Dendrimers are highly branched three-dimensional macromolecules, which properties are essentially dependent on the type of their terminal functions. Dendrimers are synthesized by iterative processes, which afford a new generation at the end of each sequence, characterized by a multiplication of the number of terminal functions. Such processes generate identical terminal functions on the surface of the dendrimers. However, it is sometimes desirable to have two types of surface functions in order to fulfil specific properties. Numerous properties have been explored already, most of them being related to catalysis, materials, or biology/nanomedicine. Strategies to get two types of terminal functions will be illustrated with polyphosphorhydrazone (PPH) dendrimers.
- dendrimer
- properties
- bifunctionalization
1. Introduction
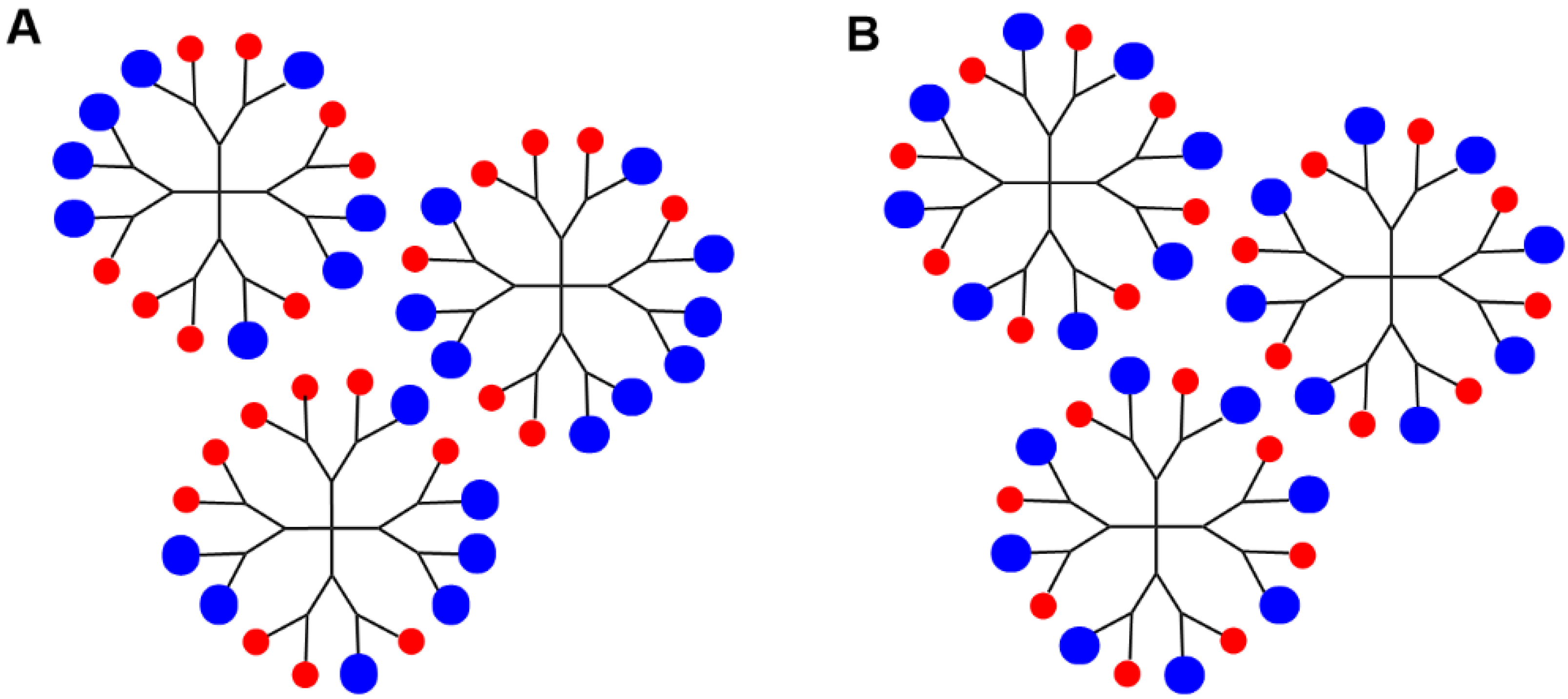
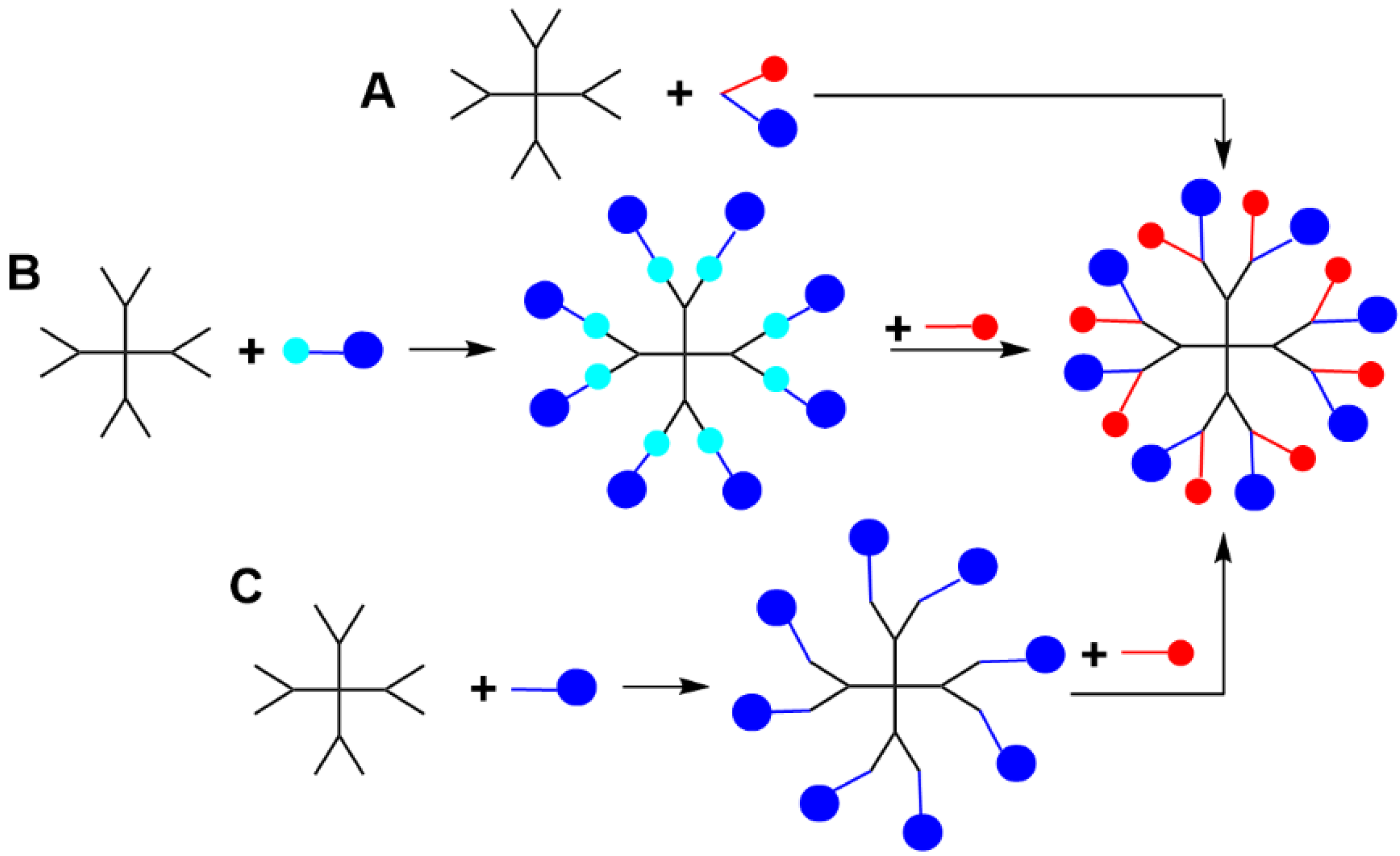
