Brainstem encephalitis refers to inflammatory diseases affecting the midbrain, pons, and medulla oblongata. The causes of brainstem encephalitis include infections, autoimmune diseases, and paraneoplastic syndromes. Listeria is a common etiology of infectious rhombencephalitis. The trigeminal nerve has been proposed as a pathway through which Listeria monocytogenes reaches the brainstem after entering damaged oropharyngeal mucosa or periodontal tissues. Listeria monocytogenes may also invade the brainstem along the vagus nerve after it infects enteric neurons in the walls of the gastrointestinal tract.
- Brainstem encephalitis
- MRI
- Listeria monocytogenes
- the vagus nerve
- axonal migration
The term “brainstem encephalitis” refers to inflammatory diseases affecting the midbrain, pons, and medulla oblongata. The rhombencephalon consists of the pons, medulla, and cerebellum but does not include the midbrain[1]. Therefore, rhombencephalitis and brainstem encephalitis are slightly different anatomically, but these terms are used interchangeably by many authors[2].
1. Introduction
The first study systematically introducing brainstem encephalitis should be a report [3] published in 1951. The causes of brainstem encephalitis include infections, autoimmune diseases, and paraneoplastic syndromes. Brainstem encephalitis is difficult to diagnose with CT scans because of beam-hardening artifacts. In comparison with CT, MRI has much better tissue characterization and spatial resolution without artifacts in the posterior fossa and is a convenient imaging tool for detecting brainstem diseases.
Brainstem encephalitis is mainly caused by autoimmune diseases and other non-infectious inflammatory conditions including demyelinating diseases, Susac syndrome, behcet disease, chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS), and Hashimoto’s encephalopathy [4]. Infectious etiologies include Listeria monocytogenes, enterovirus 71, flaviviruses, and herpes viruses. Listeria is a common etiology of infectious rhombencephalitis [2].
In 1957, brainstem encephalitis was first introduced as an infrequent type of listeria infection [5]. Listeria monocytogenes meningitis primarily occurs in patients with immunosuppression or those with coexisting diseases, the elderly, or neonates. By contrast, listeria brainstem encephalitis predominantly occurs in previously healthy people. Many clinical manifestations, such as malaise, fever, headache, vomiting, and sweating, in the prodrome (from several days to approximately two weeks) stage of listeria brainstem encephalitis are not specific. Therefore, the early recognition of listeria brainstem infection is challenging.
After the prodrome stage, patients present progressive brainstem deficits, including cranial nerve palsy and cerebellar dysfunction/ataxia. The motor and/or sensory deficits of extremities, respiratory distress, consciousness impairment, seizure, fever, and meningitis, may also be present. Several possible causes of cranial nerve signs have been suggested: invasion of a cranial nerve and then its nucleus, intra-axonal spreading from a region in the brainstem to other regions, and space-occupying effect originating from abscesses and accompanying edema in the brainstem[6].
Patients with brainstem lesions may present dysphagia or dysarthria. Most of the motor or sensory nerves supplying to the pharynx originate from the pharyngeal plexus. The plexus consists of the branches of the glossopharyngeal nerve, vagus nerves, and superior cervical sympathetic ganglia. Except for the stylopharyngeus, which is innervated by the glossopharyngeal nerve, the pharyngeal muscles are innervated from the pharyngeal plexus by the vagal pharyngeal branch [7]. Only solitary nuclei (a series of small nuclei) receive visceral afferents through the facial, glossopharyngeal, and vagus nerves and innervate the salivatory, hypoglossal, dorsal vagal motor and ambiguous nuclei. Patients with dysphagia or dysarthria may present abnormal gag reflex. The glossopharyngeal nerve is the afferent pathway of this reflex, whereas the efferent pathway comprises the glossopharyngeal and vagus nerves originating from the ambiguous nucleus [8]. The central pattern generator of deglutition is considered to be in the region of the solitary nucleus [9][10]. Electrical stimulation with ring electrodes to the pharynx can promote the recovery of dysphagia in patients with stroke [11]. This effect indicates that the sensory input from the pharynx can improve swallowing function by modulating brainstem nuclei.
