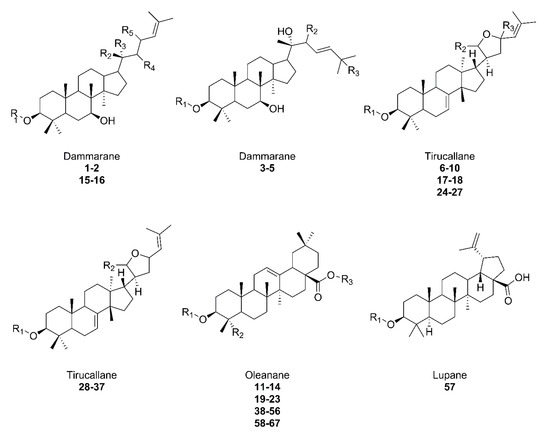
Figure 3. Overview of saponin structures present in S. mukorossi. The structure extensions are shown in Table 1 with the corresponding numbers.
Table 1.
Sapindus mukorossi
saponins present in different parts of the plant.
| No. | Chemical Name | Abbreviations | Type | Ref. |
|---|---|---|---|---|
| 1 | 3β,7β,20(S),22-tetrahydroxydammar-24-ene-3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside | R1: -Glc2 a-Rha b R2: -CH3 R3: -OH R4: -OH R5: -H |
Dammarane | [41] |
| 2 | 3β,7β,20(S),22,23-pentahydroxydammar-24-ene-3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside | R1: -Glc2-Rha R2: -CH3 R3: -OH R4: -OH R5: -OH |
||
| 3 | 3β,7β,20(S),22,25-pentahydroxydammar-23-ene-3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside | R1: -Glc2-Rha R2: -OH R3: -OH |
Dammarane | [41] |
| 4 | 25-methoxy-3β,7β,20(S),22-tetrahydroxydammar-23-ene-3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside | R1: -Glc2-Rha R2: -OH R3: -OCH3 |
||
| 5 | 25-methoxy-3β,7β,20(R)-trihydroxydammar-23-ene-3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside | R1: -Glc2-Rha R2: -H R3: -OCH3 |
||
| 6 | 21β-methoxy-3β,21(S),23(R)-epoxytirucalla-7,24-diene-3-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside | R1: -Glc6-Rha R2: β-OCH3 R3: β-H |
Tirucallane | [40] |
| 7 | 21α-methoxy-3β,21(S),23(R)-epoxytirucalla-7,24-diene-3-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside | R1: -Glc6-Rha R2: α-OCH3 R3: β-H |
||
| 8 | 21α-methoxy-3β,21(R),23(R)-epoxytirucalla-7,24-diene-3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside | R1: -Glc2-Rha R2: α-OCH3 R3: β-H |
||
| 9 | 21β-methoxy-3β,21(S),23(R)-epoxytirucalla-7,24-diene-3-O-α-l-dirhamnopyranosyl-(1→2,6)-β-d-glucopyranoside | R1: -Glc2,6-Rha,Rha R2: β-OCH3 R3: β-H |
||
| 10 | 21α-methoxy-3β,21(R),23(R)-epoxytirucalla-7,24-diene-3-O-α-l-dirhamnonopyranosyl-(1→2,6)-β-d-glucopyranoside | R1: -Glc2,6-Rha,Rha R2: α-OCH3 R3: β-H |
||
| 11 | Hederagenin-3-O-(3-O-acetyl-α-l-arabinopyranosyl)-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Ara3 c-OAc R2: -CH2OH R3: -H |
Oleanane | [38] |
| 12 | Hederagenin-3-O-(4-O-acetyl-α-l-arabinopyranosyl)-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Ara4-OAc R2: -CH2OH R3: -H |
||
| 13 | Hederagenin-3-O-(2,3-O-diacetyl-β-d-xylopyranosyl)-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl2,3 d-OAc,OAc R2: -CH2OH R3: -H |
||
| 14 | Hederagenin-3-O-(2,4-O-diacetyl-β-d-xylopyranosyl)-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl2,4-OAc,OAc R2: -CH2OH R3: -H |
||
| 15 | 3,7,20(S)-trihydroxydammar-24-ene-3-O-α-l-rhamnopyrnosyl-(1→2)-β-d-glucopyranoside | R1: -Glc2-Rha R2: -OH R3: -CH3 R4: -H R5: -H |
Dammarane | [38] |
| 16 | 3,7,20(R)-trihydroxydammar-24-ene-3-O-α-l-rhamnopyrnosyl-(1→2)-β-d-glucopyranoside | R1: -Glc2-Rha R2: -CH3 R3: -OH R4: -H R5: -H |
