You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 5 by Gallyamov Marat Olegovich and Version 4 by Gallyamov Marat Olegovich.
Supercritical CO2 (scCO2) is an alternative promising solvent that has been actively used in recent decades to simplify many processes of polymer synthesis, modification, decomposition, etc.
- polydimethylsiloxane
- silicone rubbers
- supercritical fluid
1. Obtaining Aerogels in scCO2
In 1997, Loy et al. described the first one-step sol–gel polycondensation of tetraalkoxysilanes or 1,4-bis-(triethoxysilyl) benzene (BESP) in scCO2: a mixture of alkoxysilanes or BESP and anhydrous formic acid was placed in a reactor, where scCO2 was applied. The system was exposed for 12 h and slowly decompressed, this way transforming scCO2 to a gaseous state and forming monolithic silica or polysilsesquioxane aerogel [1]. The use of scCO2 for carrying out such condensation reactions remains an urgent problem. Freed from the solvent in the absence of capillary forces, aerogels retain most of their original volume, which allows them to be effectively used as heat insulators, catalysts, etc. However, typically, low-molecular-weight liquid by-products of the condensation reaction, such as formic acid esters, require careful removal before the decompression procedure to prevent the retrograde condensation in the pores of the formed aerogel (the retrograde condensation is a counterintuitive phenomenon of liquid droplets formation in an initially homo-phase supercritical mixture during decompression) [2]. In 2015, Zou et al. demonstrated the process of obtaining elastic superhydrophobic cross-linked polydimethylsiloxane (PDMS) aerogels for water purification [3]. In the presence of Karstedt’s catalyst (divinyl-containing Pt disiloxane complexes), oligomeric vinyl-terminated dimethylsiloxane was hydrosilylated with a hydride-containing oligomer. No liquid by-products of the reaction are formed with this approach, which guarantees the absence of possible complicating phenomena, such as the retrograde condensation at the stage of removal of the supercritical solvent. Various aerogel densities obtained by using diverse initial siloxane reagents and variations in their concentrations ranged from 150 to 260 mg cm−3. The method is fast in comparison with the classical schemes for obtaining aerogels, especially those involving a stage of solvent replacement, and it requires only a few hours. In 2017, researchers applied a similar hydrosilylation method for the synthesis of organosilicone aerogels in scCO2 [4]. Commercially available hydride-containing functionalized PDMS and a wide range of divinyl PDMS oligomers of variable length were used as reagents. Speier’s catalyst (chloroplatinic acid) or scCO2-soluble organometallic compound (1,5-cyclooctadiene)dimethylplatinum (PtMe2(COD)) were chosen as catalysts among a wide range of compounds. It was shown that the properties of the resulting aerogels are primarily affected by the density of CO2 and the length of the divinyl cross-links from PDMS units. By varying these parameters and the concentration of reagents, it was possible to obtain either superhydrophobic monolithic aerogels with a density of down to ~130 mg cm−3, which is slightly lower than the values achieved in the previous work, or microgranules with a diameter of ~0.5 mm. It is important to emphasize that the use of supercritical fluid soluble catalysts opens the potential for their extraction, at least partial, separation and reuse, which is an important problem in conventional