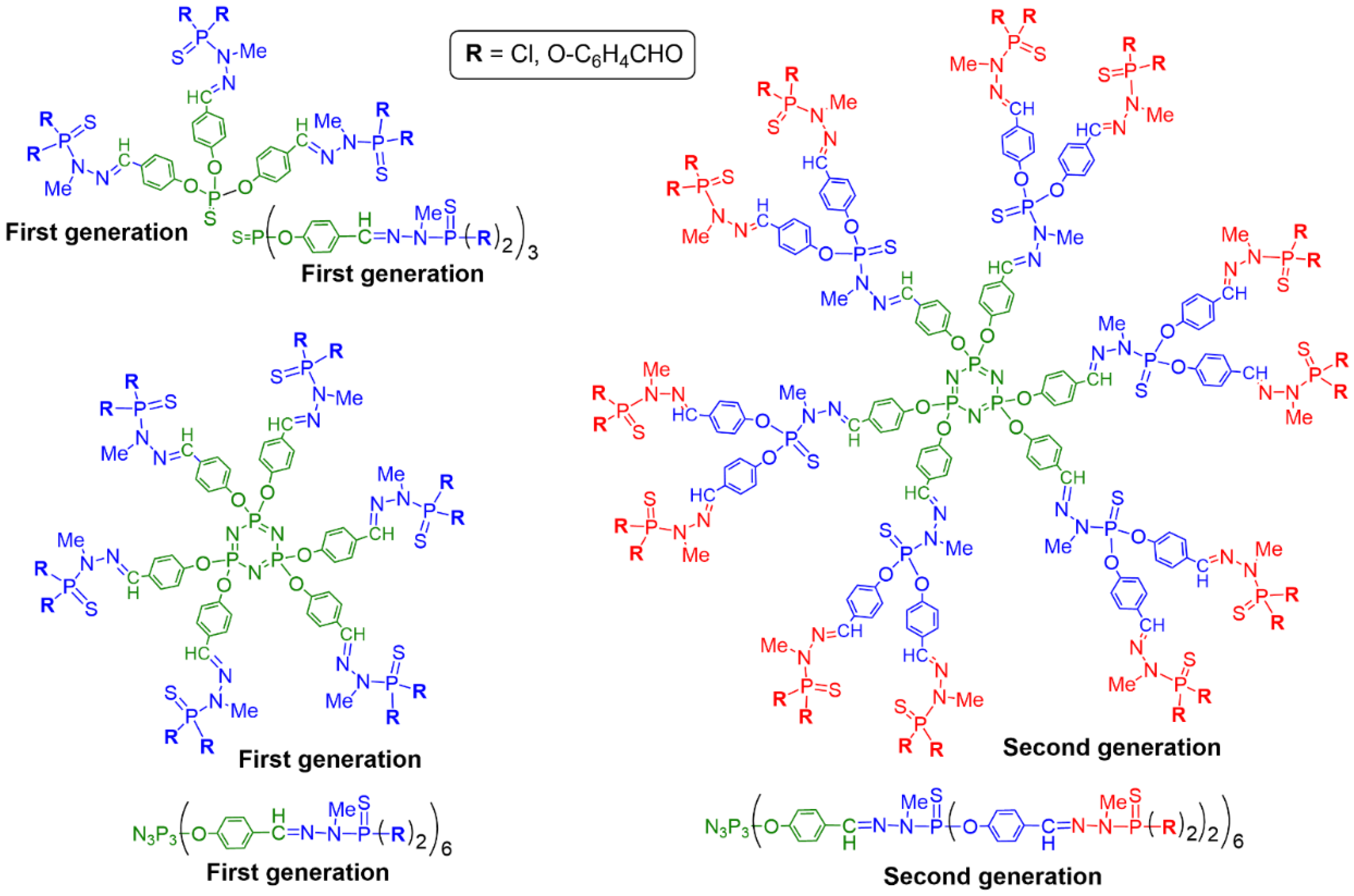
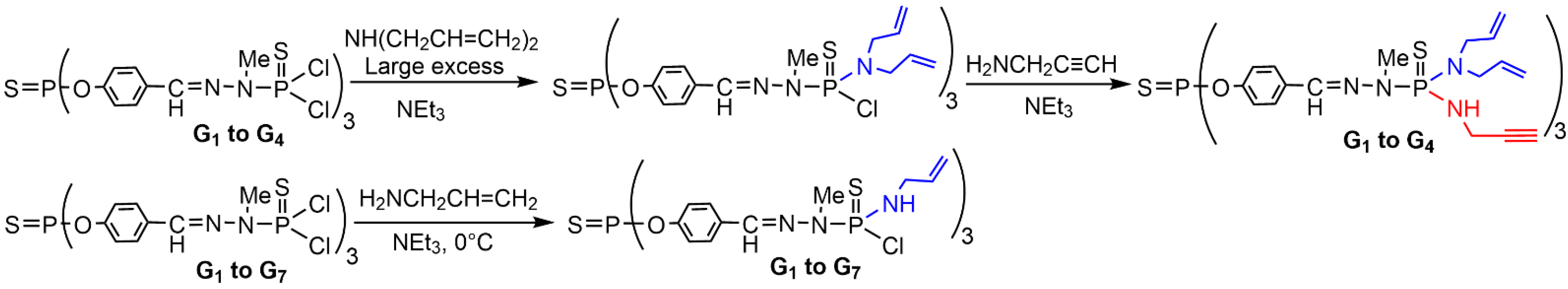
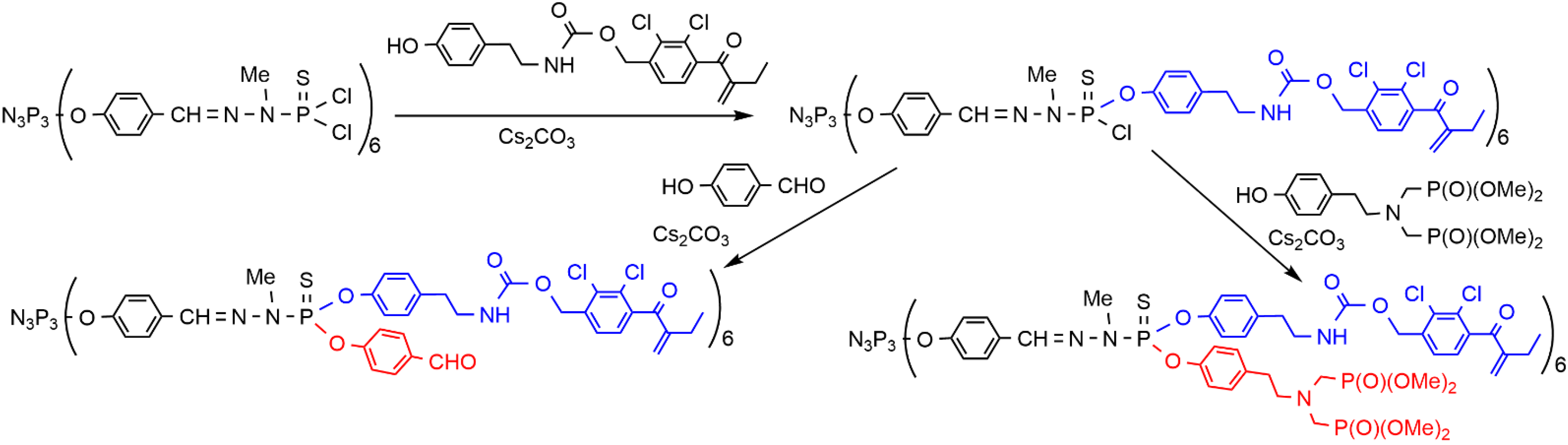
2. Sequential Grafting of a First, then a Second Function on the Surface of PPH Dendrimers
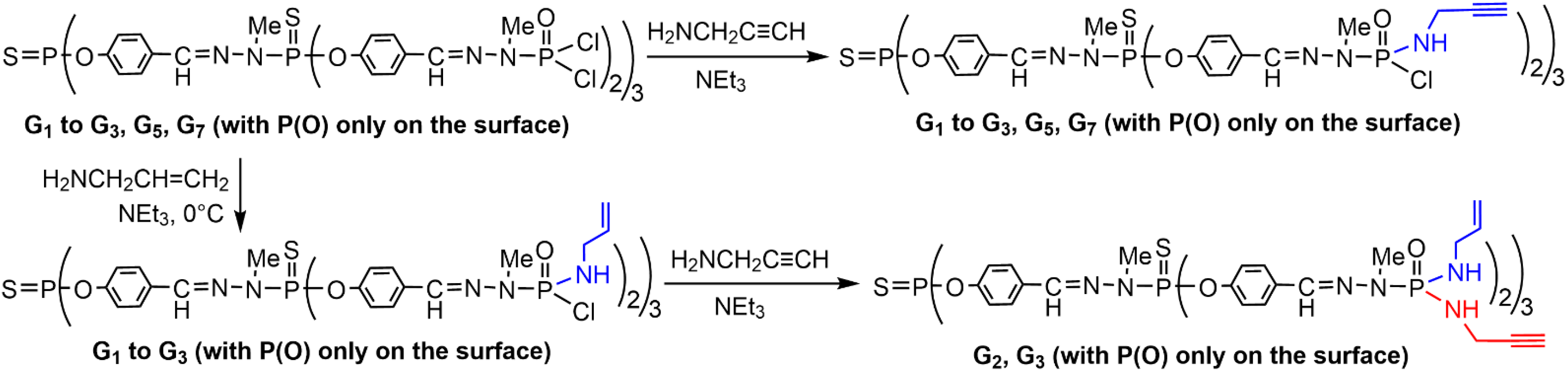
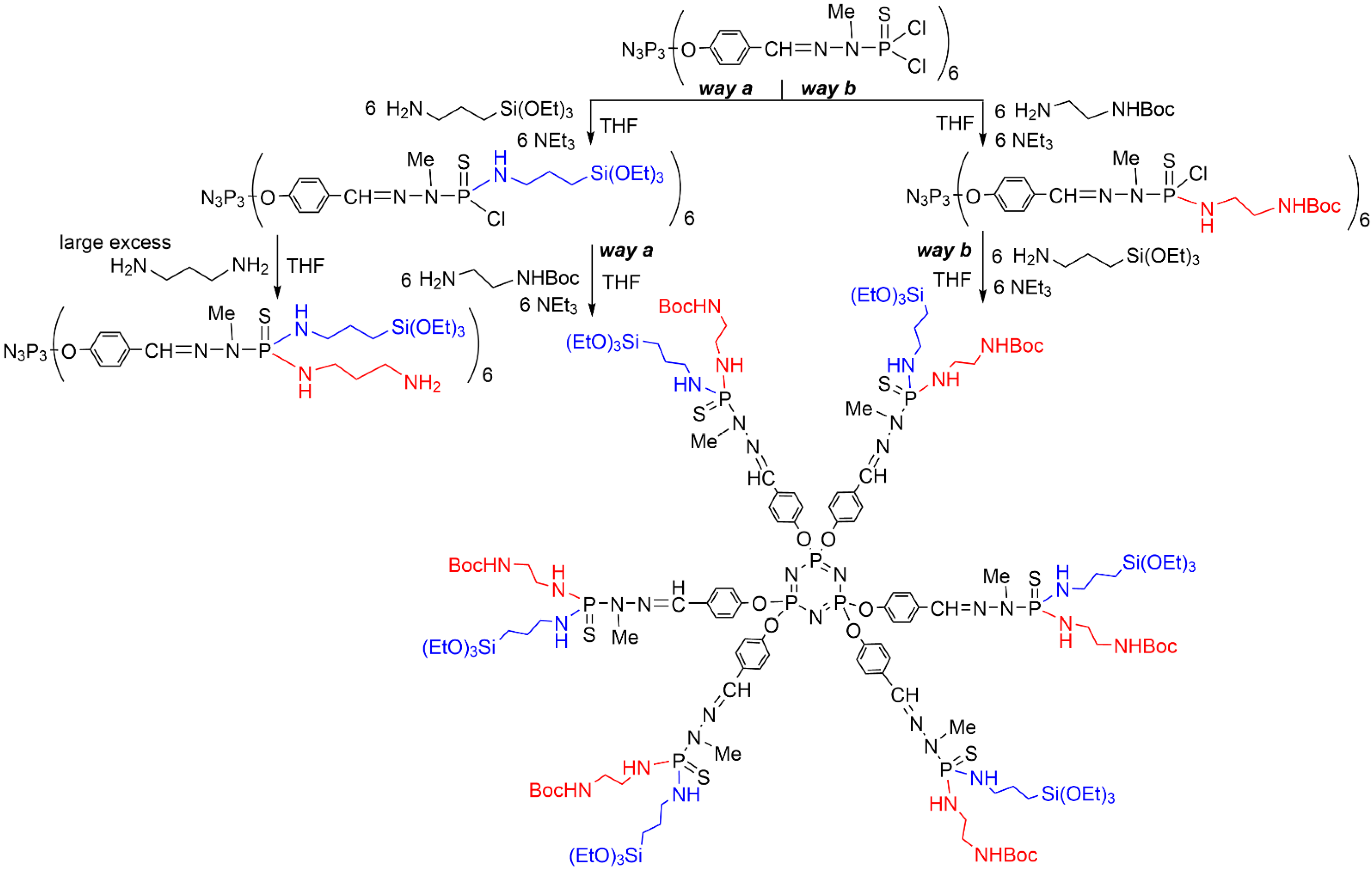
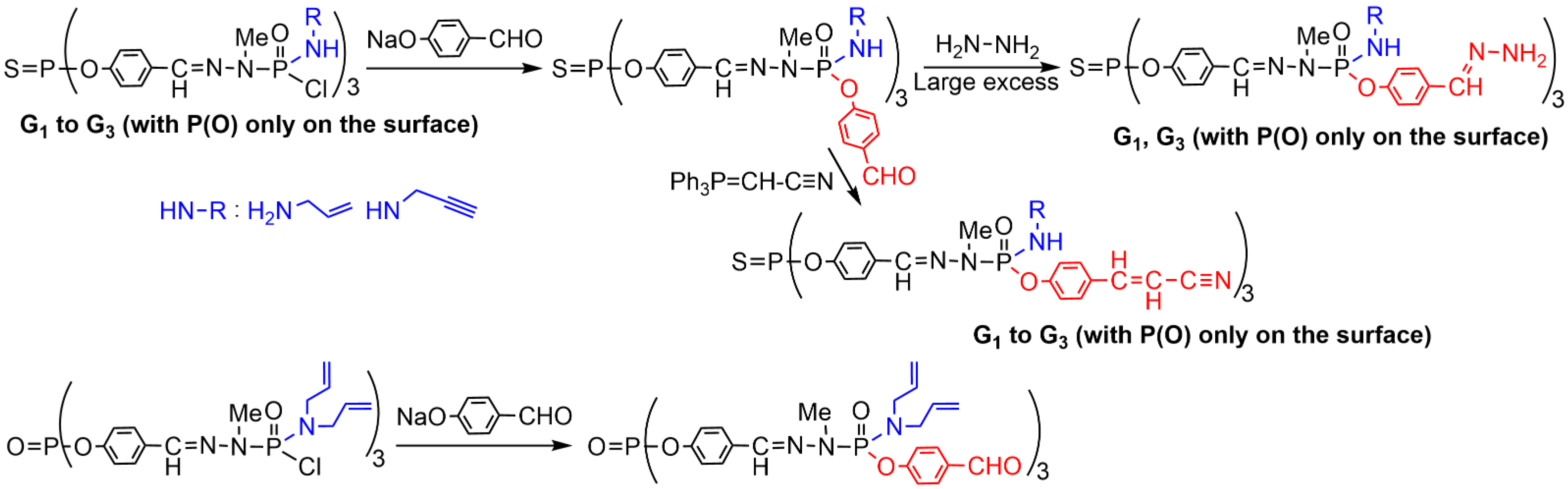
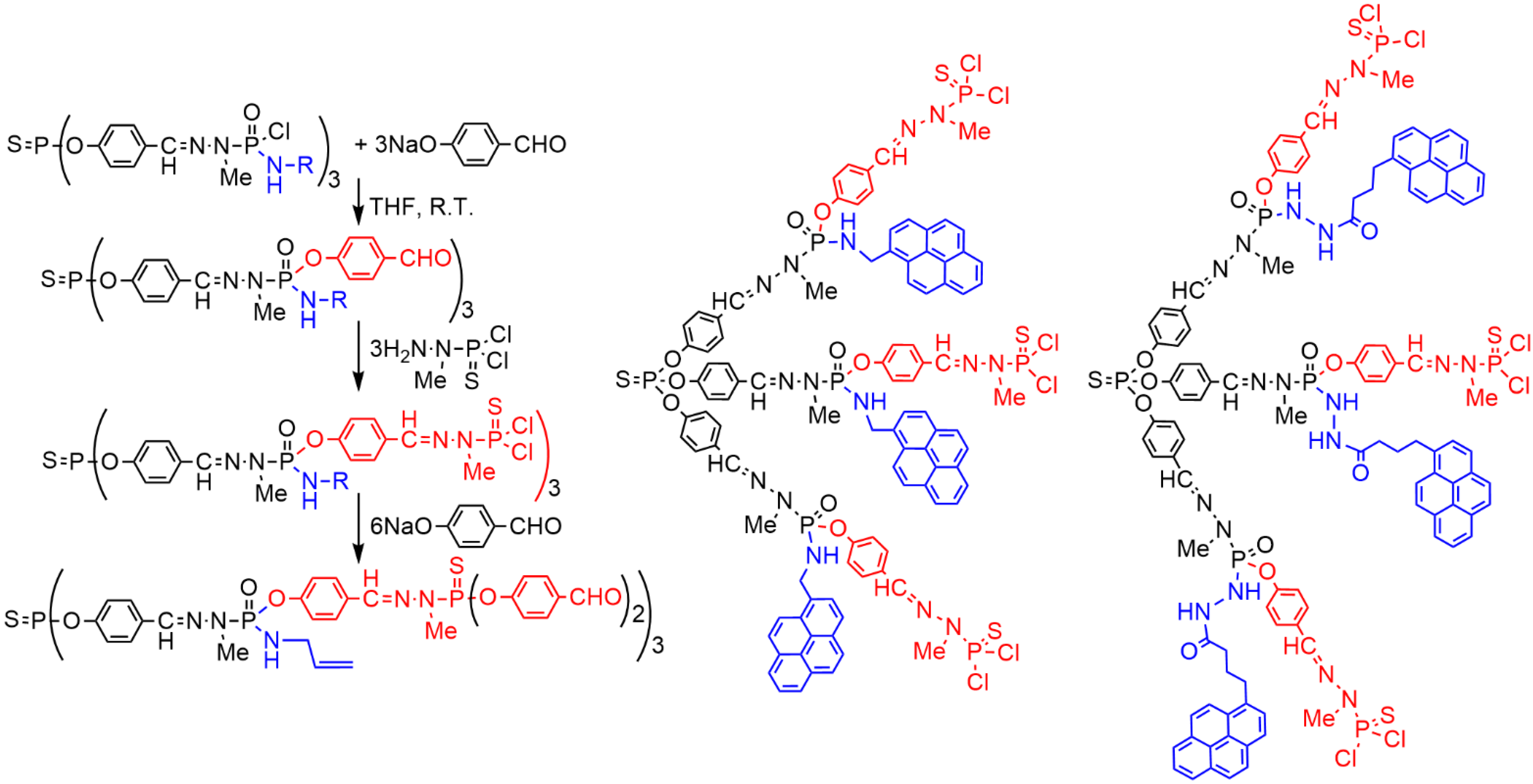
References
- Newkome, G.R.; Shreiner, C.D. Poly(amidoamine), Polypropylenimine, and Related