Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Ruben Ramirez Zegarra.
Gestational diabetes mellitus (GDM) is defined as glucose intolerance first identified during pregnancy and usually resolving after birth. Since the beginning of the COVID-19 pandemic, there has been a growing interest in the association between GDM and COVID-19.
- COVID-19
- SARS-CoV-2
- gestational diabetes
- nutrition
1. Introduction
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, China [1]. Months later, the disease caused by the virus—i.e., COVID-19—was classified by the WHO as a pandemic [2]. During the course of the disease, some of the patients affected by COVID-19 may develop a severe disease and show the deterioration of their clinical conditions. Severe COVID-19 is a life-threatening complication commonly associated with acute respiratory distress syndrome, thromboembolic complications, and disorders of the central or peripheral nervous system, resulting in multiorgan failure and death [4][3].
Gestational diabetes mellitus (GDM) is defined as glucose intolerance first identified during pregnancy and usually resolving after birth [13][4]. It is one of the most common medical complications of pregnancy, with a prevalence ranging from 5.8% (1.8–22.3%) in Europe to 12.9% (8.4–24.5%) in the Middle East and North Africa [14,15][5][6]. Although the etiology of GDM has yet to be fully understood, different risk factors for the development of the disease, including maternal age, obesity, increased gestational weight gain, and a family history of diabetes mellitus, have been identified [14,16][5][7]. Pregnant women with GDM are at increased risk of short-term adverse maternal and perinatal outcomes, such as cesarean section, shoulder dystocia, preeclampsia, fetal macrosomia, neonatal hypoglycemia, and the admission of neonates to the intensive care unit [14,17,18][5][8][9].
Since the beginning of the COVID-19 pandemic, there has been a growing interest in the association between GDM and COVID-19. On the one hand, SARS-CoV-2 may determine hyperglycemia and diabetes mellitus due to its interaction with the angiotensin-converting enzyme 2 (ACE2) receptors and the resulting damage to the pancreatic islet cells [33,34,35,36][10][11][12][13]. On the other hand, pregnant women with GDM are more likely to manifest symptoms of COVID-19 than their counterparts, due to the common presence of other comorbidities such as obesity and hypertension [37,38,39][14][15][16].
 Lifestyle modifications including physical activity are also among the first-line options for tackling GDM and poor glycemic control in women with GDM. In women with GDM, physical activity has been shown to improve glucose control and reduce insulin use [88[43][44],89], especially when combined with dietary modifications [90][45]. As mentioned above, one of the measures adopted during the COVID-19 pandemic that had a negative impact on the prevalence of GDM was lockdowns [43][46]. Lockdown periods led to unhealthy diets and reduced physical activity in some individuals [91[47][48],92], resulting in a rise in insulin resistance, total body fat, abdominal fat, and inflammatory cytokines [93][49]. Moreover, mothers suffered from psychological stress, depression, and anxiety during quarantine, which contributed further to the increase in unhealthy diets and the reduction in physical activity, worsening the rates of hyperglycemia [92][48]. These COVID-19-related containment measurements led to increased HbA1c concentrations [72][31] and poor glycemic control [73[32][50],94], which might also explain the increasing rates of insulin use among mothers with GDM during the SARS-CoV2 pandemic.
Mothers with a normal BMI and GDM on diet treatment are not at higher risk of contracting or developing symptomatic COVID-19 compared to women with pregnancies that are not complicated by GDM [37][14]. Conversely, overweight or obese mothers with GDM on diet treatment have a 35% higher risk of developing a symptomatic disease [37][14]. However, this is not reflected in the maternal or neonatal outcomes, as mothers with GDM on diet treatment maintain good glycemic control. This might explain the low rates of maternal and neonatal complications, especially in patients with a BMI < 25 kg/m2. However, it is important to underline that the available evidence is limited, and the studies so far published may not have been capable of detecting significant changes in adverse maternal and neonatal outcomes. Therefore, results should be interpreted with caution, especially when counseling and treating a pregnant patient with overweight or obesity and GDM, as obesity alone is an acknowledged risk factor for severe COVID-19 [42,95][51][52].