synthesis in the presence of liquid solvents. In subsequent work, a wider range of vinyl- and hydride-containing siloxane precursors was used, and the mechanical properties of aerogels obtained in scCO2 were studied depending on the nature of the reagents and their quantitative ratio [5]. When hyperbranched silsesquioxane hydrides are used, the Young’s modulus of the resulting aerogels is down to 3.6 kPa. On the other hand, the use of tetraallylsilane with oligomeric linear hydride-containing siloxane makes it possible to increase the Young’s modulus to 20–40 kPa and higher, depending on the ratios of the initial compounds during loading. Mahadik et al. proposed a modified method for the preparation of aerogels from tetramethylorthosilicate (TMOS), including in combination with trimethoxymethylsilane in methanol [6]. In contrast to the well-known model, when the sol is heated slowly during synthesis in order to avoid a temperature gradient, it was proposed to provide fast heating. This approach allows for a rapid transition of methanol to the supercritical state so that gelation proceeds after this transition. For this, nitrogen was preliminarily pumped to the reactor at a partial pressure of above 50 bar, which increased the boiling point of methanol. The authors claim that this approach makes it possible to transfer methanol directly to the supercritical state, bypassing phase separation and thereby eliminating capillary effects. As a consequence, aerogels with minimal shrinkage and high specific surface area can be rapidly formed. The density of the resulting aerogels, depending on the concentrations and the combination of reagents, ranged from 35 to 170 mg cm−3. It is worth discussing the success of classical processes for the synthesis of aerogels at atmospheric pressure followed by supercritical drying of the material. Thus, in 2007, the process of obtaining elastic aerogels based on methyltrimethoxysilane was described using the classical approach of supercritical drying [7][8]. Their flexible behavior is due to the silsesquioxane nature: the density of the network formed is lower than that in silica. At the same time, a high concentration of hydrophobic methyl groups, as well as a lower content of residual silanol groups, ensures the reversibility of deformations. Moreover, due to the excellent mechanical properties, it was also possible to obtain methyltrimethoxysilane-based xerogels by a simple conventional drying procedure. A milestone on the way to obtaining aerogels with high mechanical properties was the series of works by Nakanishi et al. [9][10][11]. Transparent aerogels based on polyethylsilsesquioxane (PESQ, CH3CH2SiO1.5) and polyvinylsilsesquioxane (PVSQ, CH2=CHSiO1.5) with high thermal insulation properties were obtained. In addition, it was possible to carry out additional parallel cross-linking (according to the free radical mechanism, along with hydrolysis/condensation) in the resulting PVSQ, which provided an additional increase in mechanical characteristics. It is important that due to the flexibility and strength of the formed gels, it turned out to be possible to create xerogels that are easy to obtain and similar in properties. In order to further improve the properties of thermal insulation and flexibility, aerogels based on polyvinylpolymethylsiloxane, polyallylpolymethylsiloxane, and other compounds were obtained [9][10][11]. Due to the technological complexity of obtaining aerogels, maximum optimization of each stage is needed. In 2021, a highly efficient method for