Low phosphate (Pi) availability in soils severely limits crop growth and production. Plants have evolved to have numerous physiological and molecular adaptive mechanisms to cope with Pi starvation. The release of Pi from membrane phospholipids is considered to improve plant phosphorus (P) utilization efficiency in response to Pi starvation and accompanies membrane lipid remodeling. RIn this researchersview, we summarize recent discoveries related to this topic and the molecular basis of membrane phospholipid alteration in response to Pi depletion in plants at different subcellular levels. These findings will help to further elucidate the molecular mechanisms underlying plant adaptation to Pi starvation and thus help to develop crop cultivars with high P utilization efficiency.
- lipid metabolism
- phosphate starvation
- triacylglycerol
1. Introduction
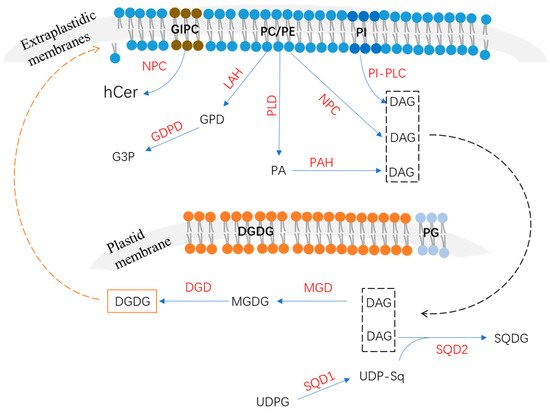
2. Phospholipids’ Degradation in Response to P Deficiency
2.1. Phospholipase C Pathway
The plant phospholipase C family includes the phosphoinositide (PI)-specific phospholipase C (PI–PLC) and non-specific phospholipase C (NPC) families according to their substrate specificity [14][15][17,18]. Accumulating evidence has shown that PI-PLCs participate in the hydrolyzation of phosphatidylinositol 4,5-bisphosphate to generate inositol 1,4,5-trisphosphate and DAG [16][19]. The plasma membrane’s perception of external stimuli may be changed and triggered to activate lipid signaling to regulate diverse cellular processes [15][18]. For example, inositol 1,4,5-trisphosphate, one product of phosphatidylinositol 4,5-bisphosphate hydrolyzed by PI-PLCs, is involved in Ca2+ release [15][18]. There are nine PI-PLC members in Arabidopsis named AtPLC1 to AtPLC9; most of them are induced by diverse environment stresses [16][17][18][19,20,21]. Among them, AtPLC2 has been identified as a primary PI-PLC and plays a role in the phospholipid metabolism and endoplasmic reticulum stress responses [19][22]. However, none of these Arabidopsis PI-PLC genes have been reported to be involved in responses to P deficiency.2.2. Phospholipase D and Phosphatidic Acid Phosphatase Pathway
Phospholipase D is also found to participate in the hydrolyzation of phospholipids to generate phosphatidic acid and soluble head groups; then, phosphatidic acid could be further hydrolyzed by phosphatidic acid phosphatase to generate DAG and release Pi [8][26][27][8,46,47]. The Arabidopsis phospholipase D gene family member AtPLDZ2 was reported to be induced by P deficiency in both shoots and roots [28][29]. AtPLDZ2 localized in the tonoplast and the relative amounts of PC and PE were significantly increased in the atpldz2 mutant compared to the WT of Arabidopsis roots under Pi-starvation conditions [28][29][29,48], suggesting that AtPLDZ2 plays a role in tonoplast membrane phospholipids’ degradation in response to Pi starvation (Table 1). Furthermore, the atpldz2 mutant also decreased the relative amounts of DGDG in Pi-limited Arabidopsis roots, suggesting that AtPLDZ2 also plays a role in membrane remodeling in roots, especially in DGDG synthesis [28][29]. AtPLDZ2 was also reported to be involved in root hair development in response to Pi starvation [30]. Under P-deficiency conditions, an increased abundance of AtPLDZ2 proteins derives more PA, which can bind to SORTING NEXIN 1 (SNX1) to decrease vacuole endocytosis and the degradation of PIN-FORMED2 (PIN2), leading to an increase in PIN2 accumulation at the plasma membrane, thus promoting root hair growth [30]. In addition, compared to AtNPC4, AtPLDZ2 also was shown to have greater effects on leaves’ lipid remodeling at later stages of Pi depletion [21][24]. PAPs have been reported to function in P-deficiency-induced membrane lipid remodeling [8]. According to their enzymatic