As a promising alloying approach, the modification of chemical composition by increasing the B content and decreasing the N content has been applied to improve the creep resistance of various 9–12% Cr heat-resistant martensitic steels. The 9–12% Cr steels have to exhibit high long-term creep strength, oxidation resistance in a high temperature steam, low cycle fatigue resistance, impact toughness, etc. The creep resistance is the main critical requirement: the minimum long-term creep rupture strength on the base of 100,000 h should be 100 MPa or higher at 650 °C.
- martensitic steels
- chemical composition
- boron
- nitrogen
1. Introduction
2. Obtaining and Heat Treatment of Steels
2.1. Chemical Composition of Steels
Advanced steels contain 2–3% Co, which is known to have a positive effect on the microstructure and creep strength of high-Cr steels [17][18][19][20][21]. The main purpose of the Co addition is to suppress the formation of undesirable δ-ferrite during normalizing. Helis et al. studied the effect of 0–5% Co on the 9Cr-3W-0.2V-0.05Nb-0.08C-0.05N steel [17]. It was found that addition of 1% Co reduced the δ-ferrite fraction from 6% to 0.4%, while 3% Co completely eliminated the δ-ferrite. This fact is due to Co being an austenite-forming element, and it extends the austenite region on the phase diagram. The absence of δ-ferrite in high-Cr steels increases the stability of the tempered martensite lath structure [17]. On the other hand, although precipitates do not contain Co, the addition of 3% Co provides an increasing amount of MX carbonitrides and M23C6 particles. It was revealed that the number of precipitates around PAG boundaries significantly increased at 3% Co from 6 (at 0% Co) to 14 per μm2 [17]. Co also affects the chemical composition of precipitates, increasing the V content in MX particles, and the Fe, Cr, and W content in M23C6 particles. Therefore, Co indirectly affects the precipitation strengthening of 9–12% Cr steels.2.1.1. The 9% Cr Steels
The 9% Cr steels are represented by the advanced CSEF steels, such as MARBN, G115, and SAVE12AD steels, and experimental 9Cr-1.5W-3Co steel. The MARBN steel is the Japanese 9% Cr steel developed by National Institute of Materials Science (NIMS), in co-operation with private companies in Japan, for application to thick section boiler components in USC power plant [9][22][23]. MARBN is a MARtensitic 9Cr steel strengthened by B boron and N nitrides [9][22][23]. Various compositions of MARBN steel are presented in literature, differing in concentrations of B and N [9][22][23][24]. The G115 steel is the 9% Cr Chinese steel developed by the China Iron and Steel Research Institute (CISRI) and Bao Steel [25][26][27]. This 9Cr-3W-3Co-1CuVNbB steel is recommended for use in USC power plants at operation temperatures up to 650 °C in China owing to its excellent overall properties. It is reported that G115 with 2.8%W–3%Co is for piping and with 3% W–3% Co for tubing [27]. Composition of the G115 is similar to MARBN steel, while approximately 1% Cu is added in order for additional strengthening by fine Cu-rich particles, by analogy with P122 steel [28][29].2.1.2. The 10% Cr Steels
The 10% Cr steels are represented by the experimental 10Cr [6][13] and 10Cr-0.2Re [30][31][32] steels designed on the base of TOS 110 steel. As known, TOS 110 steel was developed in Japan at Toshiba with the main composition of 10Cr-0.7Mo-1.8W-3Co-VNb-0.01B-0.02N for turbine rotor application at 630 °C [33][34][35]. The experimental 10Cr steel is a modification of TOS 110 steel by decreasing the N content to 0.003% and addition of Ti (0.002%) while maintaining the high B content of 0.008%. It was shown that this modification results in enhanced long-term creep rupture strength [6][13]. The NF12 [2] and HR1200 [34][36] steels were used for comparison of creep resistance of the 10Cr steel.2.1.3. The 11–12% Cr Steels
The 11–12% Cr steels are represented by the TAF650, SuperVM12 steels, and experimental 12% Cr steels. The TAF650 steel is the 12% Cr Japanese steel derived from the TAF steel [37]. The TAF steel was developed in 1956 by Toshio Fujita [38] and has the superior high temperature strength. The TAF650 steel was developed for improvement of poor weldability and hot workability. In the TAF650 steel, the part of Mo was replaced by W; Co and Ni were added; C and B contents were reduced from 0.21% to 0.1% and from 0.03% to 0.019%, respectively [39][40][41][42].2.2. Vacuum Induction Melting of Steels
In contrast to high-chromium steels with conventional N content (~0.05%), the method of vacuum induction melting is used for producing steels with decreased nitrogen content (<0.01%). It is attributed to the fact that the nitrogen content in alloy is determined by the gas pressure on the molten alloy. As is known, the solubility of the diatomic nitrogen gas (N2) in metal melt can be described by the reaction: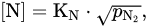 where [N] is the solubility of the nitrogen gas in metal melt at a given partial pressure of gas pN2; KN is the solubility constant (Sievert’s constant), which depends on temperature and the way concentration and pressure are expressed.
where [N] is the solubility of the nitrogen gas in metal melt at a given partial pressure of gas pN2; KN is the solubility constant (Sievert’s constant), which depends on temperature and the way concentration and pressure are expressed.
2.3. Heat Treatment
Heat treatment of the steels usually consists in normalization and tempering in order to form a tempered martensite lath structure. Normalization of the considered steels is carried out in a wide temperature range of 1050 to 1170 °C (Figure 1). Heating is carried out in the austenite region for preventing the δ-ferrite formation after normalization. Duration of heating varies from 0.5 to 1 h. During cooling (by air or oil), the austenite transforms to martensite. The normalization leads to the formation of the lath martensite structure, in which the PAGs, packets, blocks, and laths with high density of lattice dislocations are distinguished. The normalization temperature affects the PAG size. The size of PAG in the steels significantly varies from 20 to 200 μm due to different normalization temperatures.
3. The Boron/Nitrogen Ratio in the Steels
-
Preventing the formation of BN phase. On the one hand, an increased boron content enhances the coarsening resistance of M23C6-type carbides during creep. On the other hand, the presence and content of nitrogen strongly affects the efficiency of alloying with boron. Nitrogen affects the solubility of boron in the ferritic matrix. At excess nitrogen content, the undesirable BN phase is formed during normalization. The coarse BN precipitates can act as initiation sites for creep cavities that reduce the creep resistance and creep ductility. Formation of BN leads to a depletion of boron from the ferritic matrix.
-
Optimal fraction of MX phase. The nitrogen content determines the volume fraction of MX carbonitrides, namely N- and V-enriched precipitates, in steels. At optimal B content, the N content should not be too high or too low.
-
Preventing MX→Z-phase transformation. In the steels with 10–12% Cr, a low N concentration prevents the transformation of the MX phase into the undesirable coarse Z-phase particles (CrVN). Thus, in the experimental 12% Cr steels with 20 ppm N, the Z phase was not observed even after long-term creep for >20,000 h at 650 °C [15]. In the Super VM12 steel with 110 ppm N, the Z-phase was not revealed after long-term creep during 23,844 h at 650 °C [48].
