Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by ANA LAURA REYES REYES.
Lipases are efficient enzymes with promising applications in the nutraceutical and food industry, as they can offer high yields, pure products under achievable reaction conditions, and are an environmentally friendly option.
- lipases
- food
- nutraceutical industry
1. Lipases as Biocatalysts in the Food and Nutraceutical Industry
Lipases are widely used in the food industry [9,10][1][2]. Lipases (triacylglycerol hydrolases EC 3.1. 1.3) play a crucial role in numerous industrial food processes [11,12][3][4] because they participate in reactions that improve product quality and provide greater stability, solubility, durability, and better organoleptic characteristics [10,13,14][2][5][6].
1.1. Lipase Characteristics
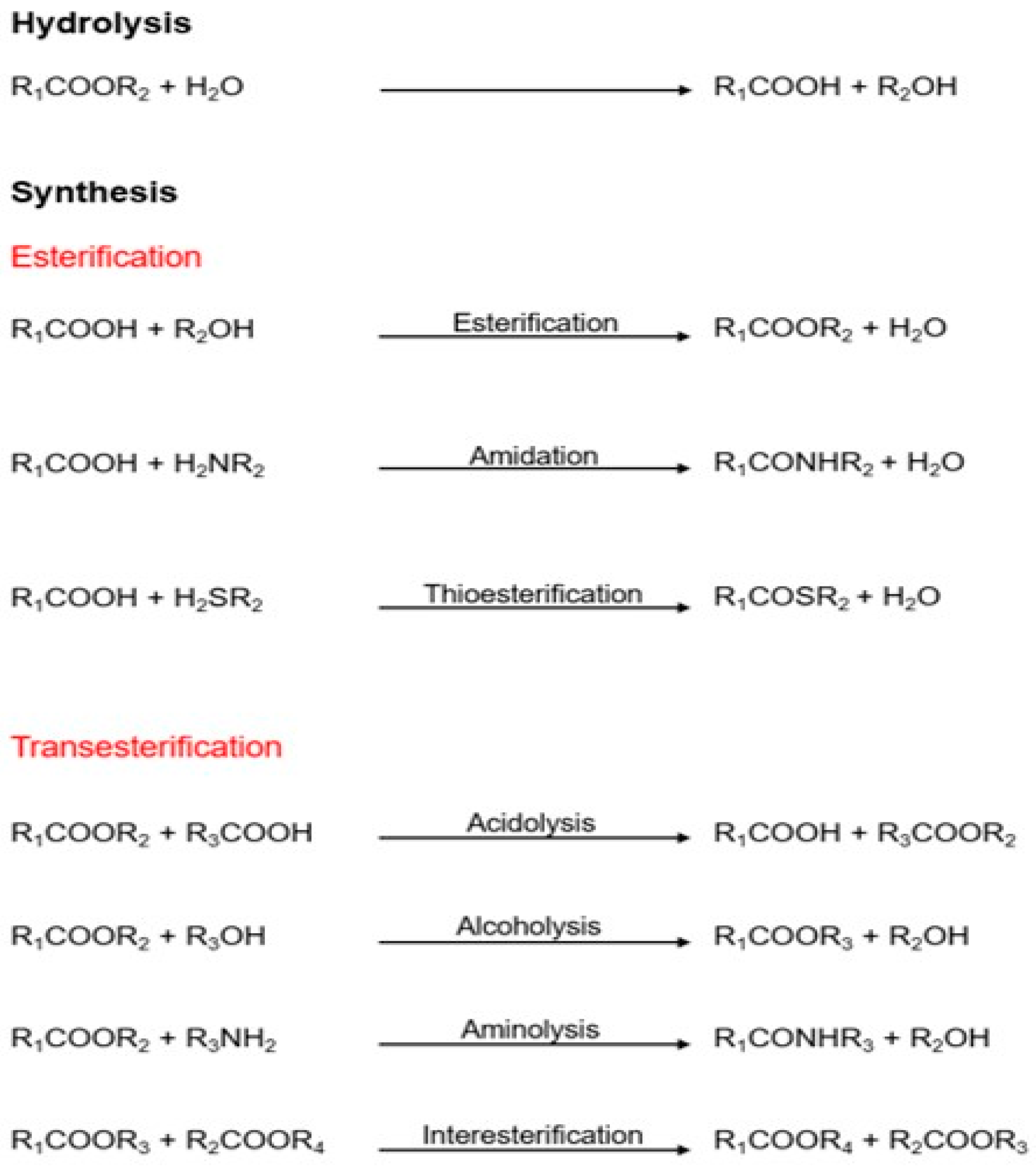
These enzymes can hydrolyze triglycerides to obtain free fatty acids, monoacylglycerols (MAGs), diacylglycerols (DAGs), and glycerol; on the other hand, they can synthesize new products in organic media by esterification, transesterification, and aminolysis mechanisms (Figure 1) [15,16][7][8]. Lipases have a highly conserved catalytic triad comprising serine as a nucleophile, an aspartate/glutamate as an acidic residue, and histidine. In their active conformation, lipases present in their active center a group of hydrophobic residues arranged around the catalytic serine that constitute an electrophilic region known as an oxyanion cavity. Lipases are also characterized by the presence of disulfide bridges that give them stability and are critical for their catalytic activity [16][8]. Some lipases also have a structural feature covering the active site, called the “lid,” that opens at hydrophobic/hydrophilic interphases. Ancient classifications denoted esterases as lipolytic enzymes lacking a lid. However, because some lipases, such as Candida antarctica lipase B (CALB), lack the lid, an alternative classification has been proposed [17][9].