obtaining siloxane aerogels was described: the gelation time (the first stage of the process) was reduced to 5 min (subsequent drying of the gel was carried out in scCO2) [12]. During the classical aerogel-obtaining method, the hydrolysis stage proceeds much faster than condensation in acid catalysis, while the situation is reversed in basic catalysis. Therefore, a combined approach is often used: acid catalysis followed by basic catalysis. However, this method does not always allow simplifying the process and reducing the time. Therefore, it was proposed to use an “amphoteric” catalyst, which would be widespread, cheap and sufficiently active. Thus, the synthesis was carried out at room temperature and atmospheric pressure, BF3·Et2O was used as the desired catalyst, and acetone was used as a solvent. Great work has been carried out to analyze the influence of various parameters, including the type of precursor, type of solvent, and concentration of components, on the morphology of the obtained samples. For example, the specific surface area of aerogels obtained from TMOS varied in the range from 800 to 1200 m2 g−1, while Young’s modulus varied from 0.1 to 6 MPa. The wide applications potential of organosilicon aerogels and the problems, primarily related to the complexity of the synthesis process and the mechanical stability of materials [13], stimulate further study of their synthesis.2. Copolymers Based on Organosilicon Compounds in scCO2
The synthesis of a poly(acrylate-siloxane) copolymer in supercritical carbon dioxide was proposed [14]. The reaction of poly(4,4′-isopropylidene-2,2′-diphenylene (tere) isophthalate) and 3-aminopropyltriethoxysilane was carried out in scCO2 at 150 bar and 100 °C. Under such conditions, aminolysis of ester bonds in the initial polymer occurred. This led to the formation of amides containing ethoxy groups and their subsequent condensation with the second chain fragment (by the reaction of ethoxy groups and terminal phenolic groups), to poly(acrylate-siloxane). The silane reagent incorporation into the structure of macromolecules made it possible to significantly improve the thermomechanical properties of the initial polymer. It is worth noting that ethoxy groups on silicon atoms remain reactive. This leads to the formation of an insoluble in standard solvents material upon exposure to air for a month. The long-term stability of Si–O–C bonds in the aromatic structural fragments of the polymer requires separate consideration. Studies on the synthesis of triblock copolymers based on PDMS and polystyrene are known [15]. The authors proposed a radical polymerization of styrene and PDMS macroinitiator (PDMS-MA) in scCO2 medium. PDMS-MA was previously obtained by the reaction of hydroxyl-terminated PDMS with 4,4-azobis(4-cyanopentanoyl chloride) (Figure 1). The bond to the silicon atom between the forming blocks is ester, which has been confirmed by the detected peak in the IR spectrum at 1720 cm−1. The Si–O–C group in the aromatic structural fragment is a weak link due to the unpredictability of its stability under various conditions. On the other hand, its presence may prove to be an advantage in the directed utilization of the polymer, acting as an element of programmable decomposition into separate fragments in the recycling process.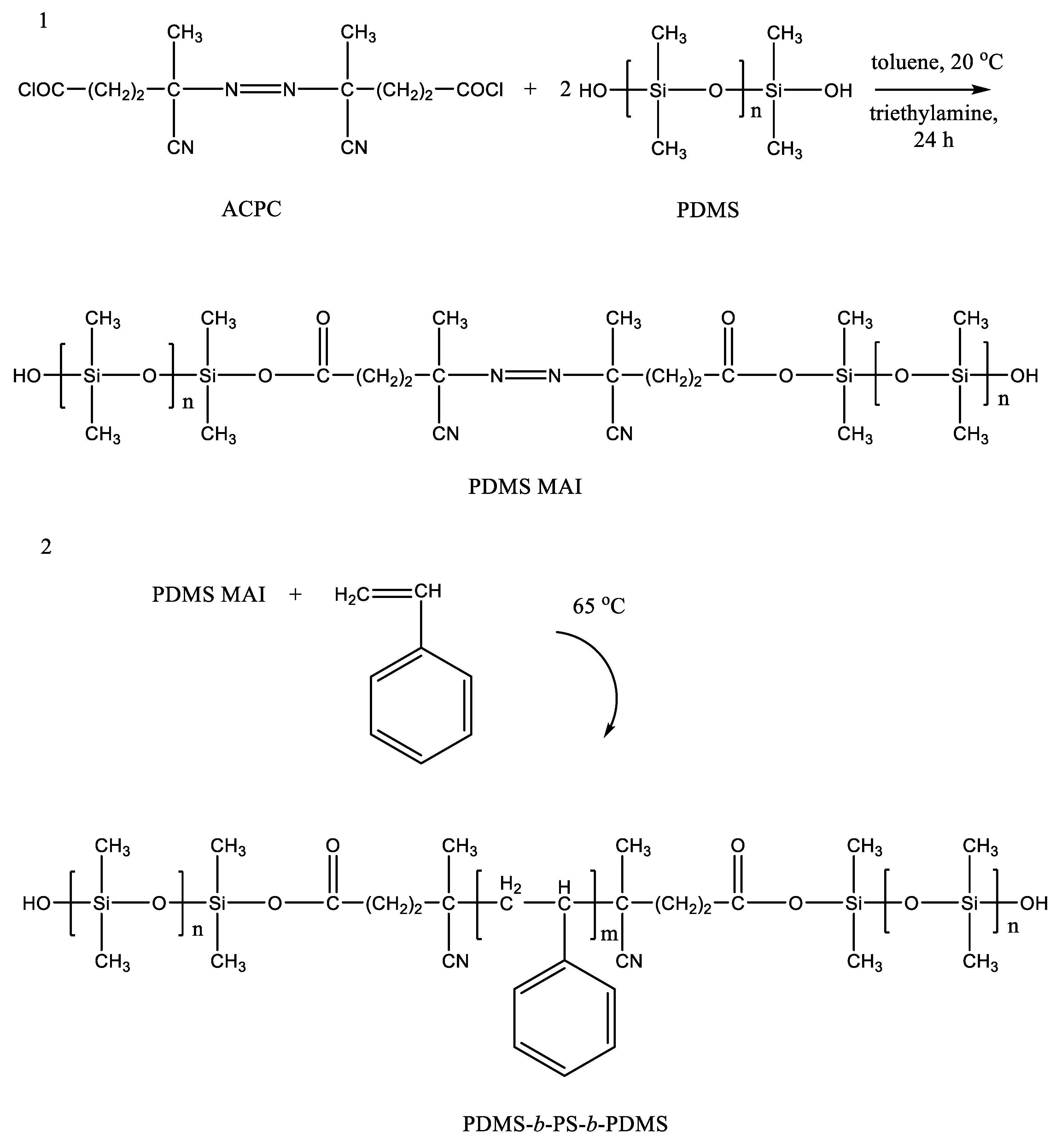
Figure 1. Stages of synthesis of the macroinitiator PDMS MAI (1) and triblock copolymer PDMS-b-PS-b-PDMS (2). Adopted with permission ofr John Wiley and Sons, copyright 2004, from Ref. [15].