Lifestyle modifications including physical activity are also among the first-line options for tackling GDM and poor glycemic control in women with GDM. In women with GDM, physical activity has been shown to improve glucose control and reduce insulin use [88[43][44],89], especially when combined with dietary modifications [90][45]. As mentioned above, one of the measures adopted during the COVID-19 pandemic that had a negative impact on the prevalence of GDM was lockdowns [43][46]. Lockdown periods led to unhealthy diets and reduced physical activity in some individuals [91[47][48],92], resulting in a rise in insulin resistance, total body fat, abdominal fat, and inflammatory cytokines [93][49]. Moreover, mothers suffered from psychological stress, depression, and anxiety during quarantine, which contributed further to the increase in unhealthy diets and the reduction in physical activity, worsening the rates of hyperglycemia [92][48]. These COVID-19-related containment measurements led to increased HbA1c concentrations [72][31] and poor glycemic control [73[32][50],94], which might also explain the increasing rates of insulin use among mothers with GDM during the SARS-CoV2 pandemic.
Mothers with a normal BMI and GDM on diet treatment are not at higher risk of contracting or developing symptomatic COVID-19 compared to women with pregnancies that are not complicated by GDM [37][14]. Conversely, overweight or obese mothers with GDM on diet treatment have a 35% higher risk of developing a symptomatic disease [37][14]. However, this is not reflected in the maternal or neonatal outcomes, as mothers with GDM on diet treatment maintain good glycemic control. This might explain the low rates of maternal and neonatal complications, especially in patients with a BMI < 25 kg/m2. However, it is important to underline that the available evidence is limited, and the studies so far published may not have been capable of detecting significant changes in adverse maternal and neonatal outcomes. Therefore, results should be interpreted with caution, especially when counseling and treating a pregnant patient with overweight or obesity and GDM, as obesity alone is an acknowledged risk factor for severe COVID-19 [42,95][51][52].
2. Gestational Diabetes Treated with Diet and COVID-19
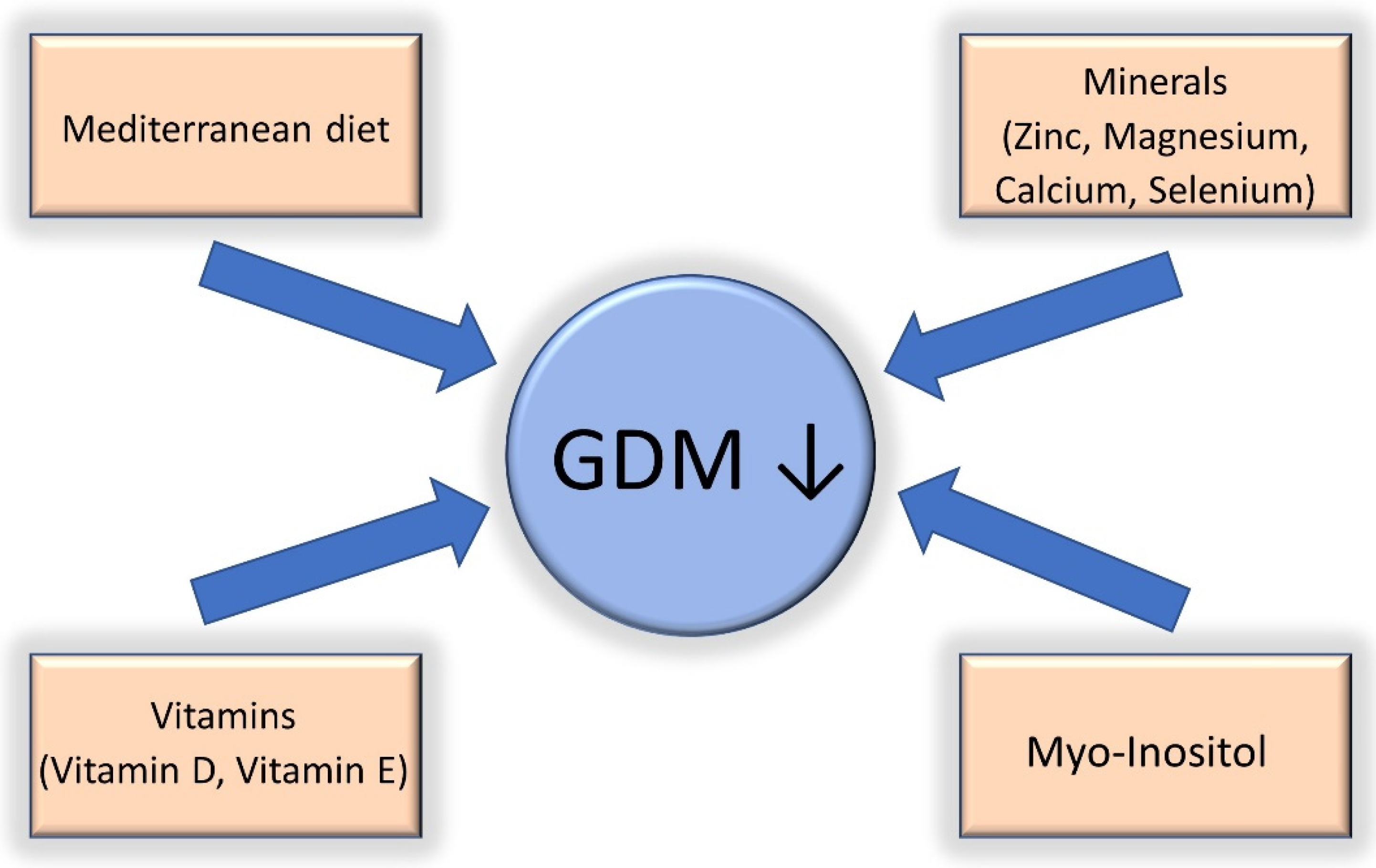