Lipases are characterized by maintaining their activity and high production in nonaqueous media [18][10], high production, and stability at pH ranges and do not require cofactors. According to their substrate specificity, lipases can be chemoselective, regioselective, or stereoselective. The first lipase type can selectively catalyze a reaction. The second type catalyzes a reaction specifically with one of the triglyceride positions (sn-1,3 regioselective, sn-2 regioselective, or nonregioselective). Additionally, the third type catalyzes reactions selecting only one of the stereoisomers from a mixture of enantiomers [10,16][2][8].
1.2. Sources and Tools to Improve Lipase-Catalyzed Reactions
Lipases are ubiquitous enzymes produced by various organisms, including microorganisms, plants, and animals [12,19,20,21,22,23,24][4][11][12][13][14][15][16]. Because of the increased commercial interest in these proteins in the food and nutraceutical industry, the use of recombinant production technology is critical.
The productivity of lipase production bioprocesses has been increasing, reducing the cost of enzymes by using cell factories for the heterologous production of lipases. Between them, Komogataella phaffi (P. pastoris) is one of the most common cell factories used [25][17].
Lipases have been improved using natural evolution techniques, protein engineering, bioinformatics design, directed evolution, saturation mutagenesis, site-directed mutagenesis, and DNA shuffling [26][18]. However, in the food industry, the native form is often preferred (Figure 2).
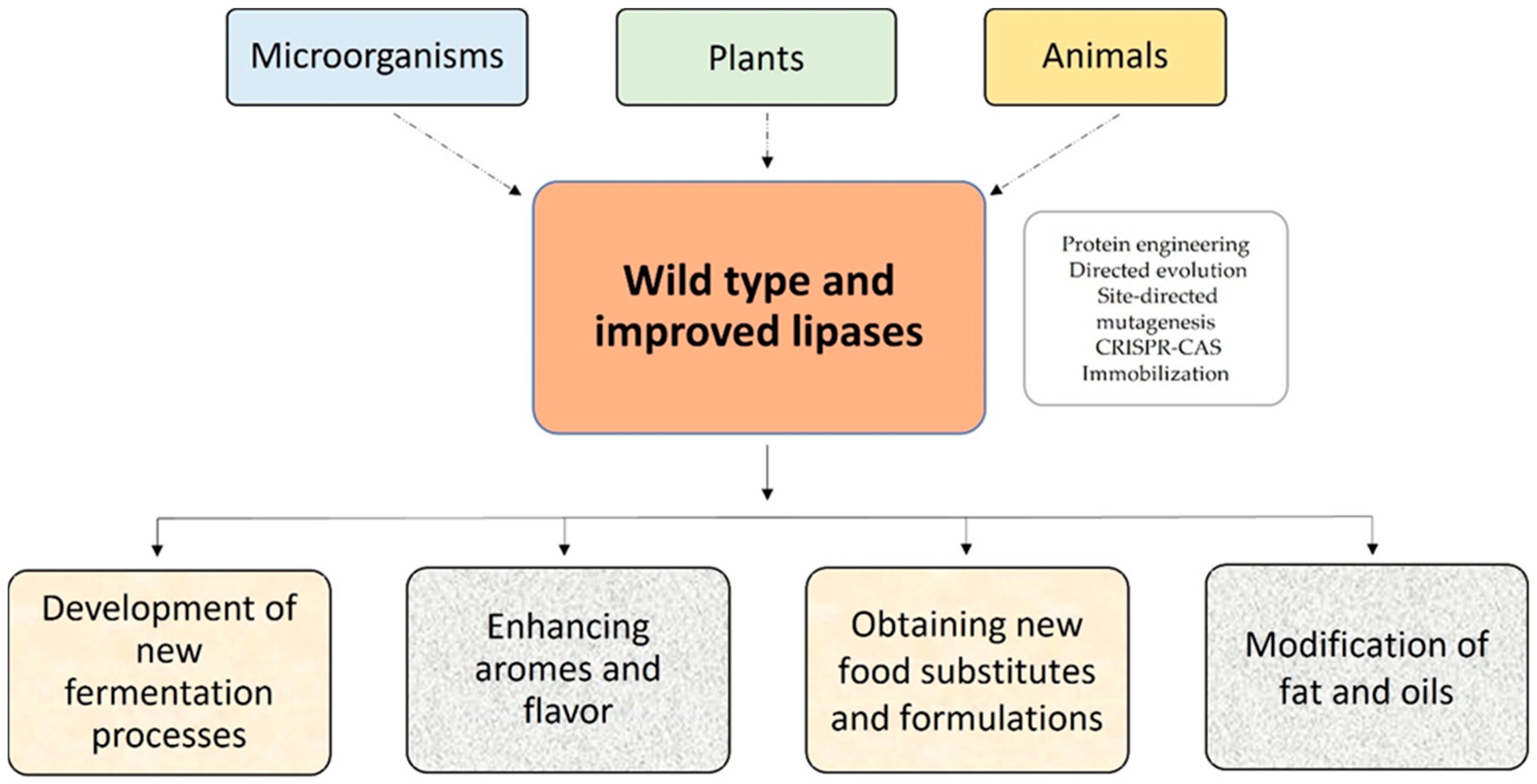
Figure 2.
Sources and use of lipases in the food industry.
Table 1 shows some microbial lipases that are commercially available and immobilized on different supports to enhance their efficiency and reuse [27,28,29,30][19][20][21][22]. Most commercially important lipase-producing yeasts belong to the class of ascomycetes, such as Candida sp. and Rhizopus sp. Novozymes® (Bagsværd, Denmark), DuPont® (Wilmington, DE, USA), Roche® (Basel, Switzerland), and Amano (Yokohama, Japan) are the main companies that produce and commercialize lipases [31][23].