4. Microstructure and Creep Properties of Advanced 9–12% Cr Steels
4.1. Creep Properties of the 9–12% Cr Steels
Figure 2 illustrates the creep data at 650 °C for the advanced 9–12% Cr steels with the increased B and decreased N contents in comparison with those for the conventional P92 and P122 steels. The data are from Refs. [6][9][14][16][18][20][22][24][28][30][49][50]. It is clearly seen that the time to rupture versus applied stress points for these steels are higher, mainly than those for the P92 and P122 steels, suggesting the higher creep resistance of new steels.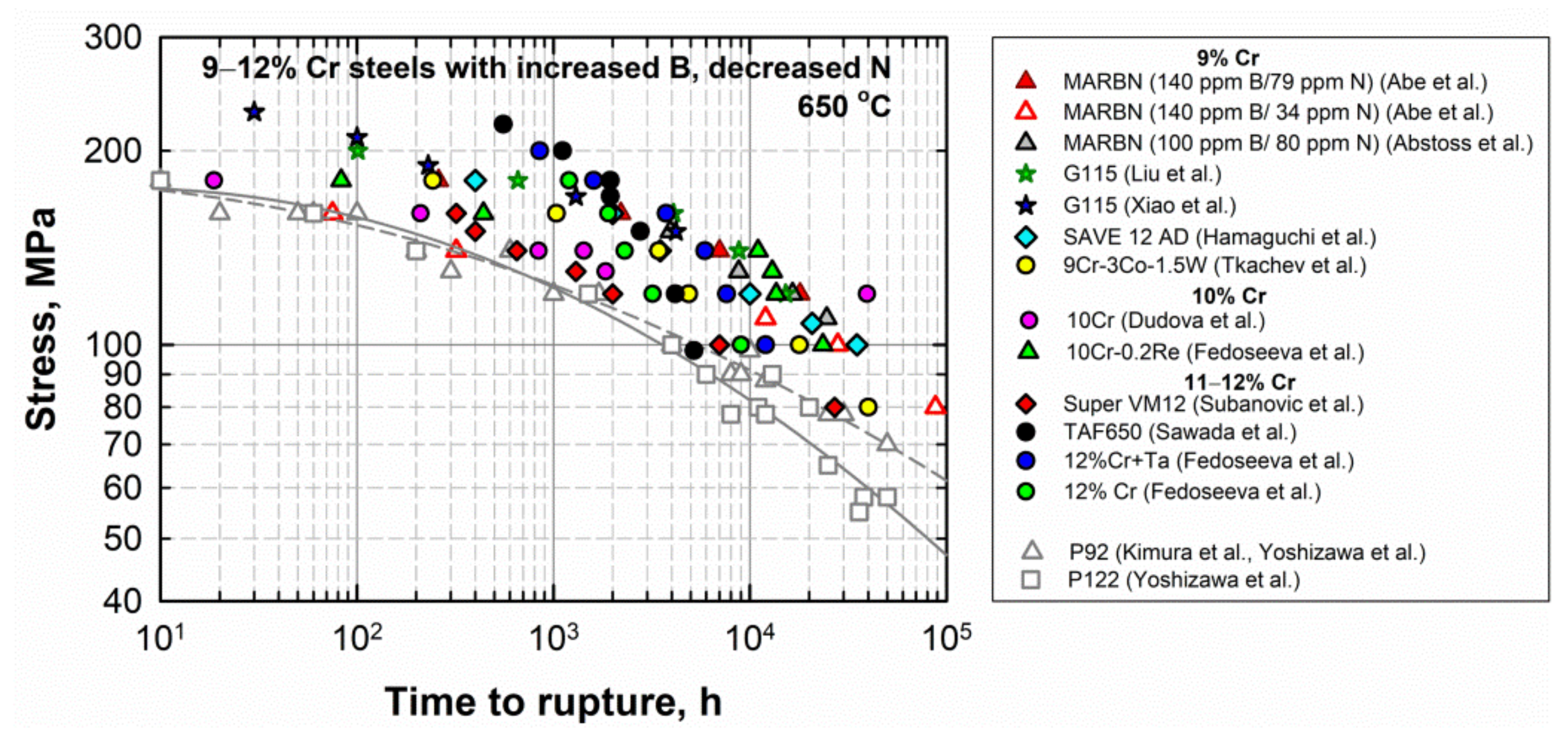 Figure 72. Time to rupture vs. stress curves of 9–12% Cr steels with the increased B and decreased N contents in comparison with the curves for the P92 and P122 steels. Data from [6][9][14][16][18][20][22][24][28][30][49][50].
Figure 72. Time to rupture vs. stress curves of 9–12% Cr steels with the increased B and decreased N contents in comparison with the curves for the P92 and P122 steels. Data from [6][9][14][16][18][20][22][24][28][30][49][50].4.2. The 9% Cr Steels
The advanced 9% Cr CSEF steels—the MARBN, G115, and SAVE12AD steels—demonstrate a high level of creep strength both at high stresses (in the short-term creep region) and at low stresses (in the long-term creep region) as compared with the conventional P92 steel (Figure 3). Regression lines of the experimental creep points predict the long-term creep rupture strength of these steels at 650 °C for 100,000 h in the range from 80 to 110 MPa [49]. These values are significantly higher than those for the P92 steel and 3%Co-modified P92 steels with 2% and 3%W (approximately 60–70 MPa) (Figure 3a).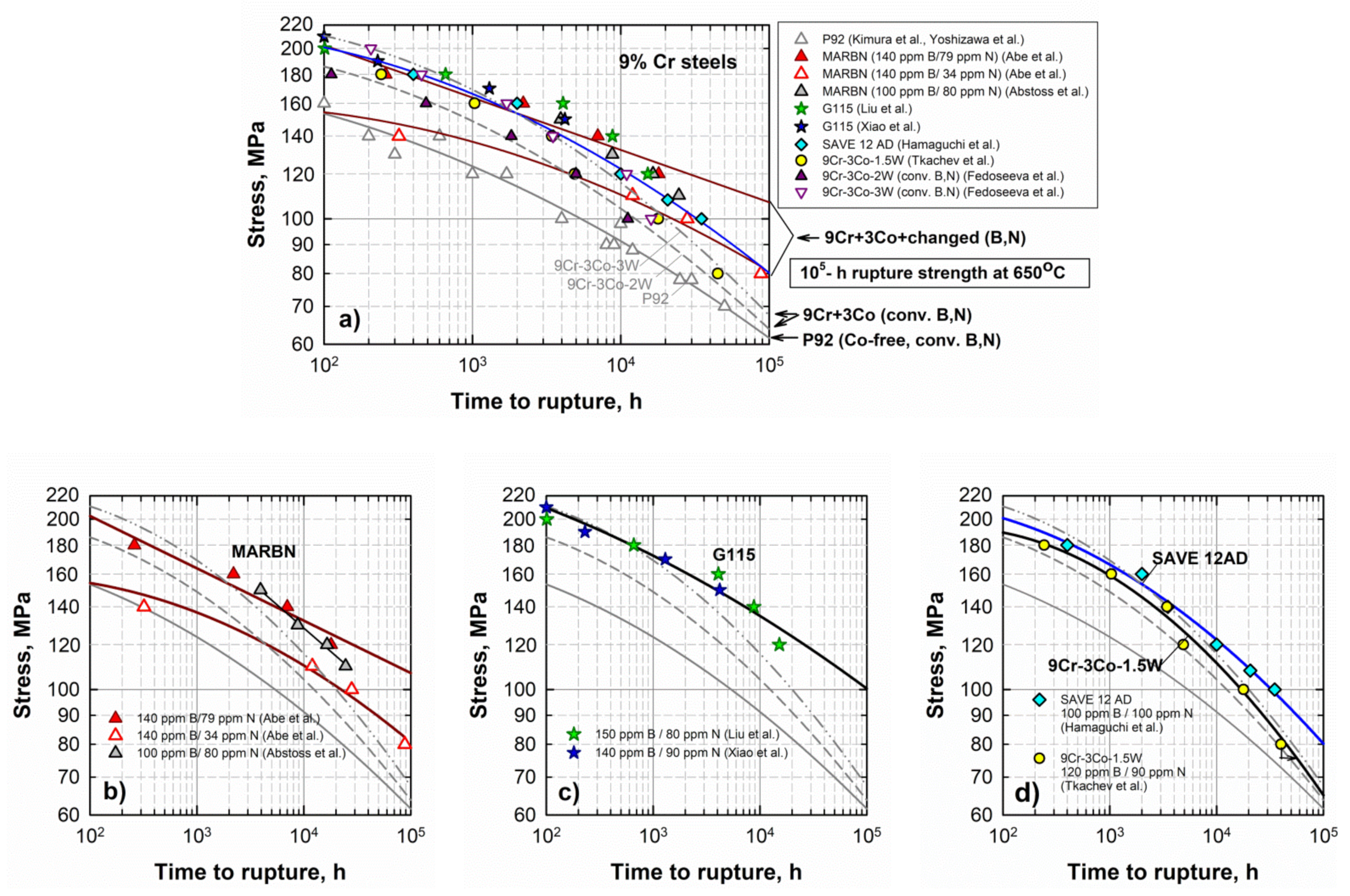 Figure 3. Time to rupture vs. stress curves of 9% Cr steels with increased B and decreased N contents in comparison with curves for 9% Cr steels with conventional B, N contents (a–d). Experimental data from [6][9][14][16][18][20][22][24][28][50].The creep strength curves for the MARBN steel vary depending on the B and N content [9][22]. The MARBN steel with 140 ppm B/79 ppm N demonstrates the highest creep resistance, whereas a lower N content of 34 ppm reduces the creep strength (Figure 3b).The G115 steels with slightly different B and N content (140–150 ppm B and 80–90 ppm N) studied by Liu et al. [14] and Xiao et al. [50][51] exhibit close stress/time to rupture points, which are approximated by a regression line predicting the long-term creep strength of 100 MPa (Figure 3c).The SAVE12AD steel is predicted to have the long-term creep rupture strength of 80 MPa at 650 °C for 100,000 h (Figure 3d) [52].