Overall, 80% of women diagnosed with GDM can maintain glycemic control through dietary advice and lifestyle modifications [31,60][17][18]. This might not hold true for patients with GDM and COVID-19, as the rates of patients treated only with diet modifications have been shown to be lower (approximately 60%) [39][16]. Dietary modification is the first-line treatment for GDM. The Mediterranean diet, which consists mainly of vegetables, legumes, nuts, cereals, and fish, is effective in improving glycemic control and reducing the risk of GDM and associated adverse outcomes [58,61][19][20]. In detail, the Mediterranean diet has been associated with lower weight gain during pregnancy [62][21] and an improvement in short- [63,64,65][22][23][24] and long-term maternal and fetal outcomes [66,67,68,69][25][26][27][28] in patients with GDM. Conversely, the Western diet, which is characterized by a high consumption of sugars, proteins, and saturated fats, is associated with obesity, type 2 diabetes mellitus, the activation of the innate immune system, and the impairment of adaptive immunity [58][19]. The latter two conditions lead to chronic inflammation and impaired host defense against viruses, such as COVID-19, and can be counted among the risk factors for severe disease [70][29]. Containment measurements adopted during the epidemic waves of SARS-CoV-2 infection, such as lockdowns, led to an increase in the consumption of sugary food and snacks, leading to poor glycemic control in women [71,72,73][30][31][32] and hence potentially worsening maternal and fetal outcomes. Another important topic to discuss when addressing nutrition in patients with GDM is nutrient deficiency. Deficiencies in vitamin D, vitamin E, zinc, and magnesium have been found in mothers with GDM and are associated with chronic low-level inflammation and oxidative stress [74,75,76,77][33][34][35][36]. Available data from one meta-analysis involving 12 randomized controlled studies found that vitamin and mineral supplementation (vitamin D, vitamin E, magnesium, zinc, calcium, and selenium) improved glycemic control and attenuated low-grade chronic inflammation and oxidative stress in mothers with GDM [78][37]. Myo-inositol, a sugar found in grains, corn, nuts, meat, legumes, and fresh citrus fruits, has also been associated with a reduction in the incidence of GDM and fetal macrosomia in normal and overweight/obese mothers [79,80,81][38][39][40]. Myo-inositol reduces serum glucose and improves insulin sensitivity [82][41] in a similar way to metformin [83][42]. Overall, a good dietary approach involving a Mediterranean diet and micronutrient/probiotic supplementation might have the potential to reduce the risk of GDM, SARS-CoV-2 infection, and severe COVID-19. More research is needed in this area to determine the real benefit of micronutrient and probiotic supplementation in patients with GDM and COVID-19. A summary of the most important dietary interventions is shown in Figure 1.
Figure 1.
Nutritional interventions that have been shown to be effective in the reduction of gestational diabetes (GDM).