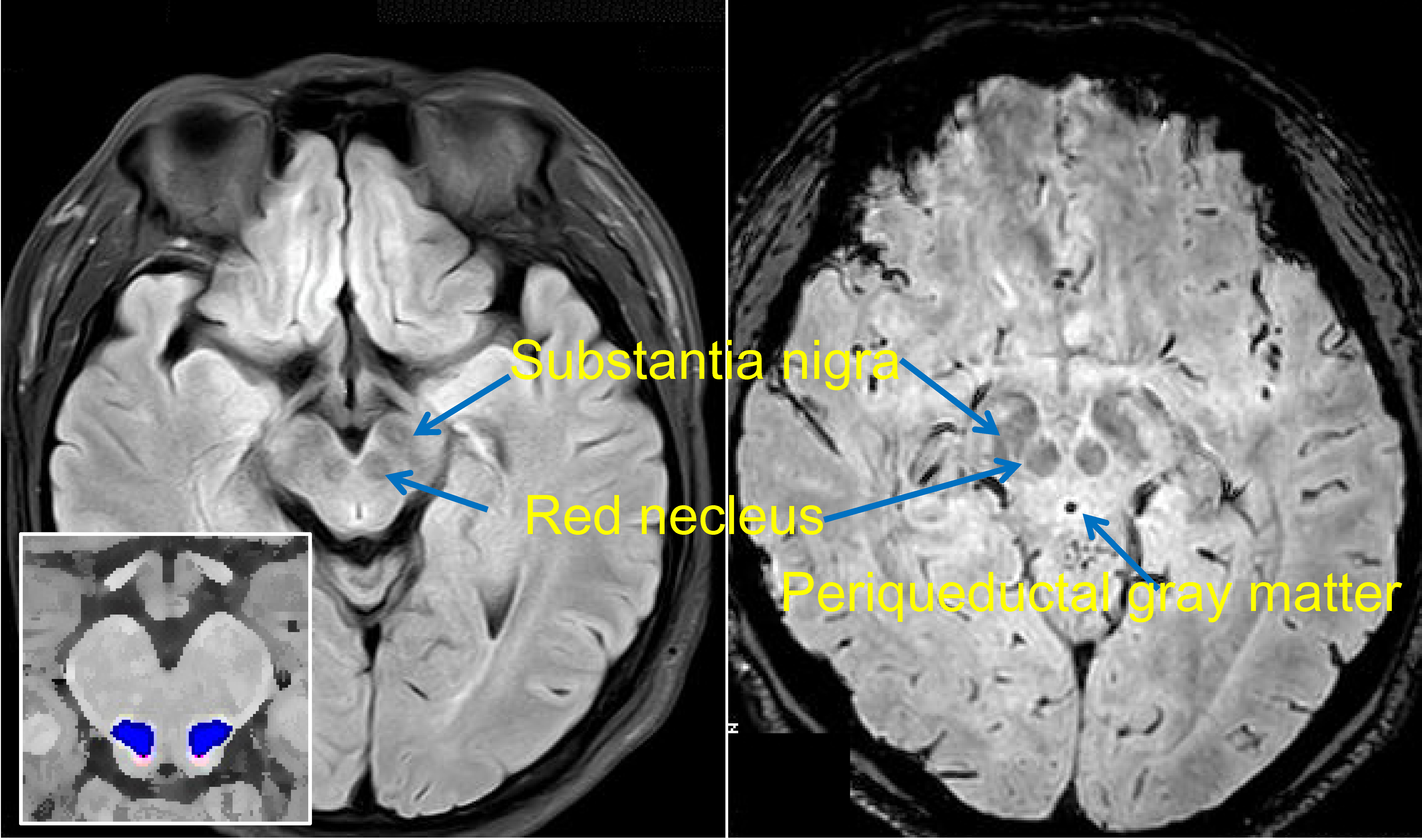
Two structures in the midbrain, the cuneiform nucleus (CN) and the pedunculopontine nucleus (PPN), participate in gait control [12][13]. The exact scopes of the CN and PPN are defined in a histochemically based atlas [14]. Lesions in these regions may result in gait disturbance[15]. The PAG is a region that is traditionally considered as a center for pain regulation. Recently, it has been regarded as a node that links the cerebellum and participates in motor control[16] (PAG, CN and PPN shown in Figure 1).