Figure 3. Time to rupture vs. stress curves of 9% Cr steels with increased B and decreased N contents in comparison with curves for 9% Cr steels with conventional B, N contents (a–d). Experimental data from [6][9][14][16][18][20][22][24][28][50].The creep strength curves for the MARBN steel vary depending on the B and N content [9][22]. The MARBN steel with 140 ppm B/79 ppm N demonstrates the highest creep resistance, whereas a lower N content of 34 ppm reduces the creep strength (Figure 3b).The G115 steels with slightly different B and N content (140–150 ppm B and 80–90 ppm N) studied by Liu et al. [14] and Xiao et al. [50][51] exhibit close stress/time to rupture points, which are approximated by a regression line predicting the long-term creep strength of 100 MPa (Figure 3c).The SAVE12AD steel is predicted to have the long-term creep rupture strength of 80 MPa at 650 °C for 100,000 h (Figure 3d) [52].4.3. The 10% Cr and 11–12% Cr Steels
The main problem of the 10–12% Cr steels is a drop in creep resistance in the long-term creep region. Creep degradation is associated with the instability of the tempered martensite lath structure under creep conditions, which can be caused by such microstructural changes as:- -
-
preferential recovery of martensitic microstructure near the PAG boundaries;
- -
-
Z-phase formation and disappearance of MX strengthening precipitates;
- -
-
Laves phase formation, etc.
5. Strengthening Factors in the 9–12% Cr Steels
5.1. Solid Solution Strengthening
The solid solution strengthening of considered steels is mainly caused by chromium, tungsten, molybdenum, and cobalt. A small amount of rhenium is also used as a solid solution strengthening element in the 10Cr-0.2Re and TOS 203 steels. Cr is the most strengthening element in the high-chromium steels. An increase in the Cr content from 9 to 12% can markedly increase the creep strength in the short-term creep region, for example, for the 11–12% Cr TAF650, 12Cr, and 12Cr-Ta steels, but not for the SuperVM12 steel. The W content varies from 1.5 to 3% in the steels, the Mo content varies from 0 to 0.7%, and Co content varies from 2 to 3%. Most of the 9% Cr advanced steels contain 3%W-0%Mo. On the other hand, the 9Cr-1.5W-0.6Mo steel shows the same creep strength in the short-term region. The 10Cr-0.2Re steel shows higher creep strength up to the