||
| 17 | 21α-methoxy- 3β,21(R),23(S)-epoxytirucall-7,24-diene-3-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside | R1: -Glc2-Glc R2: α-OCH3 R3: β-H |
Tirucallane | [39] |
| 18 | 21α-methoxy-3β,21(R),23(S)-epoxytirucall-7,24-diene-3-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside | R1: -Glc2-Glc6-Rha R2: α-OCH3 R3: β-H |
||
| 19 | Hederagenin-3-O-(3,4-O-di-acetyl-β-d-xylopyranosyl)-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl3,4-OAc,OAc R2: -CH2OH R3: -H |
Oleanane | [38] |
| 20 | Hederagenin-3-O-(2-O-acetyl-β-d-xylopyranosyl)-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl2-OAc R2: -CH2OH R3: -H |
||
| 21 | Hederagenin-3-O-(3-O-acetyl-β-d-xylopyranosyl)-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl3-OAc R2: -CH2OH R3: -H |
||
| 22 | Hederagenin-3-O-(4-O-acetyl-β-d-xylopyranosyl)-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl4-OAc R2: -CH2OH R3: -H |
||
| 23 | Hederagenin-3-O-α-l-arabinopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Ara R2: -CH2OH R3: -H |
||
| 24 | 21β-methoxy-3β,23α-epoxytirucalla-7,24-diene-3-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside | R1: -Glc6-Rha R2: β-OCH3 R3: α-H |
Tirucallane | [42] |
| 25 | 21β-methoxy-3β,23α-epoxytirucalla-7,24-diene-3-O-α-l-dirhamnopyranosyl-(1→2,6)-β-d-glucopyranoside | R1: -Glc2,6-Rha,Rha R2: β-OCH3 R3: α-H |
||
| 26 | 21α-methoxy-3β,23α-epoxytirucalla-7,24-diene-3-O-α-l-rhamnopyranosyl-(1→2)-[α-l-arabinopyranosyl-(1→3)]-β-d-glucopyranoside | R1: -Glc2,3-Rha,Ara R2: α-OCH3 R3: α-H |
||
| 27 | 21α-methoxy-3β,23α-epoxytirucalla-7,24-diene-3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside | R1: -Glc2-Rha R2: α-OCH3 R3: α-H |
||
| 28 | 3-O-α-l-rhamnopyranosyl-(1→2)-[α-l-arabinopyranosyl-(1→3)]-β-d-glucopyranosyl-21,23R-epoxyl tirucall-7,24R-diene-3β,21-diol | R1: -Glc2,3-Rha,Ara R2: -OH |
Tirucallane | [44] |
| 29 | 3-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyl-21,23R-epoxyl tirucall-7,24R-diene-3β,21-diol | R1: -Glc6-Rha R2: -OH |
||
| 30 | 3-O-α-l-rhamnopyranosyl-(1→2)-[α-l-arabinopyranosyl-(1→3)]-β-d-glucopyranosyl (21,23R)-epoxyl tirucalla-7,24-diene-(21S)-ethoxyl-3β-ol | R1: -Glc2,3-Rha,Ara R2: -OCH2CH3 |
Tirucallane | [45] |
| 31 | 3-O-α-l-rhamnopyranosyl-(1→2)-[α-l-arabinopyranosyl-(1→3)]-β-d-glucopyranosyl (21,23R)-epoxyl tirucall-7,24-diene-(21S)-methoxyl-3β-ol | R1: -Glc2,3-Rha,Ara R2: -OCH3 |
||
| 32 | 3-O-α-l-arabinopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-[α-l-arabinopyranosyl-(1→3)]-β-d-glucopyranosyl-21,23R-epoxyl tirucalla-7,24-diene-21β-ethoxy-3β-ol | R1: -Glc2,3-(Rha3-Ara),Ara R2: -OCH2CH3 |
Tirucallane | [43] |
| 33 | 3-O-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-[β-l-arabinopyranosyl-(1→3)]-β-d-glucopyranosyl-21,23R-epoxyl tirucalla-7,24-diene-21β-ethoxy-3β-ol | R1: -Glc2,3-(Rha3-Xyl),Ara R2: -OCH2CH3 |