Figure 1. Brainstem nuclei on MRI. Flair (on the left) and susceptibility-weighted (right) MR images are shown. The substantia nigra and red nucleus present hypointense signals. The approximate locations of the cuneiform nucleus (CN) and the pedunculopontine nucleus (PPN) are indicated in red and blue, respectively.
2. Data, Model, Applications and Influences
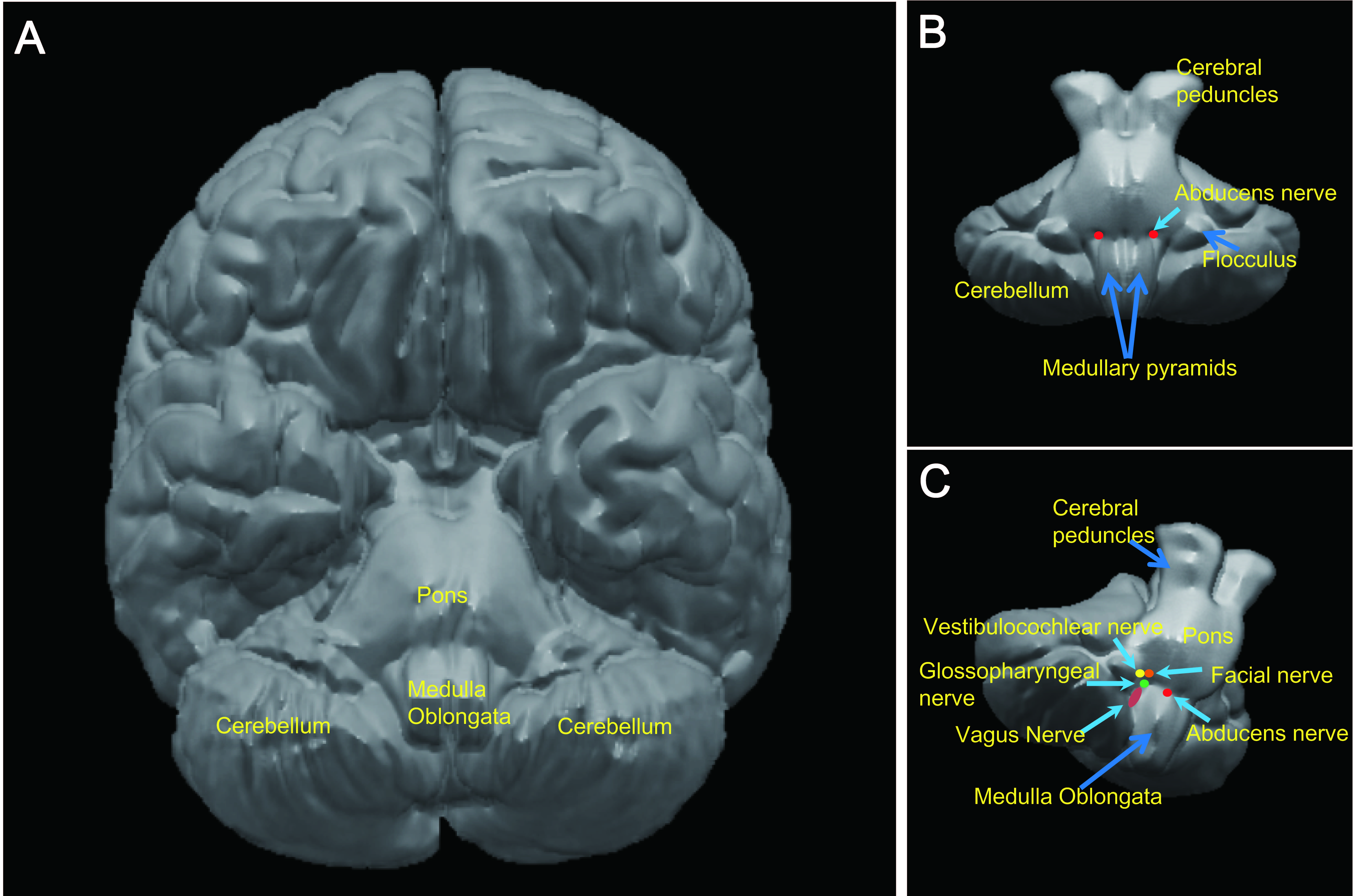
The trigeminal nerve has been proposed as a pathway through which Listeria monocytogenes reaches the brainstem after entering damaged oropharyngeal mucosa or periodontal tissues. Listeria monocytogenes may also invade the brainstem along the vagus nerve after it infects enteric neurons in the walls of the gastrointestinal tract. The roots of cranial nerves III–XII exit from the brainstem. However, only some cranial nerves can be seen in clinical MR images. Additional detailed structures of the brainstem and cranial nerves on MRI may be seen with a high spatial resolution (e.g., 0.5 mm × 0.5 mm in the axial plane, 1 mm slice thickness). Notably, among cranial nerves III–XII, the trigeminal nerve is almost conspicuous on MRI. The vestibulocochlear and facial nerves are also easily seen. The roots of the glossopharyngeal and vagus nerves are jacent and difficult to differentiate (Figure 2). The roots of the oculomotor and abducens nerves are thin but often visible. The roots of the trochlear and hypoglossal nerves are very thin and may not be seen [17].