5. Gestational Diabetes Treated with Insulin Therapy and COVID-19
The use of insulin in patients with GDM is recommended when glycemic control is not achieved within two weeks of diet and lifestyle intervention treatment [31,32][17][53]. Good glycemic control has been shown to reduce maternal and perinatal morbidity in pregnancies complicated by GDM and COVID-19 [33,34,35,48,49,50][10][11][12][54][55][56]. However, since the beginning of the COVID-19 pandemic in 2020, there has been a 30% increase in the use of insulin to control glycemia in patients with GDM compared to previous years [39,94][16][50]. This is likely due to the effect of lockdowns on physical activity and diet, resulting in an increase in serum glucose levels [91,92][47][48]. Evidence on the occurrence of complications associated with COVID-19 in pregnant patients with GDM treated with insulin is limited. Similarly to non-pregnant individuals with diabetes mellitus treated with insulin [96[57][58],97], mothers affected by GDM who are on insulin are also more likely to have a confirmed SARS-CoV-2 infection irrespective of their BMI [37][14]. Patients affected by COVID-19 and diabetes mellitus type 2 treated with insulin are at increased risk of developing severe/critical complications or dying [98][59]. Similarly, mothers with GDM on insulin treatment may undergo a more severe course of the disease and develop adverse maternal outcomes, especially if they are overweight or obese [39][16]. The mechanisms underlying this association are yet to be fully understood. One hypothesis is that insulin may increase the levels of proinflammatory cytokine from activated macrophages and promote a proinflammatory state, which may exacerbate the inflammation cascade caused by COVID-19 [99][60]. Another hypothesis is that insulin may increase the susceptibility to lung inflammation [100][61] and hence may worsen the pulmonary complications associated with COVID-19. Regarding neonatal outcomes, data from the German COVID-19 registry (CRONOS) showed an almost five-fold increased risk of adverse neonatal outcomes in mothers with GDM treated with insulin with a normal BMI.6. Gestational Diabetes Treated with Metformin and COVID-19
The use of metformin in patients with GDM is recommended when they fail to achieve glycemic goals with diet and lifestyle interventions [101,102,103][62][63][64]. These recommendations are based on randomized clinical trials [104][65] and meta-analyses [105[66][67],106], which have shown similar outcomes when compared to patients with GDM treated with insulin. There is no data regarding maternal or perinatal outcomes in patients with COVID-19 and GDM treated with metformin. Available information on the outcomes of COVID-19 in patients treated with metformin pertains to patients with type 2 diabetes mellitus. As above, several theories have been proposed to explain the association between insulin and adverse outcomes [99,100][60][61]. Moreover, the key molecular target of metformin is 5’-AMP-activated protein kinase, which mediates the expression and stability of ACE2 receptors [107,108][68][69]. The underexpression or instability of ACE2 receptors may impair the entry of SARS-CoV-2 into the host cells. Another hypothesis is related to the reduction in the levels of TNF-α that has been associated with the administration of metformin [109[70][71],110], which suggests that the drug has anti-inflammatory properties. Therefore, it is plausible that pregnancies treated with metformin instead of insulin are at a lower risk of adverse outcomes and COVID-19-related mortality [111,112,113,114][72][73][74][75]. =References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733.
- Shultz, J.M.; Perlin, A.; Saltzman, R.G.; Espinel, Z.; Galea, S. Pandemic March: 2019 Coronavirus Disease’s First Wave Circumnavigates the Globe. Disaster Med. Public Health Prep. 2020, 14, e28–e32.
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe COVID-19. N. Engl. J. Med. 2020, 383, 2451–2460.
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S14–S31.
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7.
- Nguyen, C.L.; Pham, N.M.; Binns, C.W.; Van Duong, D.; Lee, A.H. Prevalence of Gestational Diabetes Mellitus in Eastern and Southeastern Asia: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2018, 2018, 6536974.
- Jovanovic, L.; Pettitt, D.J. Gestational Diabetes Mellitus. JAMA 2001, 286, 2516.
- Ferrara, A. Increasing Prevalence of Gestational Diabetes Mellitus. Diabetes Care 2007, 30, S141–S146.
- Chiefari, E.; Arcidiacono, B.; Foti, D.; Brunetti, A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017, 40, 899–909.
- Yang, J.-K.; Lin, S.-S.; Ji, X.-J.; Guo, L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010, 47, 193–199.
- Zhao, P.; Praissman, J.L.; Grant, O.C.; Cai, Y.; Xiao, T.; Rosenbalm, K.E.; Aoki, K.; Kellman, B.P.; Bridger, R.; Barouch, D.H.; et al. Virus-Receptor Interactions of Glycosylated SARS-CoV-2 Spike and Human ACE2 Receptor. Cell Host Microbe 2020, 28, 586–601.e6.