Dendrimers and Dendrons Possessing Different 1 → 2 Branching Motifs: An overview of the Divergent Procedures. Polymer 2008, 49, 1–173. https://doi.org/10.1016/j.polymer.2007.10.021.
- Newkome, G.R.; Shreiner, C. Dendrimers Derived from 1 → 3 Branching Motifs. Chem. Rev. 2010, 110, 6338–6442. https://doi.org/10.1021/cr900341m.
- Mullen, D.G.; Borgmeier, E.L.; Desai, A.M.; van Dongen, M.A.; Barash, M.; Cheng, X.M.; Baker, J.R.; Holl, M.M.B. Isolation and Characterization of Dendrimers with Precise Numbers of Functional Groups. Chem. –A Eur. J. 2010, 16, 10675–10678. https://doi.org/10.1002/chem.201001175.
- Thomas, T.P.; Huang, B.H.; Choi, S.K.; Silpe, J.E.; Kotlyar, A.; Desai, A.M.; Zong, H.; Gam, J.; Joice, M.; Baker, J.R. Polyvalent Dendrimer-Methotrexate as a Folate Receptor-Targeted Cancer Therapeutic. Mol. Pharm. 2012, 9, 2669–2676. https://doi.org/10.1021/mp3002232.
- Thomas, K.R.J.; Thompson, A.L.; Sivakumar, A.V.; Bardeen, C.J.; Thayumanavan, S. Energy and Electron Transfer in Bifunc-tional Non-Conjugated Dendrimers. J. Am. Chem. Soc. 2005, 127, 373–383. https://doi.org/10.1021/ja044778m.
- Navath, R.S.; Menjoge, A.R.; Wang, B.; Romero, R.; Kannan, S.; Kannan, R.M. Amino Acid-Functionalized Dendrimers with Heterobifunctional Chemoselective Peripheral Groups for Drug Delivery Applications. Biomacromolecules 2010, 11, 1544–1563. https://doi.org/10.1021/bm100186b.