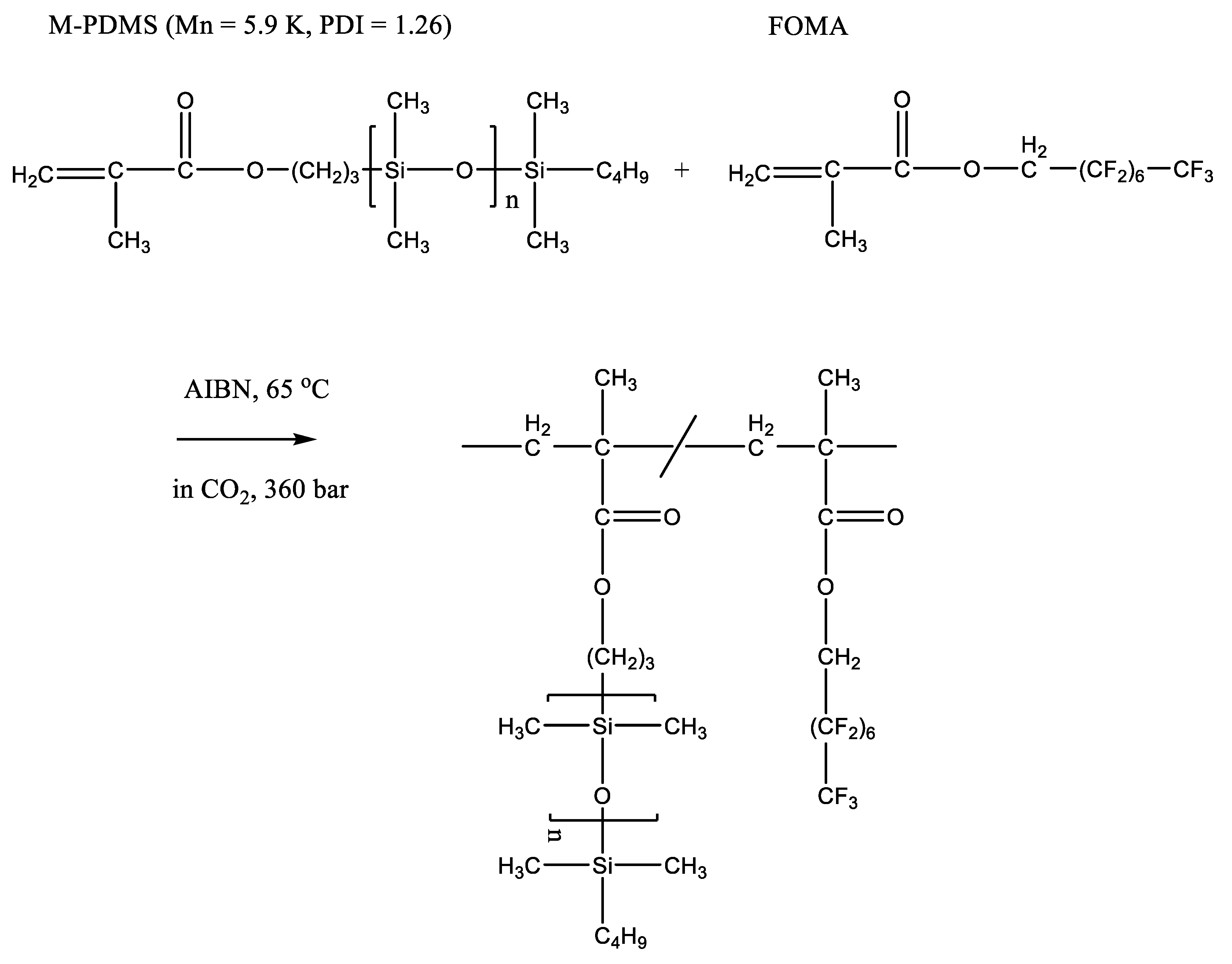
Figure 2. Copolymerization of PDMS and FOMA in supercritical CO2. Adopted permission of John Wiley and Sons, copyright 2000, from Ref. [17].