Figure 2. Spatial relationship among the roots of cranial nerves VI–X. (a) Inferior view of a 3D brain generated from an anatomical template (colin27T1_seg.nii in the SPM Anatomy toolbox) by using the MRIcroN software. (b) 3D brain (brainstem and cerebellum only) generated from an anatomical template (SUIT.nii in the SUIT toolbox). Red spots indicate the approximate locations of the roots of the abducens nerve. (c) Rotated 3D brainstem/cerebellum. Spots in different colors indicate the roots of cranial nerves. The vagus nerve is thicker than the glossopharyngeal nerve.
Due to a low spatial resolution, clinical routine MRI can hardly detect thin nerve roots such as the hypoglossal nerve. Nevertheless, thick cranial nerves, i.e., the trigeminal, vestibulocochlear, and facial nerves, are easily found. Recently, a review that included 123 cases of L. monocytogenes rhombencephalitis concluded that abnormal findings for the hypoglossal nerve are reported only occasionally, although the cranial nerves (fifth, seventh, ninth, tenth, and twelfth) supplying the oropharynx are commonly affected [6]. The introduced phenomena (i.e., the trigeminal nerve is thick and almost conspicuous; the glossopharyngeal and vagus nerves are jacent and difficult to differentiate; and the roots of the trochlear and hypoglossal nerves are too thin to be seen) may account for the numerous reports on the involvement of the trigeminal nerve and the scarcity of MRI reports on the involvement of the vagus nerve.
Efforts to isolate the bacterium from the cerebral spinal fluid (CSF) of ruminants with encephalitis always fail, suggesting that during infection, Listeria monocytogenes rarely enters the CSF [18]. Thus, hematogenous infection is unlikely to lead to listeria brainstem encephalitis [19]. Brain autopsy findings support the hypothesis that the bacterium enters the brainstem via the cranial nerves [20]. In mice, inoculating Listeria monocytogenes in a cranial nerve or facial muscle can induce clinical brainstem encephalitis with histological changes [21]. Autopsy findings in nine human cases with listeria brainstem encephalitis revealed inflammatory infiltrates primarily in the nuclei of the cranial nerves innervating the oropharynx, indicating that the pathogen invades the brainstem along the cranial nerves [20]. Two patients with brainstem encephalitis and culture-identified listeriosis bacteremia presented sensory trigeminal nerve dysfunction (paraesthesia in the face) prior to any other neurological symptoms. Additionally, MRI detected contrast-enhancing lesions were detected in the sensory trigeminal tract in the brainstem [6]. Collectively, Listeria monocytogenes may infect local nerve endings when injuries exist in the mucosa of the oropharynx and cavum nasi and then reach the brainstem via axonal migration [19]. A route via the conjunctiva[22] may also be possible.
In sheep that are cutting, changing, and losing teeth, the inoculation of Listeria monocytogenes into the endodontium follows a route from the infected trigeminal nerve at the dental terminals to the brain via an ascending neuritis [23]. Notably, dental diseases are highly prevalent across the world and pose a serious public health challenge [24] but are often under-reported [25]. Therefore, we suggest that the frequently reported involvement of the trigeminal nerve in listeria rhombencephalitis [6] may partly stem from damaged dental and periodontal defense mechanisms due to dental diseases, which provide a route for the food‐borne Listeria monocytogenes to invade the brainstem through the trigeminal nerve.