- Montanez, M.I.; Hed, Y.; Utsel, S.; Ropponen, J.; Malmstrom, E.; Wagberg, L.; Hult, A.; Malkoch, M. Bifunctional Den-dronized Cellulose Surfaces as Biosensors. Biomacromolecules 2011, 12, 2114–2125. https://doi.org/10.1021/bm200201y.
- Hed, Y.; Oberg, K.; Berg, S.; Nordberg, A.; von Holst, H.; Malkoch, M. Multipurpose Heterofunctional Dendritic Scaffolds as Crosslinkers towards Functional Soft Hydrogels and Implant Adhesives in Bone Fracture Applications. J. Mater. Chem. B 2013, 1, 6015–6019. https://doi.org/10.1039/c3tb21061h.
- Sharma, A.; Neibert, K.; Sharma, R.; Hourani, R.; Maysinger, D.; Kakkar, A. Facile Construction of Multifunctional Nanocarriers Using Sequential Click Chemistry for Applications in Biology. Macromolecules 2011, 44, 521–529. https://doi.org/10.1021/ma102354k.
- Castelar, S.; Romero, P.; Serrano, J.L.; Barbera, J.; Marcos, M. Multifunctional Ionic Hybrid Poly (propyleneimine) Den-drimers Surrounded by Carbazole Dendrons: Liquid Crystals, Optical and Electrochemical Properties. RSC Adv. 2015, 5, 65932–65941. https://doi.org/10.1039/c5ra12237f.
- Kaufman, E.A.; Tarallo, R.; Elacqua, E.; Carberry, T.P.; Weck, M. Synthesis of Well-Defined Bifunctional Newkome-Type Dendrimers. Macromolecules 2017, 50, 4897–4905. https://doi.org/10.1021/acs.macromol.7b01035.
- van der Poll, D.G.; Kieler-Ferguson, H.M.; Floyd, W.C.; Guillaudeu, S.J.; Jerger, K.; Szoka, F.C.; Frechet, J.M. Design, Syn-thesis, and Biological Evaluation of a Robust, Biodegradable Dendrimer. Bioconjugate Chem. 2010, 21, 764–773. https://doi.org/10.1021/bc900553n.
- Floyd, W.C.; Klemm, P.J.; Smiles, D.E.; Kohlgruber, A.C.; Pierre, V.C.; Mynar, J.L.; Frechet, J.M.J.; Raymond, K.N. Conjuga-tion Effects of Various Linkers on Gd(III) MRI Contrast Agents with Dendrimers: Optimizing the Hydroxypyridinonate (HOPO) Ligands with Nontoxic, Degradable Esteramide (EA) Dendrimers for High Relaxivity. J. Am. Chem. Soc. 2011, 133, 2390–2393. https://doi.org/10.1021/ja110582e.
- Klemm, P.J.; Floyd, W.C.; Smiles, D.E.; Frechet, J.M.J.; Raymond, K.N. Improving T1 and T2 Magnetic Resonance Imaging Contrast Agents through the Conjugation of an Esteramide Dendrimer to High-Water-Coordination Gd (III) Hydroxypyri-dinone Complexes. Contrast Media Mol. Imaging 2012, 7, 95–99. https://doi.org/10.1002/cmmi.483.
- Klemm, P.J.; Floyd, W.C.; Andolina, C.M.; Frechet, J.M.J.; Raymond, K.N. Conjugation to Biocompatible Dendrimers In-creases Lanthanide T2 Relaxivity of Hydroxypyridinone Complexes for Magnetic Resonance Imaging. Eur. J. Inorg. Chem. 2012, 2012, 2108–2114. https://doi.org/10.1002/ejic.201101167.
- Andolina, C.M.; Klemm, P.J.; Floyd, W.C.; Frechet, J.M.J.; Raymond, K.N. Analysis of Lanthanide Complex Dendrimer Conjugates for Bimodal NIR and MRI Imaging. Macromolecules 2012, 45, 8982–8990. https://doi.org/10.1021/ma302206g.
- Floyd, W.C.; Datta, G.K.; Imamura, S.; Kieler-Ferguson, H.M.; Jerger, K.; Patterson, A.W.; Fox, M.E.; Szoka, F.C.; Frechet, J.M.J.; Ellman, J.A. Chemotherapeutic Evaluation of a Synthetic Tubulysin Analogue-Dendrimer Conjugate in C26 Tumor Bearing Mice. Chem. Med. Chem. 2011, 6, 49–53. https://doi.org/10.1002/cmdc.201000377.
- Luo, K.; Liu, G.; She, W.C.; Wang, Q.Y.; Wang, G.; He, B.; Ai, H.; Gong, Q.Y.; Song, B.; Gu, Z.W. Gadolinium-labeled Peptide Dendrimers with Controlled Structures as Potential Magnetic Resonance Imaging Contrast Agents. Biomaterials 2011, 32, 7951–7960. https://doi.org/10.1016/j.biomaterials.2011.07.006.
- Luo, K.; Liu, G.; He, B.; Wu, Y.; Gong, Q.Y.; Song, B.; Ai, H.; Gu, Z.W. Multifunctional Gadolinium-based Dendritic Macro-molecules as Liver Targeting Imaging Probes. Biomaterials 2011, 32, 2575–2585. https://doi.org/10.1016/j.biomaterials.2010.12.049.