3. Spatially Regular Well-Defined Mesoporous Organosilicon Materials
The absence of capillary effects and high diffusion coefficient makes scCO2 a superb media to obtain porous structures of complex geometric shapes. In Ref. [18], supercritical carbon dioxide was used as a medium component for the hollow organosilicon spheres synthesis. Particles with an outer diameter of ≈5–10 microns were obtained in a CO2 emulsion in water with the addition of a poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) triblock copolymer as a surfactant and an organosilicon precursor tetraethoxysilane (TEOS). The resulting spheres had poor mechanical properties, partially collapsing during decompression. Nevertheless, the fundamental possibility of creating such structures, which are promising for problems of catalysis and drug delivery systems, was demonstrated. In addition, the authors indicated the potential possibilities of varying the properties of the obtained spheres by changing the reagents and the thermodynamic parameters of the medium. In a subsequent similar work [19], the authors also managed to obtain more complex structures, i.e., single-layer and multilayer hollow organosilicon spheres. Pluronic P123 triblock copolymer and a mixture of cationic (hexadecyltrimethylammonium bromide) and anionic (sodium dodecyl sulfate) surfactants were used as a double template for the spheres’ formation. The organosilicon precursor was 1,4-bis(triethoxysilyl)benzene. Single-layer and multilayer spheres with an average diameter of 40 to 200 nm were obtained, and varying the pressure of the medium—a mixture of the aqueous phase and compressed CO2—made it possible to change the wall thickness of the obtained particles. In 2016, the same group of authors proposed a “green” method for obtaining bifunctional periodic mesoporous organosilicas in a mixture of an aqueous phase and compressed CO2 [20]. The resulting material had hexagonally packed cylindrical pores with a diameter of 3.2–6.5 nm, which could be controlled by changing the applied CO2 pressure (from 4 to 6 MPa). The same P123 copolymer and 1,4-bis(triethoxysilyl)benzene were used together with 2,5-bis(triethoxysilyl)thiophene as two organosilicon precursors in the synthesis. The authors believe that the morphology of the resulting structures is primarily determined by the kinetics of hydrolysis and condensation processes, which, in turn, depends on the pH of the aqueous phase of the synthesis medium. The authors used the bromophenol blue indicator to detect the pH values of the aqueous phase saturated with CO2 under pressure (formation of carbonic acid [21]) by means of spectrometry. It was concluded that at CO2 pressure of 3.9 MPa or less, the pH of the medium was insufficient for the rapid hydrolysis and condensation of silicon-containing precursors, which led to the formation of less regular structures. At higher pressures, it was possible to form worm-like mesoporous periodic structures, with the pore size and wall thickness increasing with an increase in CO2 pressure. This is due to the following factors: firstly, an increase in the concentration of dissolved CO2 facilitates the penetration of gas molecules into the hydrocarbon chains regions of the micelles, contributing to their volume growth; secondly, lower pH values accelerate the sol–gel reaction, which contributes to the rapid condensation of hydrolyzed siloxanes. Furthermore, supercritical CO2 is used to create periodic mesoporous organosilicon films, which are required in sensors, detectors, separators, low permittivity materials and others [22]. Well-ordered mesoporous organosilicon films were prepared by the infusion and selective condensation of silicon alkoxides within microphase-separated block copolymer templates dilated with scCO2 [23]. Depending on the ratios of TEOS and methyltriethoxysilane (MTES), it was possible to achieve different spatial pores packing: a cubic lattice of spheres at a zero MTES content, and a hexagonal packing of cylinders at an MTES content of 25–50%. A further increase in the MTES concentration leads to the disordered structure. The authors attributed this behavior to the nature of the precursors, which affects the hydrophobic–hydrophilic balance of the structure-forming agent. In particular, the authors explained the transition from packing of cylinders to packing of spheres by a change in the spatial nature of the microphase separation of the block copolymer template. Systems of noble metal nanoparticles on various substrates are notable due to their promising optical, magnetic, and catalytic