- Rubino, F.; Amiel, S.A.; Zimmet, P.; Alberti, G.; Bornstein, S.; Eckel, R.H.; Mingrone, G.; Boehm, B.; Cooper, M.E.; Chai, Z.; et al. New-Onset Diabetes in COVID-19. N. Engl. J. Med. 2020, 383, 789–790.
- Accili, D. Can COVID-19 cause diabetes? Nat. Metab. 2021, 3, 123–125.
- Eskenazi, B.; Rauch, S.; Iurlaro, E.; Gunier, R.B.; Rego, A.; Gravett, M.G.; Cavoretto, P.I.; Deruelle, P.; García-May, P.K.; Mhatre, M.; et al. Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: The INTERCOVID study. Am. J. Obstet. Gynecol. 2021, 227, 74.e1–74.e16.
- Vousden, N.; Ramakrishnan, R.; Bunch, K.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; et al. Variant on the Severity of Maternal Infection and Perinatal Outcomes: Data From the UK Obstetric Surveillance System National Cohort. medRxiv 2021, 1–22.
- Kleinwechter, H.J.; Weber, K.S.; Mingers, N.; Ramsauer, B.; Schaefer-Graf, U.M.; Groten, T.; Kuschel, B.; Backes, C.; Banz-Jansen, C.; Berghaeuser, M.A.; et al. Gestational diabetes mellitus and COVID-19: Results from the COVID-19–Related Obstetric and Neonatal Outcome Study (CRONOS). Am. J. Obstet. Gynecol. 2022.
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 2019, 5, 47.
- Landon, M.B.; Spong, C.Y.; Thom, E.; Carpenter, M.W.; Ramin, S.M.; Casey, B.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Thorp, J.M.; et al. A Multicenter, Randomized Trial of Treatment for Mild Gestational Diabetes. N. Engl. J. Med. 2009, 361, 1339–1348.
- Fedullo, A.L.; Schiattarella, A.; Morlando, M.; Raguzzini, A.; Toti, E.; De Franciscis, P.; Peluso, I. Mediterranean Diet for the Prevention of Gestational Diabetes in the COVID-19 Era: Implications of Il-6 In Diabesity. Int. J. Mol. Sci. 2021, 22, 1213.
- Franquesa, M.; Pujol-Busquets, G.; García-Fernández, E.; Rico, L.; Shamirian-Pulido, L.; Aguilar-Martínez, A.; Medina, F.; Serra-Majem, L.; Bach-Faig, A. Mediterranean Diet and Cardiodiabesity: A Systematic Review through Evidence-Based Answers to Key Clinical Questions. Nutrients 2019, 11, 655.
- Silva-del Valle, M.A.; Sánchez-Villegas, A.; Serra-Majem, L. No TitleAssociation between the adherence to the Mediterranean diet and overweight and obesity in pregnant women in Gran Canaria. Nutr. Hosp. 2013, 28, 654–659.
- Assaf-Balut, C.; García de la Torre, N.; Fuentes, M.; Durán, A.; Bordiú, E.; del Valle, L.; Valerio, J.; Jiménez, I.; Herraiz, M.; Izquierdo, N.; et al. A High Adherence to Six Food Targets of the Mediterranean Diet in the Late First Trimester is Associated with a Reduction in the Risk of Materno-Foetal Outcomes: The St. Carlos Gestational Diabetes Mellitus Prevention Study. Nutrients 2018, 11, 66.
- Olmedo-Requena, R.; Gómez-Fernández, J.; Amezcua-Prieto, C.; Mozas-Moreno, J.; Khan, K.S.; Jiménez-Moleón, J.J. Pre-Pregnancy Adherence to the Mediterranean Diet and Gestational Diabetes Mellitus: A Case-Control Study. Nutrients 2019, 11, 1003.
- Karamanos, B.; Thanopoulou, A.; Anastasiou, E.; Assaad-Khalil, S.; Albache, N.; Bachaoui, M.; Slama, C.B.; El Ghomari, H.; Jotic, A.; Lalic, N.; et al. Relation of the Mediterranean diet with the incidence of gestational diabetes. Eur. J. Clin. Nutr. 2014, 68, 8–13.
- Melero, V.; Assaf-Balut, C.; de la Torre, N.G.; Jiménez, I.; Bordiú, E.; del Valle, L.; Valerio, J.; Familiar, C.; Durán, A.; Runkle, I.; et al. Benefits of Adhering to a Mediterranean Diet Supplemented with Extra Virgin Olive Oil and Pistachios in Pregnancy on the Health of Offspring at 2 Years of Age. Results of the San Carlos Gestational Diabetes Mellitus Prevention Study. J. Clin. Med. 2020, 9, 1454.