appearance of the creep strength breakdown in comparison with the 10Cr steel, probably due to the higher W content (3% instead of 2%), although the Mo equivalent (Mo + 1/2 W) is the same in these steels (1.7%), as well as due to the presence of Re. In the 11–12% Cr steels, there is also no distinct correlation between the content of W, Mo, and Co and the creep strength.5.2. Boundary and Sub-Boundary Strengthening
In the 9–12% Cr steels with tempered martensite lath structure, the sub-boundary hardening enhanced by fine distributions of precipitates along boundaries gives the most important strengthening mechanism for creep compared to the PAG boundaries [5]. The sub-boundary strengthening is inversely proportional to the width of lath or subgrains. The width of lath is nearly the same in most of the steels in the as-tempered state and comprises 300–400 nm (Figure 4). Evolution of lath width during creep depends on the stability of precipitates located on the lath boundaries, not only the M23C6 carbides but also the Laves phase. Figure 4 shows that the lath width slightly increases during creep. A sharp increase in the lath width or subgrain size above 1 μm usually corresponds to the transformation of the lath structure into the subgrain structure and the creep strength breakdown appearance as for the 9Cr-1.5W-3Co, 10Cr-0.2Re, and TAF650 steels.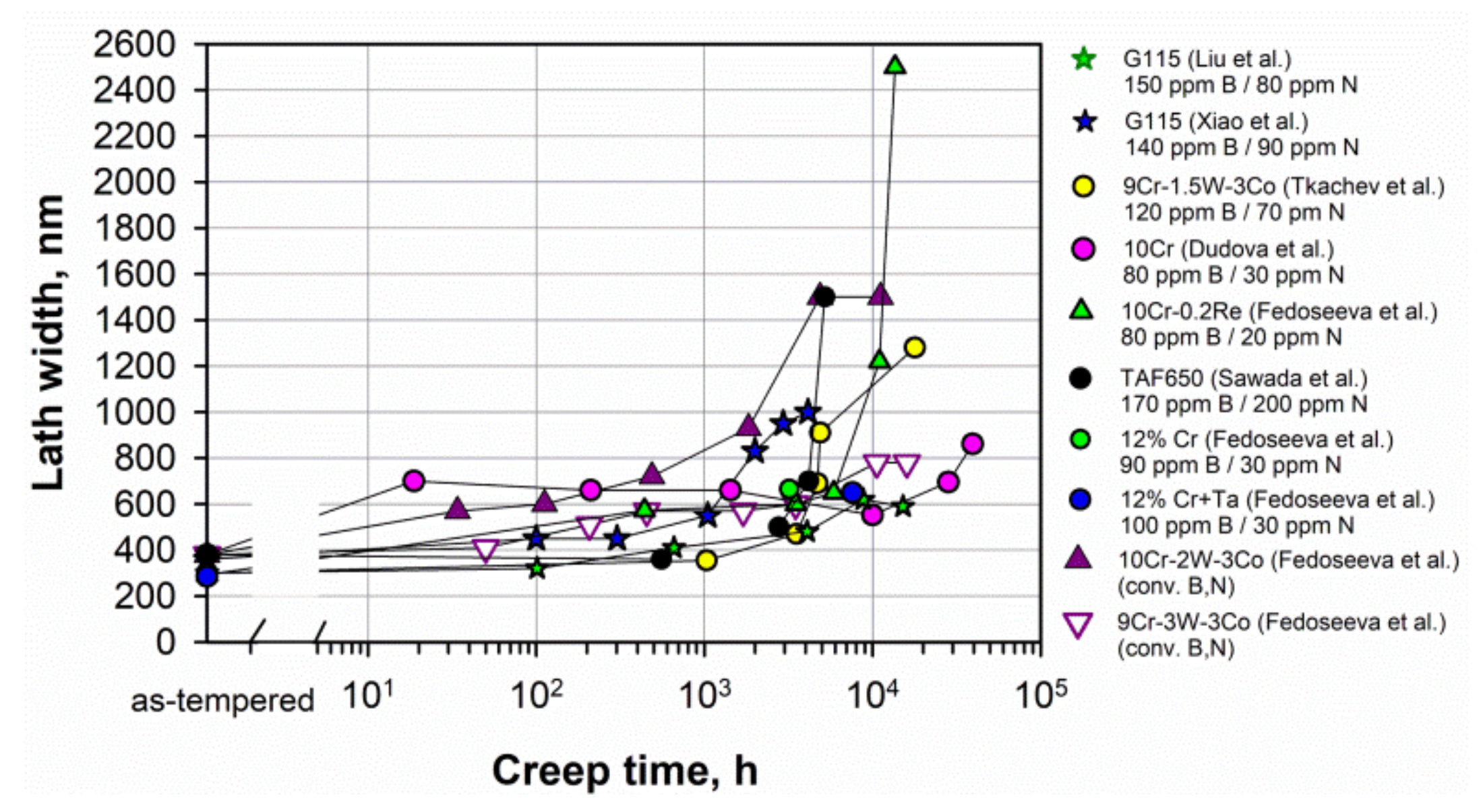 Figure 4. Evolution of the lath width during creep in the 9–12% Cr steels. Data from [6][14][15][19][20][30][39][54][55].
Figure 4. Evolution of the lath width during creep in the 9–12% Cr steels. Data from [6][14][15][19][20][30][39][54][55].5.3. Dislocation Strengthening
The dislocation density in the steels before creep depends on the tempering temperature. Figure 5 shows that the dislocation density comprised about 1–3 × 1014 m−2 in the as-tempered steels at 750–780 °C. Lower tempering temperatures increased the dislocation density, for example, up to 7.7 × 1014 m−2 in the TAF650 steel tempered at 680 °C [39].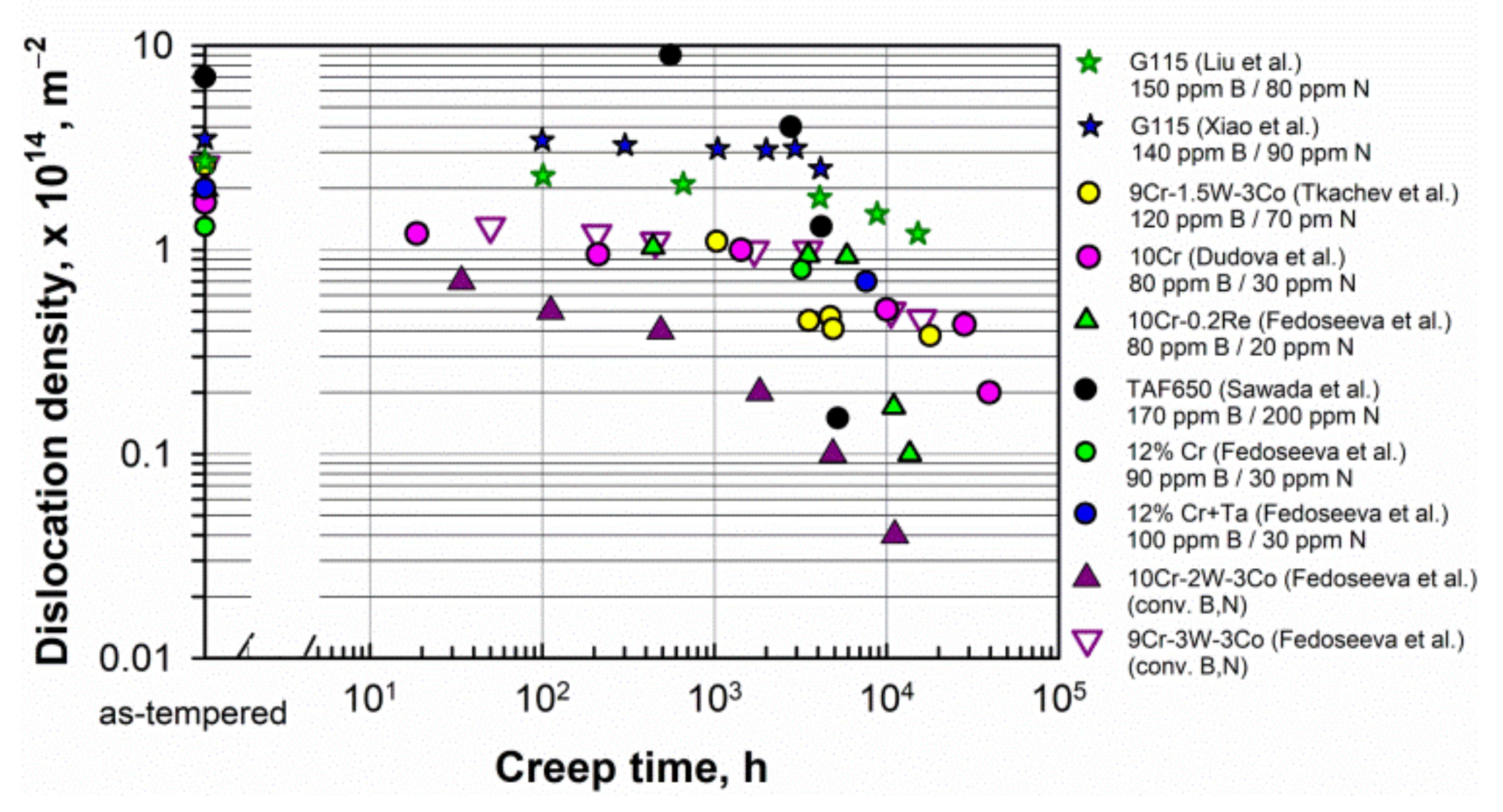 Figure 5. Evolution of the dislocation density during creep in the 9–12% Cr steels. Data from [6][14][15][19][20][30][39][54][55].
Figure 5. Evolution of the dislocation density during creep in the 9–12% Cr steels. Data from [6][14][15][19][20][30][39][54][55].5.4. Precipitation Strengthening
Threshold stress. The M23C6 carbides and Laves phase particles are the main phases precipitated on the boundaries of lath, blocks, packets and PAGs.