properties, PAPs can be divided into Mg2+-dependent or -independent types [31][49]. Phosphatidic acid phosphohydrolase (PAH) is an important Mg2+-dependent PAP [8][32][8,50]. In Arabidopsis, two PAH genes (AtPAH1 and AtPAH2) were identified and suggested to function redundantly in PA degradation in leaves’ endoplasmic reticulum (ER) [32][50]. It is still unknown whether AtPAH1 and AtPAH2 are induced by Pi starvation. However, a double mutant using atpah1 and atpah2 significantly increased the phospholipid level but decreased the DGDG level, impairing shoot and root growth under P-deficiency conditions [11], suggesting that AtPAH1 and AtPAH2 play roles in the membrane lipid metabolism and remodeling. Lipid phosphate phosphatase (LPP) has been known to belong to the Mg2+-independent PAP family and functions in the hydrolysis of diverse substrates, including PA, lyso-PA, and diacylglycerol pyrophosphate [8][9][33][8,9,51]. Although nine LPP members exist in Arabidopsis, none of them are induced by P deficiency [8][34][8,52].2.3. Lipid Acyl Hydrolase and Glycerophosphodiester Phosphodiesterase Pathway
LAH can hydrolyze phospholipids to generate fatty acids and glycerophosphodiester (GPD), which is further hydrolyzed by glycerophosphodiester phosphodiesterase (GDPD) to generate glycerol-3-phosphate (G3P) and corresponding alcohols (choline, ethanolamine, inositol, glycerol, etc.) (Figure 1) [35][31]. In Arabidopsis, several LAH (or PLA) genes were identified as up-regulated by P deficiency [36][53]; however, the functions of proteins that were involved in the phospholipid metabolism’s response to Pi starvation remain largely unknown. The Arabidopsis GDPD subfamily has six members, all of which are up-regulated by Pi starvation except for AtGDPD4 [35][31]. As a plastid-localized protein, the atgdpd1 mutant exhibited impaired growth coupled to the reduction in both glycerol-3-phosphate and Pi contents in shoots and roots under Pi-starvation conditions [35][31], suggesting that AtGDPD1 plays a role in Pi release from phospholipids in response to P deficiency (Table 1). In addition to AtGDPD1, Pi-starvation-induced AtGDPD6 also was functionally characterized to play a role in glycerophosphocholine (GPC) hydrolysis in response to P deficiency [37][32]. In Arabidopsis, the atgdpd6 mutant also exhibited decreased root growth under Pi-deficient conditions [37][32]. However, when G3P was supplemented in the medium, the inhibited root phenotype of the atgdpd6 mutant was rescued, suggesting that AtGDPD6 also plays a role in the degradation of glycerophosphodiester and sustains root growth under P-deficiency stress [37][32]. There are 13 OsGDPD members in rice. Among them, OsGDPD1/2/3/5 were up-regulated in roots after 7 days of Pi starvation and OsGDPD1/2/34/5/7/10/11 were up-regulated after 15 days of Pi starvation [38][34]. Recently, OsGDPD2 was found to be up-regulated by P deficiency in both shoots and roots, and it was suggested to be downstream of OsPHR2 [39][33]. Additionally, OsGDPD2 exhibited hydrolysis activities against several glycerophosphodiesters, including glycerophosphocholine (GPC), glycerophosphoinositol (GPI), and glycerophosphoethanolamine (GPE), and played a role in membrane remodeling in response to Pi starvation [39][33]. In addition, a total of six CaGDPD members were identified in chickpea (Cicer arietinum), of which one (CaGDPD1) was shown to be up-regulated while three (CaGDPD2/3/4) were down-regulated after 15 days of Pi starvation in roots [38][34]. Moreover, CaGDPD1 was proved to be localized in the ER and other endomembranes and exhibited high enzyme activity in GPC and glycerophosphoethanolamine (GPE) [38][34], suggesting that CaGDPD1 may play a role in chickpea roots’ adaption to P deficiency through the hydrolyzation of glycerophosphodiester.