- Renault, K.M.; Carlsen, E.M.; Nørgaard, K.; Nilas, L.; Pryds, O.; Secher, N.J.; Cortes, D.; Beck Jensen, J.-E.; Olsen, S.F.; Halldorsson, T.I. Intake of carbohydrates during pregnancy in obese women is associated with fat mass in the newborn offspring. Am. J. Clin. Nutr. 2015, 102, 1475–1481.
- Chatzi, L.; Rifas-Shiman, S.L.; Georgiou, V.; Joung, K.E.; Koinaki, S.; Chalkiadaki, G.; Margioris, A.; Sarri, K.; Vassilaki, M.; Vafeiadi, M.; et al. Adherence to the Mediterranean diet during pregnancy and offspring adiposity and cardiometabolic traits in childhood. Pediatr. Obes. 2017, 12, 47–56.
- Zhang, Y.; Lin, J.; Fu, W.; Liu, S.; Gong, C.; Dai, J. Mediterranean diet during pregnancy and childhood for asthma in children: A systematic review and meta-analysis of observational studies. Pediatr. Pulmonol. 2019, 54, 949–961.
- Butler, M.J.; Barrientos, R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain. Behav. Immun. 2020, 87, 53–54.
- Ruiz-Roso, M.B.; Knott-Torcal, C.; Matilla-Escalante, D.C.; Garcimartín, A.; Sampedro-Nuñez, M.A.; Dávalos, A.; Marazuela, M. COVID-19 Lockdown and Changes of the Dietary Pattern and Physical Activity Habits in a Cohort of Patients with Type 2 Diabetes Mellitus. Nutrients 2020, 12, 2327.
- Munekawa, C.; Hosomi, Y.; Hashimoto, Y.; Okamura, T.; Takahashi, F.; Kawano, R.; Nakajima, H.; Osaka, T.; Okada, H.; Majima, S.; et al. Effect of coronavirus disease 2019 pandemic on the lifestyle and glycemic control in patients with type 2 diabetes: A cross-section and retrospective cohort study. Endocr. J. 2021, 68, 201–210.
- Sankar, P.; Ahmed, W.N.; Mariam Koshy, V.; Jacob, R.; Sasidharan, S. Effects of COVID-19 lockdown on type 2 diabetes, lifestyle and psychosocial health: A hospital-based cross-sectional survey from South India. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1815–1819.
- Foster, M.; Samman, S. Zinc and Redox Signaling: Perturbations Associated with Cardiovascular Disease and Diabetes Mellitus. Antioxid. Redox Signal. 2010, 13, 1549–1573.
- Gommers, L.M.M.; Hoenderop, J.G.J.; Bindels, R.J.M.; de Baaij, J.H.F. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes 2016, 65, 3–13.
- Haidari, F.; Jalali, M.-T.; Shahbazian, N.; Haghighizadeh, M.-H.; Azadegan, E. Comparison of Serum Levels of Vitamin D and Inflammatory Markers Between Women With Gestational Diabetes Mellitus and Healthy Pregnant Control. J. Fam. Reprod. Health 2016, 10, 1–8.
- Grissa, O.; Atègbo, J.-M.; Yessoufou, A.; Tabka, Z.; Miled, A.; Jerbi, M.; Dramane, K.L.; Moutairou, K.; Prost, J.; Hichami, A.; et al. Antioxidant status and circulating lipids are altered in human gestational diabetes and macrosomia. Transl. Res. 2007, 150, 164–171.
- Li, D.; Cai, Z.; Pan, Z.; Yang, Y.; Zhang, J. The effects of vitamin and mineral supplementation on women with gestational diabetes mellitus. BMC Endocr. Disord. 2021, 21, 106.
- D’Anna, R.; Scilipoti, A.; Giordano, D.; Caruso, C.; Cannata, M.L.; Interdonato, M.L.; Corrado, F.; Di Benedetto, A. myo -Inositol Supplementation and Onset of Gestational Diabetes Mellitus in Pregnant Women With a Family History of Type 2 Diabetes. Diabetes Care 2013, 36, 854–857.