Table 1. Sources of lipases with applications in food and nutraceutical industry.
| Source/Commercial Name | Type | Application/Products | Reference |
|---|---|---|---|
| Candida antarctica lipase B (CALB)/Novozym 435/Lipozyme 435 | Recombinant | Flavor esters | [32][24] |
| Candida rugosa | Wild type | Glycerides, production flavor compounds | [33,34][25][26] |
| Termomyces lanuginosus/Lipozyme TL IM | Engineered | Food formulation, Interesterification of fats and oils | [35,36][27][28] |
| Aspergillus sp. | Wild type | Flavor and fragance | [37][29] |
| Aspergillus oryzae | Wild type | Interesterification of fats and oils | [36][28] |
| Geotrichum candidum | Wild type | Oil with increased unsaturation | [36][28] |
| Rhizomucor miehei/Lipozyme RM IM | Recombinant | Enhancing fruit fragrance | [38][30] |
| Modification of the amount and composition of volatile components in bovine milk | [39][31] | ||
| Ras Cheese Flavor Concentrate (RCFC) | [40][32] | ||
| Rhizopus oryzae | Wild type | Human Milk Fat Substitutes | [41][33] |
| Lactococcus chungangensis | Wild type | Flavoring in milk, cream cheese, yogurt and butter. | [42][34] |
| Lactobacillus plantarum | Wild type | Fermented food and cheese | [43,44][35][36] |
| Staphylococcus epidermidis | Wild type | Flavor-compound production | [45][37] |
| Ophiostoma piceae | Wild type | Flavor-compound production | [46][38] |
| Meyerozyma guilliermondii | Wild type | Feed industry | [47][39] |
Other important bottlenecks of the free enzymes in general and lipases are the low operational stability in synthesis reactions using solvents and substrates such as alcohols and organic acids, the high cost of the enzymes, and the need to reuse the biocatalyst minimizing product separation.