||
| 34 | 3-O-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-[α-l-arabinopyranosyl-(1→3)]-β-d-glucopyranosyl-21,23R-epoxyl tirucalla-7,24-diene-21β-methoxy-3β-ol | R1: -Glc2,3-(Rha3-Xyl),Ara R2: -OCH3 |
||
| 35 | 3-O-α-l-arabinopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-[α-l- rhamnopyranosyl-(1→3)]-β-d-glucopyranosyl-21,23R-epoxyl tirucalla-7,24-diene-21β-ethoxy-3β-ol | R1: -Glc2,3-(Rha3-Ara),Rha R2: -OCH2CH3 |
||
| 36 | 3-O-α-l-arabinopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→3)]-β-d-glucopyranosyl-21,23R-epoxyl tirucalla-7,24-diene-21β-methoxy-3β-ol | R1: -Glc2,3-(Rha3-Ara),Rha R2: -OCH3 |
||
| 37 | 3-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyl-21,23R-epoxyl tirucalla-7,24-diene-21β-ethoxyl-3β-ol | R1: -Glc6-Rha R2: -OCH2CH3 |
||
| 38 | Hederagenin-3-O-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl-28-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranosyl ester | R1: -Ara2-Rha3-Xyl R2: -CH2OH R3: -Glc2-Glc |
Oleanane | [47] |
| 39 | Hederagenin-3-O-α-l-arabinopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl-28-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranosyl ester | R1: -Ara2-Rha3-Ara R2: -CH2OH R3: -Glc2-Glc |
||
| 40 | Hederagenin-3-O-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl-28-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranosyl ester | R1: -Ara2-Rha R2: -CH2OH R3: -Glc2-Glc |
||
| 41 | Hederagenin-3-O-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha R2: -CH2OH R3: -H |
Oleanane | [49][54] |
| 42 | Hederagenin-3-O-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl R2: -CH2OH R3: -H |
||
| 43 | Hederagenin-3-O-β-d-glucopyranosyl-(1→4)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl4-Glc R2: -CH2OH R3: -H |
Oleanane | [50][57] |
| 44 | Hederagenin-3-O-β-d-glucopyranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→6)]-β-d-glucopyranosyl-(1→4)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl4-Glc2,6-Glc,Rha R2: -CH2OH R3: -H |
Oleanane | [51][56] |
| 45 | Hederagenin-3-O-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl-28-O-β-d-glucopyranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→6)]-β-d-glucopyranosyl-(1→4)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosy-(1→2)-α-l-arabinopyranosyl ester | R1: -Ara2-Rha3-Xyl R2: -CH2OH R3: -Ara2-Rha3-Xyl4-Glc2,6-Glc,Rha |
Oleanane | [52][55] |
| 46 | Hederagenin-3-O-β-d-glucopyranosyl-(1→3)-β-d-xylopyranosyl-(1→3)- β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl3-Xyl3-Glc R2: -CH2OH R3: -H |
Oleanane | [53][58] |
| 47 | Hederagenin-3-O-(3,4-O-diacetyl-α-l-arabinopyranosyl)-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Ara3,4-OAc,OAc R2: -CH2OH R3: -H |
||