- Li, Y.P.; Lin, T.Y.; Luo, Y.; Liu, Q.Q.; Xiao, W.W.; Guo, W.C.; Lac, D.; Zhang, H.Y.; Feng, C.H.; Wachsmann-Hogiu, S.; et al. A Smart and Versatile Theranostic Nanomedicine Platform Based on Nanoporphyrin. Nat. Commun. 2014, 5, 4712. https://doi.org/10.1038/ncomms5712.
- Goodwin, A.P.; Lam, S.S.; Frechet, J.M.J. Rapid, Efficient Synthesis of Heterobifunctional Biodegradable Dendrimers. J. Am. Chem. Soc. 2007, 129, 6994–6995. https://doi.org/10.1021/ja071530z.
- Patra, S.; Kozura, B.; Huang, A.Y.T.; Enciso, A.E.; Sun, X.K.; Hsieh, J.T.; Kao, C.L.; Chen, H.T.; Simanek, E.E. Dendrimers Terminated with Dichlorotriazine Groups Provide a Route to Compositional Diversity. Org. Lett. 2013, 15, 3808–3811. https://doi.org/10.1021/ol400811h.
- Lim, J.; Turkbey, B.; Bernardo, M.; Bryant, L.H.; Garzoni, M.; Pavan, G.M.; Nakajima, T.; Choyke, P.L.; Simanek, E.E.; Koba-yashi, H. Gadolinium MRI Contrast Agents Based on Triazine Dendrimers: Relaxivity and In Vivo Pharmacokinetics. Bio-conjugate Chem. 2012, 23, 2291–2299. https://doi.org/10.1021/bc300461r.
- Launay, N.; Caminade, A.M.; Lahana, R.; Majoral, J.P. A General Synthetic Strategy for Neutral Phosphorus-Containing Dendrimers. Angew. Chem. Int. Edit. Engl. 1994, 33, 1589–1592. https://doi.org/10.1002/anie.199415891.
- Launay, N.; Caminade, A.M.; Majoral, J.P. Synthesis of Bowl-Shaped Dendrimers from Generation 1 to Generation 8. J. Or-ganomet. Chem. 1997, 529, 51–58. https://doi.org/10.1016/s0022-328x(96)06293-6.
- Lartigue, M.L.; Slany, M.; Caminade, A.M.; Majoral, J.P. Phosphorus-Containing Dendrimers: Synthesis of Macromolecules with Multiple Tri- and Tetrafunctionalization. Chem. –A Eur. J. 1996, 2, 1417–1426. https://doi.org/10.1002/chem.19960021114.
- Riegert, D.; Pla-Quintana, A.; Fuchs, S.; Laurent, R.; Turrin, C.O.; Duhayon, C.; Majoral, J.P.; Chaumonnot, A.; Caminade, A.M. Diversified Strategies for the Synthesis of Bifunctional Dendrimeric Structures. Eur. J. Org. Chem. 2013, 2013, 5414–5422. https://doi.org/10.1002/ejoc.201300456.
- Riegert, D.; Bareille, L.; Laurent, R.; Majoral, J.P.; Caminade, A.M.; Chaumonnot, A. Silica Functionalized by Bifunctional Dendrimers: Hybrid Nanomaterials for Trapping CO2. Eur. J. Inorg. Chem. 2016, 2016, 3103–3110. https://doi.org/10.1002/ejic.201600426.
- Lartigue, M.L.; Caminade, A.M.; Majoral, J.P. Synthesis and Reactivity of Dendrimers Based on Phosphoryl (P= O) Groups. Phosphorus Sulfur Silicon Relat. Elem. 1997, 123, 21–34. https://doi.org/10.1080/10426509708044195.
- Severac, M.; Leclaire, J.; Sutra, P.; Caminade, A.M.; Majoral, J.P. A New Way for the Internal Functionalization of Den-drimers. Tetrahedron Lett. 2004, 45, 3019–3022. https://doi.org/10.1016/j.tetlet.2004.02.120.
- El Brahmi, N.; Mignani, S.M.; Caron, J.; El Kazzouli, S.; Bousmina, M.M.; Caminade, A.M.; Cresteil, T.; Majoral, J.P. Investi-gations on Dendrimer Space Reveal Solid and Liquid Tumor Growth-Inhibition by Original Phosphorus-Based Dendrimers and the Corresponding Monomers and Dendrons with Ethacrynic Acid Motifs. Nanoscale 2015, 7, 3915–3922. https://doi.org/10.1039/c4nr05983b.
- El Brahmi, N.; El Kazzouli, S.; Mignani, S.; Laurent, R.; Ladeira, S.; Caminade, A.M.; Bousmina, M.; Majoral, J.P. Symmetrical and Unsymmetrical Incorporation of Active Biological Monomers on the Surface of Phosphorus Dendrimers. Tetrahedron 2017, 73, 1331–1341. https://doi.org/10.1016/j.tet.2017.01.044.