properties [24][25]. The key parameters are the dispersity of metal particles as well as the morphology of the resulting system as a whole. Changes in the mesostructure usually occur through variations of reactant ratios, medium pH, salt or organic additives introduction [26][27]. Using CO2 as the synthesis medium allows avoiding the classic problems associated with the necessity for post-processing, high cost, non-environmental technology, etc. In a work, gold nanoparticles deposited on oriented mesoporous organosilicon structures formed using compressed CO2 were obtained according to the “one-pot” scheme [28]. The stages illustrated in the figure (with intermediate exposures) included: loading a structure-forming block copolymer into the reactor (Pluronic P123, water-alcohol solution), loading of organosilicon precursors (bis[3-(triethoxysilyl)propyl] tetrasulfide, tetramethoxysilane), CO2 pumping (pressure in the range of 2.9–5.9 MPa), subsequent loading of the gold precursor (HAuCl4, aqueous solution), drying and annealing (500 °C). Variation of the CO2 pressure allowed a controlled transition from the tubular and hexagonal morphology of the formed substrate to the cellular and vesicular one. As it was described in [22], CO2 dissolved in the aqueous phase catalyzes the precursors hydrolysis, and its efficiency depends on pressure. Moreover, CO2 penetrates into the region of the hydrocarbon chains of the template, increasing the volume of the micelles and affecting the structure formation as a whole.4. Composites Formed in scCO2
The high penetrating ability and diffusion rate of scCO2 are used for the synthesis of various types of composites, including those based on organosilicon compounds as well. The most common composites are network structures or dispersed particles of an inorganic phase incorporated into a polymer matrix. The benefits of using scCO2 to create such composites are described in detail in several reviews [29][30][31]; however, researchers will focus on some works not mentioned there. Such composites are successfully used as various types of selective membranes and filters. The selectivity and overall efficiency of membranes largely depends on the accuracy of control of their characteristics, which is very difficult to achieve during synthesis. It is much easier to introduce particles with the required properties into the matrix in the second stage after the synthesis. In [32][33], the authors modified polymeric perfluorosulfonic acids (analogous to Nafion) by impregnation with a silane precursor and its subsequent conversion to polysiloxane using supercritical CO2. Since the modification with CO2 allows the new silicon-containing phase introduction along both hydrophilic ion channels and hydrophobic fluoropolymer domains, as well as their interfaces, it was possible to significantly suppress methanol permeability. Such microphase-separated membranes, in which nanosized proton-conducting channels are stabilized by the silicon-containing phase of the inclusions, can show better selectivity of ion transport in different power sources, reduced permeability for reagents (methanol, etc.), and also improved performance at elevated temperatures in fuel cells due to better retention of water. Indeed, previously, researchers' team described the classical process of silica composite obtaining with a polymer template: the TEOS silica precursor dissolved in scCO2 was delivered to the nanosized proton-conducting pores of Nafion matrix, where it was subsequently hydrolyzed and condensed in the presence of residual water due to the release of protons of sulfonic groups to catalyze the reaction [34]. It was possible to improve the thermomechanical properties and durability of the polymer and increase the hygroscopicity of the membrane, which led to enhanced and selective proton conductivity under low humidity conditions. The membranes with improved ion selectivity have shown promising performance in the operating cells of vanadium redox flow batteries.References
- Loy, D.A.; Russick, E.M.; Yamanaka, S.A.; Baugher, B.M.; Shea, K.J. Direct Formation of Aerogels by Sol-Gel Polymerizations of Alkoxysilanes in Supercritical Carbon Dioxide. Chem. Mater. 1997, 9, 2264–2268.
- Park, S.J.; Kwak, T.Y.; Mansoori, G.A. Statistical mechanical description of supercritical fluid extraction and retrograde condensation. Int. J. Thermophys. 1987, 8, 449–471.
- Zou, F.; Peng, L.; Fu, W.; Zhang, J.; Li, Z. Flexible superhydrophobic polysiloxane aerogels for oil-water separation via one-pot synthesis in supercritical CO2. RSC Adv. 2015, 5, 76346–76351.