The stresses created by small precipitates cause the threshold stress for the onset of creep. Dudova et al. examined the creep behavior of the 10Cr steel in terms of threshold stress and found a high threshold stress of 111.5 MPa, which is about 30% larger than that in the P92-type steel [6]. The calculation of the stresses required for a dislocation to pass through particles at the minimum creep rate stage was carried out. The Orowan mechanism (to bow a dislocation between two particles), climb mechanism (to generate the additional length of dislocation to climb over an obstacle), and detachment mechanism (to detach the dislocation from an attractive particle after finishing the climb) were taken into account. It was revealed that the threshold stress is associated with the stress required for detachment of dislocations from M23C6 carbides, MX carbonitrides, and Laves phase particles after finishing the climb. Further, essentially stable M23C6 carbides exert the main part of threshold stress. The detachment stress can be calculated by equation [56]:
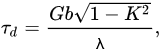
where G is the shear modulus, b is the Burgers vector, K—relaxation parameter, λ is the mean interparticle spacing, which is determined as [57]:
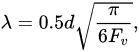
where d is the mean size of particles, Fv is the volume fraction of particles.
M23C6 carbides. Figure 6 shows that the mean sizes of M23C6 carbides in the as-tempered steels are in the range 50–100 nm. In most of the considered high B and low N steels, there is only a slight increase in the mean size at long-term creep over 10,000 h to about 120–150 nm, for example, in the MARBN, G115, 9Cr-1.5W-3Co, 10Cr, 10Cr-0.2Re, and SuperVM12 steels. Whereas, in the steels with conventional B content (the 9Cr-2W-3Co and 9Cr-3W-3Co steels), the pronounced coarsening of carbides to 200–300 nm occurs. Therefore, this confirms that the enrichment of steels by boron leads to a decrease in the size of carbides and an increase in their coarsening resistance under creep conditions.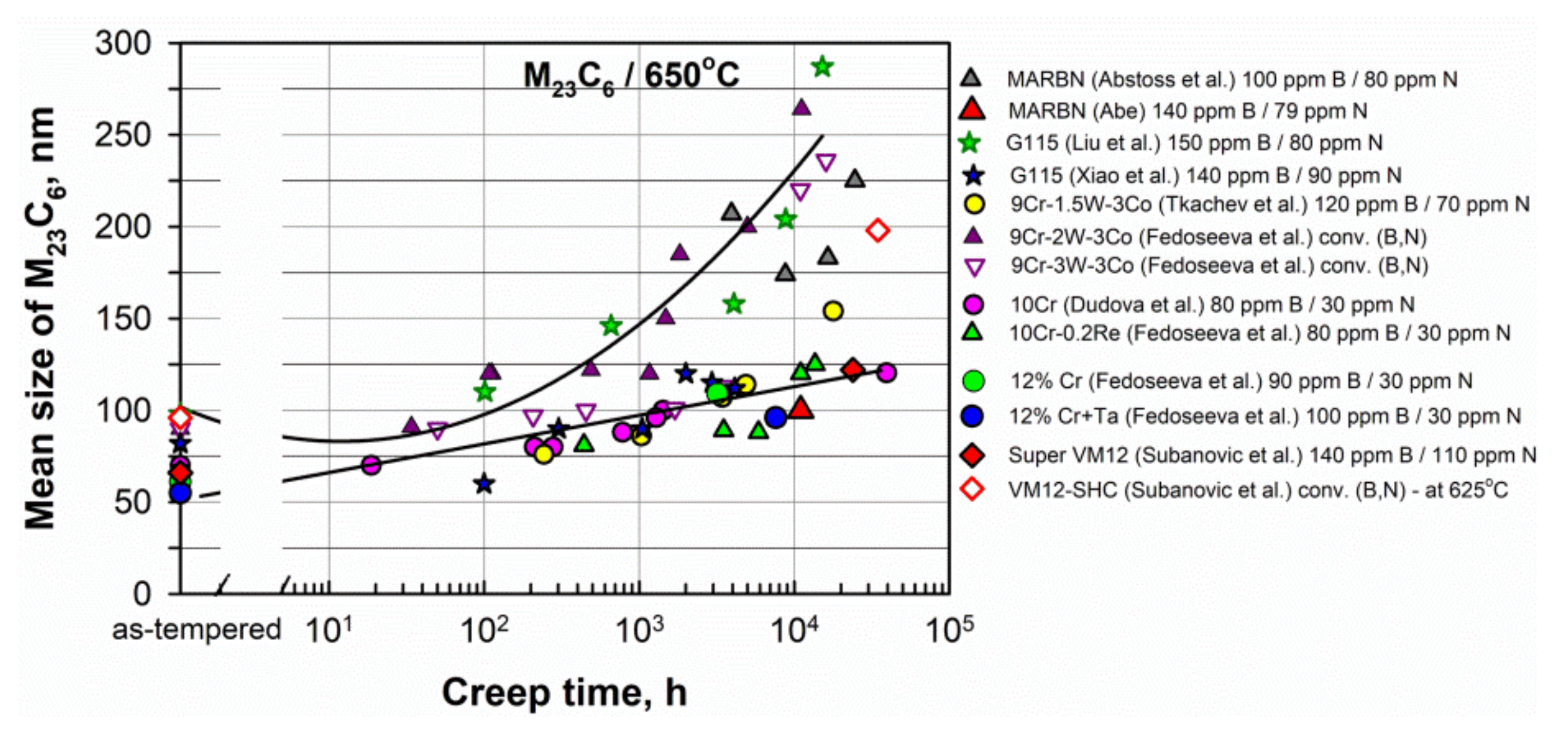 Figure 6. Evolution of the mean size of M23C6 carbides during creep in the 9–12% Cr steels. Data from [5][6][14][15][19][20][24][30][39][48][54][55].Laves phase. Laves phase particles grow during creep deformation, and their coarsening negatively affects the creep strength. Figure 7 presents the evolution of the mean size of Laves phase particles in some 9–12%Cr steels. Laves phase particles slightly grow up to approximately 1000 h of creep, and then the rapid coarsening occurs.