- D’Anna, R.; Di Benedetto, A.; Scilipoti, A.; Santamaria, A.; Interdonato, M.L.; Petrella, E.; Neri, I.; Pintaudi, B.; Corrado, F.; Facchinetti, F. Myo-inositol Supplementation for Prevention of Gestational Diabetes in Obese Pregnant Women. Obstet. Gynecol. 2015, 126, 310–315.
- Crawford, T.J.; Crowther, C.A.; Alsweiler, J.; Brown, J. Antenatal dietary supplementation with myo-inositol in women during pregnancy for preventing gestational diabetes. Cochrane Database Syst. Rev. 2015, 2015, CD011507.
- Bizzarri, M.; Carlomagno, G. Inositol: History of an effective therapy for Polycystic Ovary Syndrome. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1896–1903.
- Cabrera-Cruz, H.; Oróstica, L.; Plaza-Parrochia, F.; Torres-Pinto, I.; Romero, C.; Vega, M. The insulin-sensitizing mechanism of myo-inositol is associated with AMPK activation and GLUT-4 expression in human endometrial cells exposed to a PCOS environment. Am. J. Physiol. Metab. 2020, 318, E237–E248.
- Bo, S.; Rosato, R.; Ciccone, G.; Canil, S.; Gambino, R.; Poala, C.B.; Leone, F.; Valla, A.; Grassi, G.; Ghigo, E.; et al. Simple lifestyle recommendations and the outcomes of gestational diabetes. A 2×2 factorial randomized trial. Diabetes Obes. Metab. 2014, 16, 1032–1035.
- Padayachee, C. Exercise guidelines for gestational diabetes mellitus. World J. Diabetes 2015, 6, 1033.
- Mitanchez, D.; Ciangura, C.; Jacqueminet, S. How Can Maternal Lifestyle Interventions Modify the Effects of Gestational Diabetes in the Neonate and the Offspring? A Systematic Review of Meta-Analyses. Nutrients 2020, 12, 353.
- Zanardo, V.; Tortora, D.; Sandri, A.; Severino, L.; Mesirca, P.; Straface, G. COVID-19 pandemic: Impact on gestational diabetes mellitus prevalence. Diabetes Res. Clin. Pract. 2022, 183, 109149.
- Zupo, R.; Castellana, F.; Sardone, R.; Sila, A.; Giagulli, V.A.; Triggiani, V.; Cincione, R.I.; Giannelli, G.; De Pergola, G. Preliminary Trajectories in Dietary Behaviors during the COVID-19 Pandemic: A Public Health Call to Action to Face Obesity. Int. J. Environ. Res. Public Health 2020, 17, 7073.
- Mattioli, A.V.; Sciomer, S.; Cocchi, C.; Maffei, S.; Gallina, S. Quarantine during COVID-19 outbreak: Changes in diet and physical activity increase the risk of cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1409–1417.
- Martinez-Ferran, M.; de la Guía-Galipienso, F.; Sanchis-Gomar, F.; Pareja-Galeano, H. Metabolic Impacts of Confinement during the COVID-19 Pandemic Due to Modified Diet and Physical Activity Habits. Nutrients 2020, 12, 1549.
- Ghesquière, L.; Garabedian, C.; Drumez, E.; Lemaître, M.; Cazaubiel, M.; Bengler, C.; Vambergue, A. Effects of COVID-19 pandemic lockdown on gestational diabetes mellitus: A retrospective study. Diabetes Metab. 2021, 47, 101201.
- Radan, A.-P.; Fluri, M.-M.; Nirgianakis, K.; Mosimann, B.; Schlatter, B.; Raio, L.; Surbek, D. Gestational diabetes is associated with SARS-CoV-2 infection during pregnancy: A case-control study. Diabetes Metab. 2022, 48, 101351.
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes Metab. Res. Rev. 2021, 37, e3377.
- Society of Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM Statement: Pharmacological treatment of gestational diabetes. Am. J. Obstet. Gynecol. 2018, 218, B2–B4.
- Cuschieri, S.; Grech, S. COVID-19 and diabetes: The why, the what and the how. J. Diabetes Complicat. 2020, 34, 107637.
- Hill, M.A.; Mantzoros, C.; Sowers, J.R. Commentary: COVID-19 in patients with diabetes. Metabolism 2020, 107, 154217.
- Philips, B.J.; Meguer, J.-X.; Redman, J.; Baker, E.H. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003, 29, 2204–2210.