- Elmanovich, I.V.; Pryakhina, T.A.; Vasil’ev, V.G.; Gallyamov, M.O.; Muzafarov, A.M. A study of the hydrosilylation approach to a one-pot synthesis of silicone aerogels in supercritical CO2. J. Supercrit. Fluids 2018, 133, 512–518.
- Elmanovich, I.V.; Pryakhina, T.A.; Gallyamov, M.O.; Migulin, D.A.; Meshkov, I.B.; Vasil’ev, V.G.; Muzafarov, A.M. Silicone aerogels with tunable mechanical properties obtained via hydrosilylation reaction in supercritical CO2. J. Supercrit. Fluids 2019, 149, 120–126.
- Mahadik, D.B.; Lee, Y.K.; Chavan, N.K.; Mahadik, S.A.; Park, H.H. Monolithic and shrinkage-free hydrophobic silica aerogels via new rapid supercritical extraction process. J. Supercrit. Fluids 2016, 107, 84–91.
- Kanamori, B.K.; Aizawa, M.; Nakanishi, K.; Hanada, T. New Transparent Methylsilsesquioxane Aerogels and Xerogels with Improved Mechanical Properties. Adv. Mater. 2007, 19, 1589–1593.
- Kanamori, K.; Nakanishi, K.; Hanada, T. Elastic aerogels and xerogels synthesized from methyltrimethoxysilane (MTMS). MRS Proc. 2009, 1134, 1134-BB07-06.
- Zu, G.; Kanamori, K.; Maeno, A.; Kaji, H.; Nakanishi, K. Superflexible Multifunctional Polyvinylpolydimethylsiloxane-Based Aerogels as Efficient Absorbents, Thermal Superinsulators, and Strain Sensors. Angew. Chemie 2018, 57, 9722–9727.
- Zu, G.; Kanamori, K.; Shimizu, T.; Zhu, Y.; Maeno, A.; Kaji, H.; Nakanishi, K.; Shen, J. Versatile Double-Cross-Linking Approach to Transparent, Machinable, Supercompressible, Highly Bendable Aerogel Thermal Superinsulators. Chem. Mater. 2018, 30, 2759–2770.
- Zu, G.; Kanamori, K.; Wang, X.; Nakanishi, K.; Shen, J. Superelastic Triple-Network Polyorganosiloxane-Based Aerogels as Transparent Thermal Superinsulators and Efficient Separators. Chem. Mater. 2020, 32, 1595–1604.
- Kholodkov, D.N.; Arzumanyan, A.V.; Novikov, R.A.; Kashin, A.S.; Polezhaev, A.V.; Vasil’Ev, V.G.; Muzafarov, A.M. Silica-Based Aerogels with Tunable Properties: The Highly Efficient BF3-Catalyzed Preparation and Look inside Their Structure. Macromolecules 2021, 54, 1961–1975.
- Gurav, J.L.; Jung, I.K.; Park, H.H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 409310.
- Stakhanov, A.I.; Said-Galiev, E.E.; Izmailov, B.A.; Vasnev, V.A.; Khokhlov, A.R. Synthesis of poly(arylate-siloxane)s in supercritical carbon dioxide. Polym. Sci. Ser. B 2008, 50, 11–15.
- Akgün, M.; Deniz, S.; Baran, N.; Uzun, N.I.; Akgün, N.A.; Dinçer, S. Synthesis of polydimethylsiloxane-block-polystryrene-block- polydimethylsiloxane via polysiloxane-based macroinitiator in supercritical CO2. Polym. Int. 2005, 54, 374–380.
- Hu, H.; Chen, M.; Cheng, R. Siloxane-modified poly(acrylic acid) synthesized in supercritical CO2. Polymer 2002, 44, 341–345.
- Shiho, H.; DeSimone, J.M. Preparation of Silicone-Graft Copolymers by Homogeneous Radical Copolymerization in Supercritical Carbon Dioxide. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1139–1145.
- Wang, J.; Xia, Y.; Wang, W.; Mokaya, R.; Poliakoff, M. Synthesis of siliceous hollow spheres with large mesopore wall structure by supercritical CO2-in-water interface templating. Chem. Commun. 2005, 210–212.