An autopsy study of more than 200 natural cases of listeria encephalitis in cattle, sheep, or goats found that except for the frequent involvement of the trigeminal nerve, lesions are also detected in solitary nuclei and oculomotor and facial nerves. These findings suggested that Listeria monocytogenes may enter the brainstem via axonal migration along other nerves [26].
In humans, rhombencephalitis caused by Listeria monocytogenes often present gastrointestinal symptoms, such as nausea and vomiting [19]. Two of the three reported cases with Listeria monocytogenes rhombencephalitis presented a history of gastroenteritis (1 month for one patient and no definite duration for the other patient) prior to the involvement of the cranial nerves[6]. Nausea and vomiting are frequently seen in another group of 14 adult patients with Listeria monocytogenes rhombencephalitis [27]. This phenomenon is difficult to attribute to the infection of the brainstem through intra-axonal transport along the trigeminal nerve or other cranial nerves. We thus propose that the vagus nerve connecting the gastrointestinal tract and brainstem may be a route of the intra-axonal transport of Listeria monocytogenes.
The long distance of the vagus nerve can account for the delay (approximately 4 weeks) between gastrointestinal tract infection and brainstem involvement in certain cases. The slow transition of an enteric pathogen to the brain through the vagal nerve is proposed as the cause of Parkinson's disease [28]. In a mouse model, the vagus nerve in the small intestine or colon infected by a modified rabies virus transports the virus into the nucleus tractus solitarius of the brainstem[29], thereby proving the existence of such a pathway. Prior to listeria brainstem encephalitis, during the prodrome stage that presents nausea, vomiting, fever, and sweating, the bacterium may be transported along the vagus nerve. After arriving in the nucleus tractus solitarius, it can spread to other parts of the brainstem. The mechanisms by which Listeria monocytogenes intrudes into the brainstem through the trigeminal nerve also permit the entry of the bacterium in the brainstem along the vagus nerve.
The trigeminal nerve is thicker than the vagus nerve and other cranial nerves on brainstem MRI. Hence, the hyperintensities of the trigeminal nerve are more frequently observed and reported in MR images than those of other cranial nerves. The roots of the glossopharyngeal and vagus nerves may not be seen in clinical MRI and are difficult to differentiate even by high-resolution MRI. This difficulty may account for the low rate of positive findings for thin and vagus nerves on MRI examination. High resolution MRI may have greater probability of detecting abnormalities of the vagus nerve.
References
- Ramachandran VS. Encyclopedia of the Human Brain, Volume 4. 2002. 1st. San Diego. Academic Press. 461.
- Jubelt B, Mihai C, Li TM, Veerapaneni P. Rhombencephalitis / brainstem encephalitis. Curr Neurol Neurosci Rep. 2011. 11(6): 543-52.
- BICKERSTAFF ER, CLOAKE PC. Mesencephalitis and rhombencephalitis. Br Med J. 1951. 2(4723): 77-81.
- Tan IL, Mowry EM, Steele SU, et al. Brainstem encephalitis: etiologies, treatment, and predictors of outcome. J Neurol. 2013. 260(9): 2312-9.
- ECK H. Encephalomyelitis listeriaca apostematosa. Schweiz Med Wochenschr. 1957. 87(9): 210-4.
- Karlsson WK, Harboe ZB, Roed C, et al. Early trigeminal nerve involvement in Listeria monocytogenes rhombencephalitis: case series and systematic review. J Neurol. 2017. 264(9): 1875-1884.
- Standring S. Gray's Anatomy. 2004. 39. UK. Churchill Livingstone. 441-724.
- Haines DE. Neuroanatomy: an atlas of structures, sections, and systems. 2003. 6th ed. New York. Lippincott Williams & Wilkins. 186.
- Sörös, P.; Inamoto, Y.; Martin, R.E. Functional brain imaging of swallowing: An activation likelihood estimation meta-analysis. Hum. Brain Mapp. 2009, 30, 2426–2439.
- Restivo DA, Hamdy S. Pharyngeal electrical stimulation device for the treatment of neurogenic dysphagia: technology update. Med Devices (Auckl). 2018. 11: 21-26.