Figure 6. Evolution of the mean size of M23C6 carbides during creep in the 9–12% Cr steels. Data from [5][6][14][15][19][20][24][30][39][48][54][55].Laves phase. Laves phase particles grow during creep deformation, and their coarsening negatively affects the creep strength. Figure 7 presents the evolution of the mean size of Laves phase particles in some 9–12%Cr steels. Laves phase particles slightly grow up to approximately 1000 h of creep, and then the rapid coarsening occurs.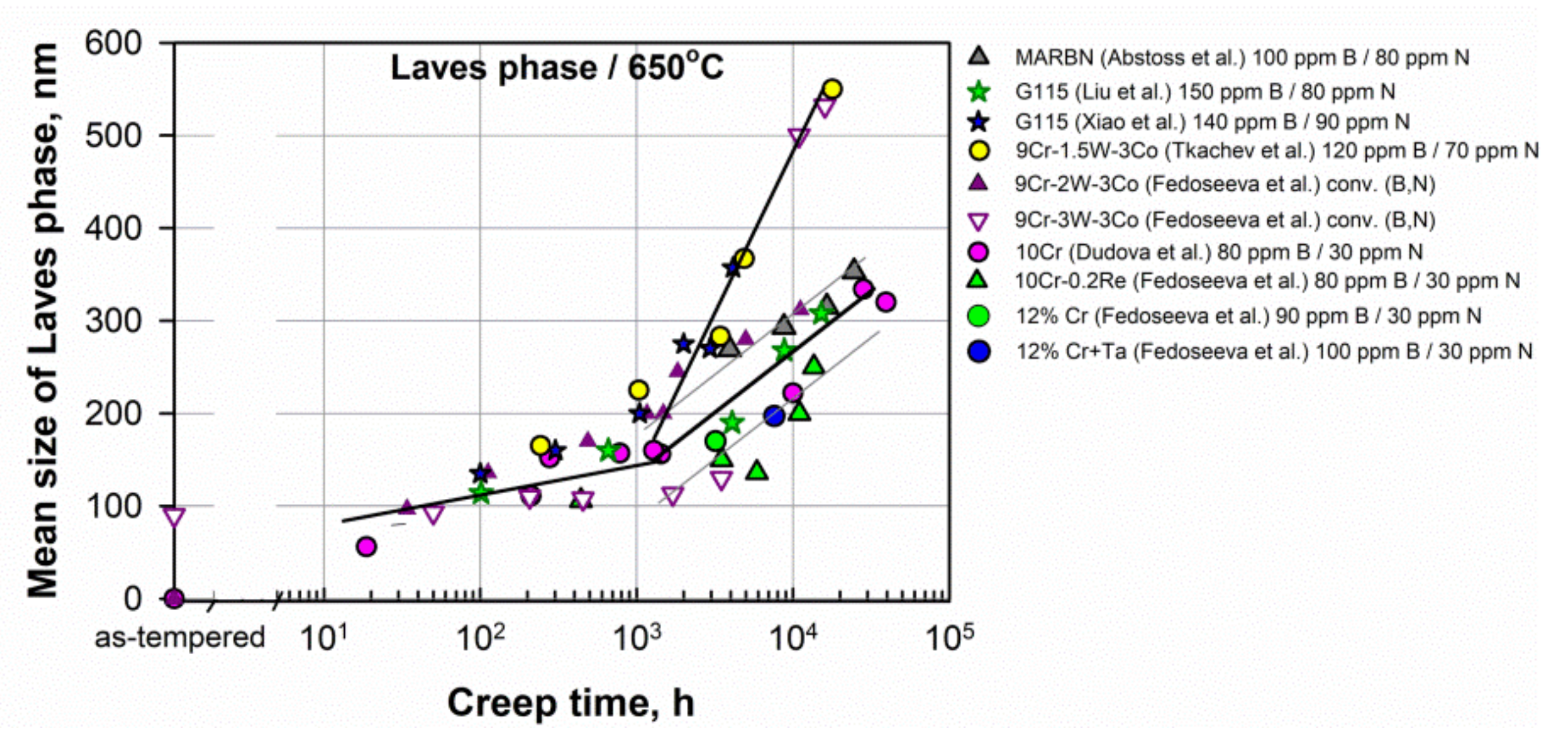 Figure 7. Evolution of the mean size of Laves phase particles during creep in the 9–12% Cr steels. Data from [6][14][15][19][20][24][30][39][54][55].MX phase. A decrease in the N content can lead to a decrease in the fraction of the MX phase in the steels with a high B and a low N content as compared to steels with conventional B and N contents. Consequently, the precipitation strengthening is reduced due to a small fraction of fine, homogeneously distributed in the lath interiors, MX particles. It can mainly concern the steels with a N content of lower than 70 ppm.Pinning pressure. Fine and stable boundary precipitates effectively prevent the migration of lath boundaries due to high pinning pressure [6][14][16].
Figure 7. Evolution of the mean size of Laves phase particles during creep in the 9–12% Cr steels. Data from [6][14][15][19][20][24][30][39][54][55].MX phase. A decrease in the N content can lead to a decrease in the fraction of the MX phase in the steels with a high B and a low N content as compared to steels with conventional B and N contents. Consequently, the precipitation strengthening is reduced due to a small fraction of fine, homogeneously distributed in the lath interiors, MX particles. It can mainly concern the steels with a N content of lower than 70 ppm.Pinning pressure. Fine and stable boundary precipitates effectively prevent the migration of lath boundaries due to high pinning pressure [6][14][16].6. Summary
The review of the 9–12% Cr heat-resistant martensitic steels with increased boron and decreased nitrogen contents in comparison with similar steels with conventional B/N contents shows the following:- -
-
The approach to alloying by the increased B (80–150 ppm) and decreased N (30–100 ppm) contents is successfully applied to advanced 9% Cr, as well as 10–12% Cr martensitic steels. The predicted long-term creep rupture strength at 650 °C for 100,000 h attained:
-
- ○
-
80–110 MPa for the 9% Cr steels, such as the MARBN (9Cr-3Co-3W-100–140 ppm B/30–80 ppm N), G115 (9Cr-3Co-3W-1Cu-150 ppm B/80 ppm N), and SAVE12AD (9Cr-3Co-3W-0.04Nd-100 ppm B/100 ppm N) steels, which sufficiently exceeds the creep strength for the 9% Cr Co-free P92 steel (~60 MPa) and 3% Co-modified P92 steels (~65–70 MPa) with conventional B (~50 ppm) and N (~500 ppm) contents;
-
- ○
-
110 MPa for the 10% Cr experimental steel (10Cr-3Co-2W-0.7Mo-80 ppm B/30 ppm N), which sufficiently exceeds the creep strength for the 11%Cr Co-free P122 steel (~45 MPa), 10% Cr Co-containing NF12 steel (10Cr-2Co-2.5W-50 ppm B/200 ppm N) (40 MPa), and advanced 10Cr-3Co-1.8W-100 ppm B/200 ppm N (TOS 110) steel (80 MPa);
-
- ○
-
Approximately 65 MPa for the 11–12% Cr steels, such as SuperVM12 steel (11Cr-1.8Co-2W-0.5Mo-140 ppm B/110 ppm N), which is sufficiently higher than that for the previous VM12-SHC steel (~50 MPa), 11%Cr Co-free P122 steel (~45 MPa), and slightly higher than that for 9% Cr P92 steel (~60 MPa) with conventional B (~50 ppm) and N (~500–600 ppm) contents;
-
- -
-
An increase in the B content and a decrease in the N content enhance the creep resistance in the long-term region at a low stress, while the creep strength in the short-term region at the higher stresses corresponds to that for the steels with conventional B and N contents;
- -
-
A high B content at a low N content effectively increases the coarsening resistance of M23C6 carbides during creep at 650 °C in all considered steels;
- -
-
A positive effect of B on the creep strength is associated with enrichment of M23C6 carbides located near the PAG boundaries, which increases their coarsening resistance during creep. Stable M23C6 carbides are able to impede the recovery of the lath structure in the vicinity of PAG boundaries and, hence, retard the local deformation in the PAG boundary regions;
- -
-
Even if the B content was already high in steel, then lowering the N content less than the solubility limit can increase the long-term creep rupture strength due to full utilization of soluble boron in the matrix and M23(B,C)6-type carbides;
- -
-
Precipitation of small MX particles during the transient stage of long-term creep effectively reduces the creep rate and increases the time to rupture, as was shown in the MARBN steel with 140 ppm B and 79 ppm N and the 10Cr experimental steel with 80 ppm B and 30 ppm N;
- -
-
An increase in the B content and a decrease in the N content is an effective way to enhance the dispersion strengthening by: fine and highly coarsening-resistant M23C6 carbides, which, in turn, can provide the slower coarsening of Laves phase particles; moreover, the MX phase, despite the low N content, can enhance the creep strength in the long-term region. This provides a stable tempered martensite lath structure over a long creep time and prevents the transformation of the lath structure into a subgrain structure.