Different approaches are being applied (Figure 2) to solve these drawbacks. The use of enzyme immobilization methods normally increases biocatalyst stability, specificity and selectivity, allows the reutilization of the enzyme, and minimizes downstream processes, and has been reflected in the number of articles and patents published in this field [48][40].
Advances in the study of lipases seek to develop more efficient processes and, for this purpose, their stability under certain temperatures, solvents, and pH conditions, among others. The development of a specific reaction medium to increase the activity, stability, and productivity of biocatalysts has been a recurring topic of research over the last three decades. The remarkable properties and useful applications of enzymes, particularly lipases, have inspired various strategies to improve their performance in near-anhydrous media. Therefore, medium engineering can be used to modulate the activity and selectivity of lipase-catalyzed reactions [49][41].
Ionic liquids (ILs) are molten salts that originate from the association of organic cations and organic/inorganic anions. The use of ILs as solvents in biocatalysis processes has recently received increased attention, and substantial progress has been made, particularly in lipase-catalyzed reactions. ILs have the advantages of low volatility, low inflammability, and a low melting point [50][42]. Deep eutectic solvents [51][43] are eutectic mixtures of salts and hydrogen bond donors with sufficiently low melting points to act as solvents. DESs were demonstrated to be a viable alternative to traditional organic solvents and ILs in many biocatalytic processes, particularly for lipases. DESs have additional advantages over ILs in simple preparation and lower costs because of their renewable and readily available raw materials [52][44].
2. Established Applications of Lipases in the Food and Nutraceutical Industry
Lipases in the food industry and nutraceutical production can be used in aqueous extracts and purified, immobilized, or whole cells to exploit the available raw material and increase their economic and nutritional value. These enzymes can be used to modify fats and oils and synthesize structured lipids or antioxidants with increased antioxidant power or modified lipophilicity, flavors, and aromas [53,54][45][46].2.1. Fats and Oils
Patent searches suggest that lipase has an impressive number of applications in the modification of fats and oils and enhancement of flavor in food products—e.g., cheese, butter, milk, and chocolate [55][47]. Some applications of lipases in dairy products and the synthesis of structured lipids are described in the following sections.2.1.1. Dairy Products
In the dairy sector, lipases are used to provide desirable aromatic characteristics to cheddar, provolone, and Romano cheeses conferred by these free short-chain fatty acids generated in the hydrolysis of fats [40,56][32][48]. Recent advances have allowed the biosynthesis of short-chain ethyl esters with fruity notes in whole milk by coupling ethanolic fermentation with transesterification using the commercial lipase Palatase. For fermentation, the following microorganisms were used: Kluyveromyces marxianus, Lactobacillus fermentum, and L. Paracasei. Many esters were obtained in ethanolic fermentation using K. marxianus yeast and lipase. This method of milk fermentation and lipase addition represents a new alternative for flavoring milk [57][49].2.1.2. Structured Lipids
In recent years, structured lipids have become a topic of great importance in the food and nutraceutical industry because technological advances allow a generation of products of better quality and that better meet consumer demands. Within this innovation in food processes, structured lipids (SLs) have been generated [58,59][50][51]. Structured lipids are fats and oils whose fatty acid composition has been modified for nutritional purposes to achieve greater bioavailability because they are not naturally occurring. In several cases, lipids have certain limitations of use in their original state because of the specific composition of their fatty acids [60][52]. In other cases, even when they are available as raw materials, they cannot always meet nutritional demand, e.g., restrictions on the daily intake of saturated fatty acids and trans fatty acids have been increased because they are related to cardiovascular diseases [61,62][53][54]. Another clear example would be access to cocoa butter; its availability may be limited by external factors such as climate change, fluctuating prices, and availability [63,64][55][56]. Therefore, the search for alternatives to address these major issues is justified. In principle, deciding which type of fatty acids to use and in which position of the molecule to restructure is possible by obtaining structured lipids [58][50]. For this procedure, the use of stereospecific enzymes allows new lipids with a stable structure to be obtained. Lipases can hydrolyze a triglyceride in an aqueous