- Dziewas, R.; Stellato, R.; van der Tweel, I.; Walther, E.; Werner, C.J.; Braun, T.; Citerio, G.; Jandl, M.; Friedrichs, M.; Nötzel, K; et al. Pharyngeal electrical stimulation for early decannulation in tracheotomised patients with neurogenic dysphagia after stroke (PHAST-TRAC): A prospective, single-blinded, randomised trial. Lancet Neurol. 2018, 17, 849–859.
- Caggiano V, Leiras R, Goni-Erro H, et al. Midbrain circuits that set locomotor speed and gait selection. Nature. 2018. 553(7689): 455-460.
- Ferreira-Pinto MJ, Ruder L, Capelli P, Arber S. Connecting Circuits for Supraspinal Control of Locomotion. Neuron. 2018. 100(2): 361-374 LID - S0896-6273(18)30788-8.
- Paxinos G, Huang XF. Atlas of the human brainstem. 1995. 1st. San Diego. ACADEMIC PRESS.
- Alam M, Schwabe K, Krauss JK. The pedunculopontine nucleus area: critical evaluation of interspecies differences relevant for its use as a target for deep brain stimulation. Brain. 2011. 134(Pt 1): 11-23.
- VCEAO, Brown ST, RIMAO. Cerebellar modulation of synaptic input to freezing-related neurons in the periaqueductal gray. Elife. 2020. 9.
- Wei P BR, Fan Y. Brainstem Encephalitis Caused by Listeria monocytogenes. Pathogens. 2020. 9(9): 715.
- Peters M, Pohlenz J, Jaton K, Ninet B, Bille J. Studies of the detection of Listeria monocytogenes by culture and PCR in cerebrospinal fluid samples from ruminants with listeric encephalitis. Zentralbl Veterinarmed B. 1995. 42(2): 84-8.
- Oevermann A, Zurbriggen A, Vandevelde M. Rhombencephalitis Caused by Listeria monocytogenes in Humans and Ruminants: A Zoonosis on the Rise. Interdiscip Perspect Infect Dis. 2010. 2010: 632513.
- Antal, E.A.; Løberg, E.M.; Dietrichs, E.; Maehlen, J. Neuropathological findings in 9 cases of listeria monocytogenes brain stem encephalitis. Brain Pathol. 2005, 15, 187–191.
- Antal, E.A.; Løberg, E.M.; Bracht, P.; Melby, K.K.; Maehlen, J. Evidence for intraaxonal spread of Listeria monocytogenes from the periphery to the central nervous system. Brain Pathol. 2001, 11, 432–438.
- Revold, T.; Abayneh, T.; Brun-Hansen, H.; Kleppe, S.L.; Ropstad, E.O.; Hellings, R.A.; Sørum, H. Listeria monocytogenes associated kerato-conjunctivitis in four horses in Norway. Acta Vet. Scand. 2015, 57, 76.
- Barlow RM, McGorum B. Ovine listerial encephalitis: analysis, hypothesis and synthesis. Vet Rec. 1985. 116(9): 233-6.
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: A systematic analysis for the global burden of diseases, injuries, and risk factors. J. Dent. Res. 2017, 96, 380–387.
- Hammoud K, Lanfranchi M, Adams D, Bedi HS, Mehan WA. Prevalence and Reporting Rates of Incidental Dental Disease on Head CT Examinations. Acad Radiol. 2018. 25(10): 1318-1324.
- Oevermann A, Di Palma S, Doherr MG, Abril C, Zurbriggen A, Vandevelde M. Neuropathogenesis of naturally occurring encephalitis caused by Listeria monocytogenes in ruminants. Brain Pathol. 2010. 20(2): 378-90.
- Uldry PA, Kuntzer T, Bogousslavsky J, et al. Early symptoms and outcome of Listeria monocytogenes rhombencephalitis: 14 adult cases. J Neurol. 1993. 240(4): 235-42.
- Svensson, E.; Horváth-Puhó, E.; Thomsen, R.W.; Djurhuus, J.C.; Pedersen, L.; Borghammer, P.; Sørensen, H.T. Vagotomy and subsequent risk of Parkinson's disease. Ann. Neurol. 2015, 78, 522–529.
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, eaat5236