-
-
-
-
References
- Abe, F. Research and development of heat-resistant materials for advanced USC power plants with steam temperatures of 700 °C and above. Engineering 2015, 1, 211–224.
- Abe, F.; Kern, T.-U.; Viswanathan, R. Creep-Resistant Steels; Woodhead Publishing: Cambridge, UK, 2008.
- Xie, X.; Wu, Y.; Chi, C.; Zhang, M. Superalloys for Advanced Ultra-Super-Critical Fossil Power Plant Application. In Superalloys; Aliofkhazraei, M., Ed.; IntechOpen: London, UK, 2015; pp. 51–76.
- Maruyama, K.; Sawada, K.; Koike, J. Strengthening mechanisms of creep resistant tempered martensitic steel. ISIJ Int. 2001, 41, 641–653.
- Abe, F. New martensitic steels. In Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants; Di Gianfrancesco, A., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 323–374.
- Dudova, N.; Mishnev, R.; Kaibyshev, R. Creep behavior of a 10%Cr heat-resistant martensitic steel with low nitrogen and high boron contents at 650 °C. Mater. Sci. Eng. A 2019, 766, 138353.
- Mitsuhara, M.; Yamasaki, S.; Miake, M.; Nakashima, H.; Nishida, M.; Kusumoto, J.; Kanaya, A. Creep strengthening by lath boundaries in 9Cr ferritic heat resistant steel. Philos. Mag. Lett. 2016, 96, 76–83.
- Dudko, V.; Belykov, A.; Kaibyshev, R. Evolution of lath substructure and internal stresses in a 9% Cr steel during creep. ISIJ Int. 2017, 57, 540–549.
- Semba, H.; Abe, F. Alloy design and creep strength of advanced 9%Cr USC boiler steels containing high concentration of boron. Energy Mater. 2007, 1, 238–244.
- Hald, J. Microstructure and long-term creep properties of 9–12% Cr steels. Int. J. Press. Vessels Pip. 2008, 85, 30–37.
- Abe, F. Effect of boron on microstructure and creep strength of advanced ferritic power plant steels. Procedia Eng. 2011, 10, 94–99.
- Horiuchi, T.; Igarashi, M.; Abe, F. Improved utilization of added B in 9Cr heat-resistant steels containing W. ISIJ Int. 2002, 42, S67–S71.
- Mishnev, R.; Dudova, N.; Kaibyshev, R. On the origin of the superior long-term creep resistance of a 10% Cr steel. Mater. Sci. Eng. A 2018, 713, 161–173.
- Liu, Z.; Liu, Z.; Chen, Z.; Wang, X.; Bao, H.; Dong, C. Microstructure and creep strength evolution in G115 steel during creep at 650 °C. Mater. Res. Express 2020, 7, 016528.
- Fedoseeva, A.; Nikitin, I.; Fedoseev, A.; Kaibyshev, R. Evolution of the tempered lath structure of the 12%Cr steels with low N and high B contents during creep. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1014, 012011.
- Tkachev, E.; Belyakov, A.; Kaibyshev, R. Creep behavior and microstructural evolution of a 9%Cr steel with high B and low N contents. Mater. Sci. Eng. A 2018, 725, 228–241.
- Helis, L.; Toda, Y.; Hara, T. Effect of cobalt on the microstructure of tempered martensitic 9Cr steel for ultra-supercritical power plants. Mater. Sci. Eng. A 2009, 510, 88–94.
- Dudova, N.; Plotnikova, A.; Molodov, D.; Belyakov, A.; Kaibyshev, R. Structural changes of tempered martensitic 9%Cr–2%W–3%Co steel during creep at 650 °C. Mater. Sci. Eng. A 2012, 534, 632–639.
- Fedoseeva, A.; Dudova, N.; Kaibyshev, R. Creep strength breakdown and microstructure evolution in a 3%Co modified P92 steel. Mater. Sci. Eng. A 2016, 654, 1–12.
- Fedoseeva, A.; Dudova, N.; Kaibyshev, R. Creep behavior and microstructure of a 9Cr–3Co–3W martensitic steel. J. Mater. Sci. 2016, 52, 2974–2988.
- Kipelova, A.; Odnobokova, M.; Belyakov, A.; Kaibyshev, R. Effect of Co on creep behavior of a P911 steel. Metall. Mater. Trans. A 2012, 44, 577–583.
- Abe, F.; Tabuchi, M.; Tsukamoto, S. Mechanisms for boron effect on microstructure and creep strength of ferritic power plant steels. Energy Mater. 2012, 4, 166–174.
- Abe, F.; Tabuchi, M.; Semba, H.; Igarashi, M.; Yoshizawa, M.; Komai, N.; Fujita, A. Feasibility of MARBN steel for application to thick section boiler components in USC power plant at 650 °C. In Proceedings of the 5th EPRI International Conference, Marco Island, FL, USA, 3–5 October 2007; pp. 92–106.
- Abstoss, K.G.; Schmigalla, S.; Schultze, S.; Mayr, P. Microstructural changes during creep and aging of a heat resistant MARBN steel and their effect on the electrochemical behaviour. Mater. Sci. Eng. A 2019, 743, 233–242.
- Yan, P.; Liu, Z.-D.; Liu, W.; Bao, H.-S.; Weng, Y.-Q.J. Hot Deformation Behavior of a New 9% Cr Heat Resistant Steel G115. J. Iron Steel Res. Int. 2013, 20, 73–79.
- Yan, P.; Liu, Z.; Bao, H.; Weng, Y.; Liu, W. Effect of normalizing temperature on the strength of 9Cr–3W–3Co martensitic heat resistant steel. Mater. Sci. Eng. A 2014, 597, 148–156.
- Liu, Z.; Bao, H.; Chen, Z.; Xu, S.; Zhao, H.; Wang, Q. Creep Strength and Oxidation Resistance of Industrially Made G115 Steel Pipe. In Energy Materials 2017; Liu, X., Liu, Z., Brinkman, K., Das, S., Dryepondt, S., Fergus, J.W., Guo, Z., Han, M., Hawk, J.A., Horita, T., et al., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2017; pp. 153–159.
- Yoshizawa, M.; Igarashi, M.; Moriguchi, K.; Iseda, A.; Armaki, H.G.; Maruyama, K. Effect of precipitates on long-term creep deformation properties of P92 and P122 type advanced ferritic steels for USC power plants. Mater. Sci. Eng. A 2009, 510–511, 162–168.
- Igarashi, M. Alloy design philosophy of creep-resistant steels. In Creep-Resistant Steels; Woodhead Publishing Series in Metals and Surface Engineering; Woodhead Publishing: Cambridge, UK, 2008; pp. 539–572.
- Fedoseeva, A.; Nikitin, I.; Dudova, N.; Kaibyshev, R. Coarsening of Laves phase and creep behaviour of a Re-containing 10% Cr-3% Co-3% W steel. Mater. Sci. Eng. A 2021, 812, 141137.
- Fedoseeva, A.; Nikitin, I.; Tkachev, E.; Mishnev, R.; Dudova, N.; Kaibyshev, R. Effect of alloying on the nucleation and growth of Laves phase in the 9–10%Cr-3%Co martensitic steels during creep. Metals 2021, 11, 60.
- Fedoseeva, A.; Nikitin, I.; Dudova, N.; Kaibyshev, R. Strain and temperature contributions to structural evolution in a Re-containing 10% Cr-3% Co-3% W steel during creep. Mater. High Temp. 2021, 38, 237–246.