medium, but they also catalyze the binding of a fatty acid to a glycerol molecule in an anhydrous reaction medium [65][57]. Recently, the use of immobilized biocatalysts has minimized the production costs of structured lipids through reusing them in successive batches [58][50]. Human milk fat substitute (HMFS) is synthesized by enzymatic interesterification of vegetable oils, animal fats, or oil mixtures, commonly using an immobilized regioselective lipase in either solvent or solvent-free media [70,73][58][59]. A recently reported lipase/acyltransferase from C. parapsilosis was used as a biocatalyst to synthesize HMFS by interesterification of ethyl oleate with tripalmitin in solvent-free media representing a new alternative to commercial immobilized lipases [70][58]. Because human milk is one of the most complex mixtures of natural lipids, studies using this approach will continue to advance steadily.2.2. Vitamin Esters
Food contains components known as bioactive compounds that, when consumed, provide energy to the body, promote good health and minimize the risk of disease. The bioactive compounds that are extracted from the original food and maintain their beneficial properties for health are called nutraceuticals [2][60]. For consumption and consumer acceptance, the functionality of bioactive compounds, safety, and nontoxicity must be guaranteed beforehand [74][61]. Highlighting a representative example, antioxidants play a crucial role in the food industry because, during food processing, the matrices used mostly incorporate lipids as emulsifiers or additives, making lipid oxidation a challenge to consider [75,76,77,78][62][63][64][65]. Lipid oxidation involves the attack of molecular oxygen on unsaturated fat molecules, which can generate undesirable volatile flavoring compounds that contribute to rancidity [79][66]. Even when quality controls are followed during food product preparation and packaging, the rate of lipid oxidation is influenced by several endogenous and exogenous parameters, including oxygen, light metals, and polyunsaturated lipids, primarily because the latter are prone to oxidation [80,81][67][68]. Antioxidants are used to mitigate this effect, meaning molecules that reduce, neutralize, or deplete molecular oxygen, remove pro-oxidative metal ions, and scavenge reactive oxygen species (ROS), hydrogen peroxide or superoxide anion radicals [82,83,84,85][69][70][71][72]. Antioxidants occur naturally, and the best known are ascorbic acid (vitamin C), tocopherols (vitamin E), carotenoids, and thiols [86][73]. During their absorption in the body, they complement the defense action as they help to slow down the use of endogenous antioxidants and improve the body’s ability to avoid oxidative stress [82,87,88,89,90][69][74][75][76][77]. The lack of action of endogenous antioxidants, either by diminution or stress, is related to the modification of lipid membrane components [91][78], resulting in neurodegenerative, cardiovascular, inflammatory diseases, diabetes, male infertility, and cancers of the breast, lung, liver, colon, prostate, ovary and brain [87,92,93,94,95,96,97][74][79][80][81][82][83][84]. The excessive presence of reactive oxygen species promotes the expression of oncogenic genes [93][80]. Antioxidants, as nutraceuticals, play a key role in the nutritional base because of their close relationship with biological processes; thus, skin benefits are also attributed to them for delaying aging [98,99,100][85][86][87]. Although the concept of nutraceuticals is not new, the trend to use antioxidants with biochemical properties of high stability and biocompatibility as a complementary ingredient has become interesting [84,101,102][71][88][89].2.3. Bakery Products
New requirements in bakery products make the development of new formulations that conform to what would be green or less harmful labels. In bakery products, lipases have been successfully applied to improve dough processing, strength, volume, structure, and softness, decrease stickiness, and increase the quality and shelf life [138,139][90][91]. With a focus on the intermediate product of bread, dough plays an indispensable role in becoming the final product because it is a semisolid foam that is converted into a solid cellular sponge upon baking so that the mixture of the lipid fraction of wheat, eggs, or baker’s fat exerts major roles in gas incorporation and its stabilization, which are necessary to achieve a fluffy product [140][92]. Although wheat flour contains low levels of lipids, they affect the quality of fresh bread because they are related to storage duration. Briefly, the studies are directed toward knowledge of the relationship of the flours or their reformulation by adding lipids from other sources and their effect on quality. Recently, lipases have been successfully applied to investigate how endogenous or exogenous lipids affect bread making. Lipases hydrolyze galactolipids, and their presence in the dough improves bread volume. The flour was defatted and subsequently