Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jessica Chacon and Version 2 by Conner Chen.
The era of personalized cancer therapy is here. Advances in the field of immunotherapy have paved the way for the development of individualized neoantigen-based therapies that can translate into favorable treatment outcomes and fewer side effects for patients.
- neoantigen
- immunotherapy
- tumor-associated antigens
1. Introduction
Immunotherapy has rapidly become a primary mode of cancer treatment due to the remarkable success and clinical improvement seen in patients with advanced-stage or aggressive, recurrent cancers such as melanoma, ovarian cancer, breast cancer, and gastrointestinal cancers [1]. A significant number of therapies that have seen success to date involve the utilization of T cells. This is because of the tumor cell’s unique ability to suppress the immune system by modulating its surrounding microenvironment, also known as the tumor microenvironment (TME). By targeting the TME with antigen-specific immune cells, we are it is able to essentially activate the immune system to mount an anti-cancer attack.
There has been much interest in identifying, developing, and producing immune cells and proteins that are able to mount a potent immune response against the tumor cells and their TME. This vested interest has led to the production of various immunotherapies including monoclonal antibodies (mAbs), tumor-infiltrating lymphocytes (TIL), and chimeric antigen receptor T-cells (CAR-T). Despite these advancements, due to the metabolic reprogramming and adaptability of cancer cells, these immunotherapies have been met with both innate and acquired resistance [2][3][2,3]. This has led to the search for new immunotherapy targets and has opened a new hallmark of cancer immunotherapy.
Neoantigens are tumor-specific proteins synthesized by tumor cells as protein by-products [4][5][4,5]. The rapid division and proliferation of tumor cells lead to various mutations in coding and non-coding loci [4][5][4,5]. The changes in the amino acid sequence that occur due to mutations in coding regions leads to the production of proteins that are not found in normal cells and are unique to tumor cells [4][5][6][4,5,6]. These tumor-specific by-products termed neoantigens are unique in that multiple patients can share the same neoantigens (shared neoantigens), but they can also be specific to an individual (personalized neoantigens) [4][5][6][4,5,6]. In addition, neoantigens are highly immunogenic, making them a favorable immunotherapy target [5][7][5,7].
2. Cancer and Immunotherapy
The relationship between cancer and the immune system is very dynamic. Cancer cells modulate the immune system to survive, proliferate, and metastasize. For instance, tumor cells express immuno-suppressive ligands on the cell surface such as programmed death ligand-1 (PDL-1) to evade immune cell recognition and inhibit the mounting of an immune response. In addition, tumor cells can also hijack and employ crosstalk to mediate immune cells and inflammation to aid with metastasis [8][9][8,9]. The basis of immunotherapy is to target these specific areas of crosstalk, intervene, and modulate the immune response to target the cancer cells. The ultimate goal of immunotherapy is to enhance recognition, target, and mount a toxic response against the cancer cells [4]. Monoclonal antibodies (mAbs) are a type of targeted immunotherapy that has shown promising therapeutic results that is currently being used to treat various cancers including breast cancer, colorectal cancer, and leukemias [5][7][10][5,7,10]. For instance, mAbs can mount a potent immune response and can be employed to trigger cytotoxicity and inhibit further tumorigenesis (Figure 1A) [1][11][12][1,11,12]. They are able to recognize both cell surface antigens and secreted antigens with high specificity, indicating their versality [10].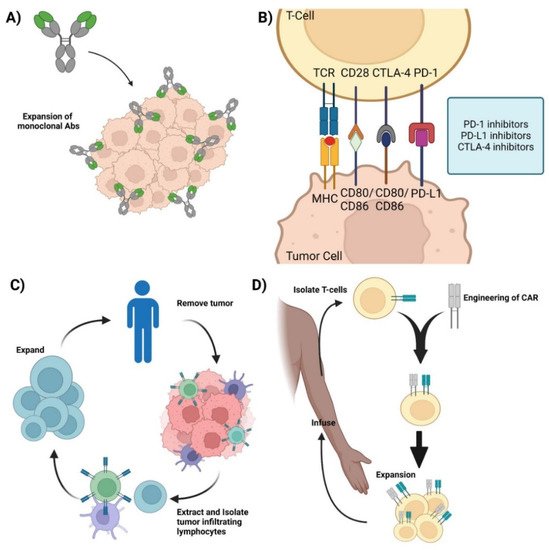
Figure 1. Current immunotherapies. (A) Monoclonal antibodies—representation of mAbs and the mechanism involved in mounting an immune response against cancer cells. (B) Immune checkpoint inhibitors—Graphic showcasing the immune crosstalk that occurs between T cells and tumor cells and the respective ligands and receptors targeted using mAbs. (C) Adoptive Cell Therapy—Graphic showing how TILs are synthesized by removing the respective tumor from the patient, isolating, extracting, and expanding the appropriate TILs, and its re-infusion back into the patient for therapy. (D) Chimeric Antigen Receptor T-cell therapy—Representation of CAR-T synthesis and production. Isolate T cells from the patient and mount a synthetic CAR onto the host T cells. Expand the modified T cell population and re-infuse back into the patient. Credits: Images were created using BioRender.