- Tsuda, T.; Yamada, M.; Ishii, R.; Watanabe, O. Advances in Turbine Materials, Design and Manufacturing. In Proceedings of the Fourth International Charles Parsons Turbine Conference, Newcastle upon Tyne, UK, 4–6 November 1997; Strang, A., Ed.; CRC Press: Boca Raton, FL, USA, 1997; Volume 689.
- Viswanathan, R.; Bakker, W. Materials for Ultrasupercritical Coal Power Plants—Turbine Materials: Part II. J. Mater. Eng. Perform. 2001, 10, 96–101.
- Takasawa, K.; Miki, K. Development of high-and intermediate-pressure steam turbine rotors for efficient fossil power generation technology. JSW Tech. Rev. 2018, 20, 15–22. Available online: https://www.jsw.co.jp/en/product/technology/technical_review/technical_review1542456266489048621/main/0/link/File024476424.pdf (accessed on 1 January 2020).
- Hidaka, K.; Fukui, Y.; Nakamura, S.; Kaneko, R.; Tanaka, Y.; Fujita, T. Development of Heat Resistant 12CrWCoB Steel Rotor for USC Power Plant. In Advanced Heat Resistant Steels for Power Generation; Viswanathan, R., Nutting, J., Eds.; The Institute of Materials: London, UK, 1999; pp. 482–493.
- Fujita, T. Advances in 9–12%Cr heat resistant steels for power plant. In Proceedings of the 3rd Conference on Advances in Material Technology for Fossil Power Plants, London, UK, 5–6 April 2001; Viswanathan, R., Bakker, W.T., Parker, J.D., Eds.; ASM International: Materials Park, OH, USA; pp. 33–65.
- Fujita, T.; Takahashi, N. The Effects of V and Nb on the Long Period Creep Rupture Strength of 12 %Cr Heat-resisting Steel Containing Mo and B. Trans. Iron Steel Inst. Jpn. 1978, 18, 269–278.
- Sawada, K.; Takeda, M.; Maruyama, K.; Ishii, R.; Yamada, M.; Nagae, Y.; Komine, R. Effect of W on recovery of lath structure during creep of high chromium martensitic steels. Mater. Sci. Eng. A 1999, 267, 19–25.
- Sklenicka, V.; Kucharova, M.; Svoboda, M.; Kloc, L.; Bursık, J.; Kroupa, A. Long-term creep behavior of 9–12%Cr power plant steels. Mat. Char. 2003, 51, 35–48.
- Svoboda, M.; Dlouhý, A.; Podstranská, I.; Sklenička, V.; Mayer, K.H. Microstructural changes in creep of TAF 650 steel at 650 °C. In Proceedings of the 9th International Metallurgical Conference METAL 2000, Ostrava, Czech Republic, 16–18 May 2000; pp. 1–8.
- Svoboda, M.; Bursik, J.; Podstranska, I.; Krouppa, A.; Sklenicka, V.; Mayer, K.H. High temperature creep behaviour and microstructural changes of TAF 650 steel. In Materials for Advanced Power Engineering; Lecomte-Beckers, J., Carton, M., Schubert, F., Ennis, P.J., Eds.; Forschungszentrum Jülich GmbH: Liege, Belgium, 2002; pp. 1521–1530.
- Sieverts, A.; Zapf, G. Eisen und Stickstoff. Z. Phys. Chem. 1935, 172, 314–315.
- El-Kashif, E.; Asakura, K.; Shibata, K. Effects of Nitrogen in 9Cr–3W–3Co Ferritic Heat Resistant Steels Containing Boron. ISIJ Int. 2002, 42, 1468–1476.
- Sakuraya, K.; Okada, H.; Abe, F. BN type inclusions formed in high Cr ferritic heat resistant steel. Energy Mater. 2006, 1, 158–166.
- Abe, F.; Tabuchi, M.; Tsukamoto, S.; Liu, Y. Alloy design of tempered martensitic 9Cr-boron steel for A-USC boilers. In Proceedings of the 7th EPRI Conference on Advances in Materials Technology for Fossil Power Plants, Waikoloa, HI, USA, 22–25 October 2013; pp. 1127–1138.
- Abe, F.; Ohba, T.; Miyazaki, H.; Toda, Y.; Tabuchi, M. Effect of boron nitrides and aluminum nitrides on long-term creep life and rupture ductility of martensitic 9 to 12Cr steels. In Proceedings of the Joint EPRI–123HiMAT International Conference on Advances in High Temperature Materials, Nagasaki, Japan, 21–24 October 2019; Shingledecker, J., Takeyama, M., Eds.; ASM International: Materials Park, OH, USA, 2019; pp. 336–347.
- Subanović, M.; Abellán, J.P.; Gauss, A.; Jarrar, M.; Schneider, A. Super VM12—A new 12% Cr boiler steel. In Proceedings of the Joint EPRI–123HiMAT International Conference on Advances in High Temperature Materials, Nagasaki, Japan, 21–24 October 2019; Shingledecker, J., Takeyama, M., Eds.; ASM International: Materials Park, OH, USA, 2019; pp. 205–216.
- Masuyama, F.; Yamaguchi, T. New ferritic steel beyond Grade 92 and its creep degradation assessment by hardness method for Grade 91. In Proceedings of the ASME 2014 Symposium on Elevated Temperature Application of Materials for Fossil, Nuclear, and Petrochemical Industries, Seattle, WA, USA, 25–27 March 2014; No. ETAM2014-1007. pp. 54–61.
- Xiao, B.; Xu, L.; Zhao, L.; Jing, H.; Han, Y. Deformation-mechanism-based creep model and damage mechanism of G115 steel over a wide stress range. Mater. Sci. Eng. A 2019, 743, 280–293.
- Xiao, B.; Xu, L.; Tang, Z.; Zhao, L.; Jing, H.; Han, Y.; Li, H. A physical-based yield strength model for the microstructural degradation of G115 steel during long-term creep. Mater. Sci. Eng. A 2019, 747, 161–176.
- Hamaguchi, T.; Okada, H.; Hirata, H.; Kurihara, S.; Semba, H.; Yoshizawa, M. Creep Rupture Strength and Microstructures of SAVE12AD Welded Joints; Technical Report No. 119; Nippon Steel & Sumitomo Metal: Tokyo, Japan, 2018; pp. 32–38.
- Fukuda, M.; Tsuda, Y.; Yamashita, K.; Shinozaki, Y.; Takanashi, T. Materials and design for advanced high temperature steam turbines. In Proceedings of the Fourth International Conference on Advances in Materials Technology for Fossil Power Plants, Hilton Head Island, SC, USA, 25–28 October 2004; ASM International: Materials Park, OH, USA, 2005; pp. 491–505.
- Fedoseeva, A.; Nikitin, I.; Fedoseev, A.; Kaibyshev, R. Degradation of the creep resistance of a Re-containing 10%Cr steel upon creep testing at low applied stress. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1014, 012012.
- Tkachev, E.; Belyakov, A.; Kaibyshev, R. Creep strength breakdown and microstructure in a 9%Cr steel with high B and low N contents. Mater. Sci. Eng. A 2020, 772, 138821.
- Rosler, J.; Arzt, E. A new model-based creep equation for dispersion strengthened materials. Acta Metall. Mater. 1990, 38, 671–683.
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomena, 2nd ed.; Elsevier Ltd.: Kidlington, UK, 2004.