- Huang, X.; Li, W.; Wang, M.; Tan, X.; Wang, Q.; Zhang, M.; Wang, C.; Zhang, H. Synthesis of multiple-shelled organosilica hollow nanospheres via a dual-template method by using compressed CO2. Microporous Mesoporous Mater. 2017, 247, 66–74.
- Li, W.; Yang, Y.; Huang, X.; Wang, Q.; Liu, L.; Wang, M.; Tan, X.; Luo, T.; Patil, A.J. Compressed CO2 mediated synthesis of bifunctional periodic mesoporous organosilicas with tunable porosity. Chem. Commun. 2016, 52, 9668–9671.
- Pigaleva, M.A.; Elmanovich, I.V.; Kononevich, Y.N.; Gallyamov, M.O.; Muzafarov, A.M. A biphase H2O/CO2 system as a versatile reaction medium for organic synthesis. RSC Adv. 2015, 5, 103573–103608.
- Pai, R.A.; Humayun, R.; Schulberg, M.T.; Sengupta, A.; Sun, J.N.; Watkins, J.J. Mesoporous Silicates Prepared Using Preorganized Templates in Supercritical Fluids. Science 2004, 303, 507–510.
- Pai, R.A.; Watkins, J.J. Synthesis of mesoporous organosilicate films in supercritical carbon dioxide. Adv. Mater. 2006, 18, 241–245.
- El-Sayed, M.A. Small is different: Shape-, size-, and composition-dependent properties of some colloidal semiconductor nanocrystals. Acc. Chem. Res. 2004, 37, 326–333.
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016.
- Thomas, A. Functional materials: From hard to soft porous frameworks. Angew. Chemie 2010, 49, 8328–8344.
- Qiao, S.Z.; Yu, C.Z.; Xing, W.; Hu, Q.H.; Djojoputro, H.; Lu, G.Q. Synthesis and bio-adsorptive properties of large-pore periodic mesoporous organosilica rods. Chem. Mater. 2005, 17, 6172–6176.
- Huang, X.; Zhang, M.; Wang, M.; Li, W.; Wang, C.; Hou, X.; Luan, S.; Wang, Q. Gold/Periodic Mesoporous Organosilicas with Controllable Mesostructure by Using Compressed CO2. Langmuir 2018, 34, 3642–3653.
- Pigaleva, M.A.; Elmanovich, I.V.; Temnikov, M.N.; Gallyamov, M.O.; Muzafarov, A.M. Organosilicon compounds in supercritical carbon dioxide: Synthesis, polymerization, modification, and production of new materials. Polym. Sci. Ser. B 2016, 58, 235–270.
- Kazarian, S.G. Polymer Processing with Supercritical Fluids. Polym. Sci. Ser. C 2000, 42, 78–101.
- Alekseev, E.S.; Alentiev, A.Y.; Belova, A.S.; Bogdan, V.I.; Bogdan, T.V.; Bystrova, A.V.; Gafarova, E.R.; Golubeva, E.N.; Grebenik, E.A.; Gromov, O.I.; et al. Supercritical fluids in analytical chemistry. Russ. Chem. Rev. 2020, 89, 1337–1427.
- Su, L.; Pei, S.; Li, L.; Li, H.; Zhang, Y.; Yu, W.; Zhou, C. Preparation of polysiloxane/perfluorosulfonic acid nanocomposite membranes in supercritical carbon dioxide system for direct methanol fuel cell. Int. J. Hydrogen Energy 2009, 34, 6892–6901.
- Su, L.; Li, L.; Li, H.; Tang, J.; Zhang, Y.; Yu, W.; Zhou, C. Preparation of polysiloxane modified perfluorosulfonic acid composite membranes assisted by supercritical carbon dioxide for direct methanol fuel cell. J. Power Sources 2009, 194, 220–225.
- Simonov, A.S.; Kondratenko, M.S.; Elmanovich, I.V.; Sizov, V.E.; Kharitonova, E.P.; Abramchuk, S.S.; Nikolaev, A.Y.; Fedosov, D.A.; Gallyamov, M.O.; Khokhlov, A.R. Modification of Nafion with silica nanoparticles in supercritical carbon dioxide for electrochemical applications. J. Memb. Sci. 2018, 564, 106–114.
More