- McGurnaghan, S.J.; Weir, A.; Bishop, J.; Kennedy, S.; Blackbourn, L.A.K.; McAllister, D.A.; Hutchinson, S.; Caparrotta, T.M.; Mellor, J.; Jeyam, A.; et al. Risks of and risk factors for COVID-19 disease in people with diabetes: A cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021, 9, 82–93.
- Chun, S.-Y.; Kim, D.W.; Lee, S.A.; Lee, S.J.; Chang, J.H.; Choi, Y.J.; Kim, S.W.; Song, S.O. Does Diabetes Increase the Risk of Contracting COVID-19? A Population-Based Study in Korea. Diabetes Metab. J. 2020, 44, 897–907.
- Yang, Y.; Cai, Z.; Zhang, J. Insulin Treatment May Increase Adverse Outcomes in Patients With COVID-19 and Diabetes: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 696087.
- Brundage, S.I.; Kirilcuk, N.N.; Lam, J.C.; Spain, D.A.; Zautke, N.A. Insulin Increases the Release of Proinflammatory Mediators. J. Trauma Inj. Infect. Crit. Care 2008, 65, 367–372.
- Filgueiras, L.R.; Capelozzi, V.L.; Martins, J.O.; Jancar, S. Sepsis-induced lung inflammation is modulated by insulin. BMC Pulm. Med. 2014, 14, 177.
- National Institute for Health and Clinical Excellence (NICE). Diabetes in Pregnancy: Management from Preconception to the Postnatal Period. Available online: https://www.nice.org.uk/guidance/ng3 (accessed on 25 February 2022).
- American College of Obstetricians and Gynecologists (ACOG). Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet. Gynecol. 2013, 122, 406–416.
- American Diabetes Association (ADA). Standards of Medical Care in Diabetes—2017: Summary of Revisions. Diabetes Care 2017, 40, S4–S5.
- Rowan, J.A.; Hague, W.M.; Gao, W.; Battin, M.R.; Moore, M.P. Metformin versus Insulin for the Treatment of Gestational Diabetes. N. Engl. J. Med. 2008, 358, 2003–2015.
- Jiang, Y.-F.; Chen, X.-Y.; Ding, T.; Wang, X.-F.; Zhu, Z.-N.; Su, S.-W. Comparative Efficacy and Safety of OADs in Management of GDM: Network Meta-analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2015, 100, 2071–2080.
- Butalia, S.; Gutierrez, L.; Lodha, A.; Aitken, E.; Zakariasen, A.; Donovan, L. Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: A systematic review and meta-analysis. Diabet. Med. 2017, 34, 27–36.
- Zhang, J.; Dong, J.; Martin, M.; He, M.; Gongol, B.; Marin, T.L.; Chen, L.; Shi, X.; Yin, Y.; Shang, F.; et al. AMP-activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 509–520.
- Ursini, F.; Ciaffi, J.; Landini, M.P.; Meliconi, R. COVID-19 and diabetes: Is metformin a friend or foe? Diabetes Res. Clin. Pract. 2020, 164, 108167.
- Matsiukevich, D.; Piraino, G.; Lahni, P.; Hake, P.W.; Wolfe, V.; O’Connor, M.; James, J.; Zingarelli, B. Metformin ameliorates gender-and age-dependent hemodynamic instability and myocardial injury in murine hemorrhagic shock. Biochim. Biophys. Acta—Mol. Basis Dis. 2017, 1863, 2680–2691.
- Park, J.W.; Lee, J.H.; Park, Y.H.; Park, S.J.; Cheon, J.H.; Kim, W.H.; Kim, T. Il Sex-dependent difference in the effect of metformin on colorectal cancer-specific mortality of diabetic colorectal cancer patients. World J. Gastroenterol. 2017, 23, 5196.
- Chen, Y.; Yang, D.; Cheng, B.; Chen, J.; Peng, A.; Yang, C.; Liu, C.; Xiong, M.; Deng, A.; Zhang, Y.; et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Diabetes Care 2020, 43, 1399–1407.
- Kow, C.S.; Hasan, S.S. Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: A meta-analysis. J. Med. Virol. 2021, 93, 695–697.
- Lukito, A.A.; Pranata, R.; Henrina, J.; Lim, M.A.; Lawrensia, S.; Suastika, K. The Effect of Metformin Consumption on Mortality in Hospitalized COVID-19 patients: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 2177–2183.
- Li, Y.; Yang, X.; Yan, P.; Sun, T.; Zeng, Z.; Li, S. Metformin in Patients With COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 704666.
More