reconstituted by adding different fractions of these lipids to determine the relationship of endogenous lipids in wheat flour and their impact on bread volume. The hydrolysis of endogenous lipids and their enzymatically released products are responsible for the positive effects on bread [138,141][90][93]. To understand the role of endogenous wheat lipids on the evolution of bread crumb firmness during storage, three lipases—Lipopan F, Lecitase Ultra, and Lipolase—were evaluated, and sodium lactylate stearoyl surfactant (SSL) was used as a surfactant. By forming amylose-lipid (AM-L) inclusion complexes, the surfactants retarded bread crumb firming. Some endogenous wheat lipids have surfactant-like structures, so the use of enzymes in bread making would increase the level of free fatty acids that allow the formation of amylose-lipid complexes. The evaluation of three enzymes showed that lipases and SSL similarly affected the texture of breadcrumbs during storage. However, after seven days of storage, the sample containing Lipolase significantly reduced amylopectin retrogradation, evidencing the importance of the formation of amylose-lipid inclusion complexes. Therefore, lipases have been proposed as alternatives to surfactants because they produce molecules in situ that possess hydrophilic and hydrophobic structures like those of surfactants [141,142][93][94].2.4. Flavors and Fragances
In the world market, a high demand exists for fragrance and flavor esters for different industries, including food, cosmetics, and pharma, as ingredients of many products (food, beverages, candies, jellies, jams, wines, dairy products, perfumes, body lotions, shampoos, and other toiletries) [143,144][95][96]. The flavor and fragrance market was valued at $28 billion in 2019 and is expected to expand at a compound annual growth rate (CAGR) of 4.7% to $35 billion from 2021 to 2027 [145][97]. Another characteristic is that many of these products are chiral [146][98]. This potential chiral product can be consulted in the database [147][99]. Many of these products are obtained after extraction from their natural sources (plants, fruits, and flowers). However, the low concentration of these products in their natural sources, climatic dependence of the source, and low yield and high production cost of the extraction and purification phases make it challenging to assume an increased world demand [143][95]. A wide range of flavors and fragrances can be obtained by chemical synthesis, solving the of raw material producing the same products at a lower cost. However, these products have not been labeled as natural according to European legislation (EC 1334/2008), and obtaining pure chiral compounds is challenging. In this context, the substitution of a chemical using biotechnology (microbial biosynthesis or applied biocatalysis) is being widely explored because the products can be labeled as natural if the employed reactants are labeled as natural. The resolution of chiral compounds is generally higher with no problems in selectivity, reaching higher yields and with an easier downstream due to the absence of undesirable side reactions. However, the operational conditions (P, T) are softer than those of the chemical approach. A marketplace of bioflavors is actually 100–500 $/kg, and more than 100 flavor products are commercialized [148][100]. In 2019, the global biotech flavor market was close to 0.5 billion US$, approximately 1.5% of the estimated global market in the same year and is expected to grow at a compound annual growth rate (CAGR) of 9.3% from 2020 to 2027. Similarly, biotech vanillin represents ca. 3% of the total vanillin market, and it is speculated to increase at a CAGR exceeding 13% by 2023 [149][101]. Thus, the significant demand for these esters has boosted the need for greener production routes and food safety aspects for human consumption, making enzymatic synthesis a favorable alternative to chemical catalysts [150,151][102][103]. Approximately 4000 enzymes are known, and close to 200 have been mainly commercialized for stereoselective organic synthesis and the biotechnological production of flavor compounds [148][100]. Between them, lipases are the most applied enzyme family to produce flavor and fragrances. Although their natural biocatalysis is the hydrolysis of lipids to produce free fatty acids, glycerol, or other alcohols, they also work in reactions of esterification and trans- and interesterification and the transfer of acyl groups from esters to other nucleophiles (e.g., amines and thiols) [143,152][95][104].References
- Borrelli, M.; Trono, D. Recombinant Lipases and Phospholipases and Their Use as Biocatalysts for Industrial Applications. Int. J. Mol. Sci. 2015, 16, 20774–20840.
- Yao, W.; Liu, K.; Liu, H.; Jiang, Y.; Wang, R.; Wang, W.; Wang, T. A Valuable Product of Microbial Cell Factories: Microbial Lipase. Front. Microbiol. 2021, 12, 743377.
- Khan, U.; Selamoglu, Z. Use of Enzymes in Dairy Industry: A Review of Current Progress. Arch. Razi Inst. 2020, 75, 131–136.