| 48 | 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-xylopyranosyl-(1→6)-β-d-glucopyranosyl-(1→3)-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranosyl oleanolic acid | R1: -Ara2-Rha3-Xyl3-Glc6-Xyl2-Rha R2: -CH3 R3: -H |
Oleanane | [46] |
| 49 | Hederagenin 3-O-(2,4-O-di-acetyl-α-l-arabinopyranosyl)-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Ara2,4-OAc,OAc R2: -CH2OH R3: -H |
Oleanane | [54][59] |
| 50 | Hederagenin 3-O-α-l-arabinopyranoside | R1: -Ara R2: -CH2OH R3: -H |
||
| 51 | Hederagenin-3-O-β-d-xylopyranosyl-(2→1)-[3-O-acetyl-α-l-arabinopyranosyl]-28-O-α-l-rhamnopyranosylester | R1: -Xyl2-Ara3-OAc R2: -CH2OH R3: -Rha |
Oleanane | [48] |
| 52 | Hederagenin 3-O-α-l-rhamnopyranosyl (3→1)-[2,4-O-diacetyl-α-l-arabinopyranosyl]-28-O-β-d-glucopyranosyl-(2→1) [3-O-acetyl-β-d-glucopyranosyl] ester | R1: -Rha3-Ara2,4-OAc,OAc R2: -CH2OH R3: -Glc2-Glc3-OAc |
Oleanane | [55][50] |
| 53 | Oleanolic acid 3-O-α-l-arabinofuranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Ara e R2: -CH3 R3: -H |
Oleanane | [22] |
| 54 | Hederagenin 3-O-5‴-O-acetyl-α-l-arabinofuranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Ara5 e-OAc R2: -CH2OH R3: -H |
||
| 55 | 23-O-acetyl-hederagenin 3-O-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl R2: -CH2OAc R3: -H |
||
| 56 | Gypsogenin 3-O-α-l-arabinopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Ara R2: -CH2O R3: -H |
||
| 57 | Betulinic acid 3-O-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl | Lupane | [22] |
| 58 | Hederagenin-3-O-β-d-glucopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Glc R2: -CH2OH R2: -H |
Oleanane | [36] |
| 59 | Hederagenin-3-O-α-l-rhamnopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Rha R2: -CH2OH R3: -H |
Oleanane | [36] |
| 60 | Hederagenin-3-O-β-d-xylopyranosyl-(1→3)-α-l-arabinopyranoside | R1: -Ara2-Xyl R2: -CH2OH R3: -H |
||
| 61 | Hederagenin-3-O-(4-O-acetyl-β-d-glucopyranosyl)-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Glc4-OAc R2: -CH2OH R3: -H |
||
| 62 | 3-O-β-d-glucopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranosyl oleanolic acid | R1: -Glc2-Rha3-Rha2-Glc R2: -CH3 R3: -H |
||
| 63 | 3-O-β-d-xylopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranosyl oleanolic acid | R1: -Glc2-Rha3-Rha2-Xyl R2: -CH3 R3: -H |
||
| 64 | Oleanolic acid 3-O-(4-O-acetyl-α-l-arabinopyranosyl)-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Ara4-OAc R2: -CH3 R3: -H |
||
| 65 | Gypsogenin 3-O-α-l-rhamnopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Rha R2: -CHO R3: -H |
||
| 66 | Oleanolic acid 3-O-β-d-xylopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Xyl R2: -CH3 R3: -H |
Oleanane | [22] |
| 67 | Oleanolic acid 3-O-α-l-arabinopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside | R1: -Ara2-Rha3-Ara R2: -CH3 R3: -H |
a β-d-Glucopyranosyl, b α-l-Rhamnopyranosyl, c α-l-Arabinopyranosyl, d β-d-Xylopyranosyl, e α-l-Arabinofuranosyl.