- Szymczak, T.; Cybulska, J.; Podleśny, M.; Frąc, M. Various Perspectives on Microbial Lipase Production Using Agri-Food Waste and Renewable Products. Agriculture 2021, 11, 540.
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Factories 2020, 19, 169.
- Bilal, M.; Fernandes, C.D.; Mehmood, T.; Nadeem, F.; Tabassam, Q.; Ferreira, L.F.R. Immobilized lipases-based nano-biocatalytic systems—A versatile platform with incredible biotechnological potential. Int. J. Biol. Macromol. 2021, 175, 108–122.
- Casas, L.; Duquesne, S.; Bordes, F.; Sandoval, G.; Marty, A. Lipases: An Overview. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 3–30.
- Sandoval, G. Lipases and Phospholipases, 2nd ed.; Springer: Berlin, Germany, 2018; Volume 1835, p. 437.
- Ali, Y.; Verger, R.; Abousalham, A. Lipases or esterases: Does it really matter? Toward a new bio-physico-chemical classification. Methods Mol. Biol. 2012, 861, 31–51.
- Priyanka, P.; Tan, Y.; Kinsella, G.; Henehan, G.; Ryan, B. Solvent stable microbial lipases: Current understanding and biotechnological applications. Biotechnol. Lett. 2019, 41, 203–220.
- Lee, H.; Park, O. Lipases associated with plant defense against pathogens. Plant Sci. 2019, 279, 51–58.
- Rivera, I.; Mateos, J.; Sandoval, G. Plant Lipases: Partial Purification of Carica papaya Lipase. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 115–122.
- Gao, M.; Yin, X.; Yang, W.; Lam, S.; Tong, X.; Liu, J.; Wang, X.; Li, Q.; Shui, G.; He, Z. GDSL lipases modulate immunity through lipid homeostasis in rice. PLoS Pathog. 2017, 13, e1006724.
- Rivera, I.; Gutiérrez, A.; Sandoval, G. Functional Expression of Plant Lipases: The Case of CpLip1 from Carica papaya. Methods Mol. Biol. 2018, 1835, 169–178.
- Villeneuve, P. Plant lipases and their applications in oils and fats modification. Eur. J. Lipid Sci. Technol. 2003, 105, 308–317.
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.; Abraham, A.; Mathew, A.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of Microbial Enzymes in Food Industry. Food Technol. Biotechnol. 2018, 56, 16–30.
- Valero, F. Heterologous Expression Systems for Lipases: A Review. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 161–178.
- Hamdan, S.H.; Maiangwa, J.; Ali, M.S.M.; Normi, Y.M.; Sabri, S.; Leow, T.C. Thermostable lipases and their dynamics of improved enzymatic properties. Appl. Microbiol. Biotechnol. 2021, 105, 7069–7094.
- Zhang, J.; Gao, B.; Lv, K.; Kumissay, L.; Wu, B.; Chu, J.; He, B. Specific immobilization of lipase on functionalized 3D printing scaffolds via enhanced hydrophobic interaction for efficient resolution of racemic 1-indanol. Biochem. Biophys. Res. Commun. 2021, 546, 111–117.
- Monteiro, R.; Virgen, J.; Berenguer, Á.; da Rocha, T.; dos Santos, J.; Alcántara, A.; Fernandez, R. Biotechnological relevance of the lipase A from Candida antarctica. Catal. Today 2021, 362, 141–154.
- Javed, S.; Azeem, F.; Hussain, S.; Rasul, I.; Siddique, M.; Riaz, M.; Afzal, M.; Kouser, A.; Nadeem, H. Bacterial lipases: A review on purification and characterization. Prog. Biophys. Mol. Biol. 2018, 132, 23–34.
- Juturu, V.; Wu, J. Heterologous Protein Expression in Pichia pastoris: Latest Research Progress and Applications. ChemBioChem 2018, 19, 7–21.
- Hee, K.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the brain: Metabolism and function. Prog. Lipid Res. 2014, 56, 1–18.
- Souza, M.; Santos, K.; Freire, R.; Barreto, A.; Fechine, P.; Gonçalves, L. Production of flavor esters catalyzed by lipase B from Candida antarctica immobilized on magnetic nanoparticles. Braz. J. Chem. Eng. 2017, 34, 681–690.
- Kurtovic, I.; Nalder, T.D.; Cleaver, H.; Marshall, S. Immobilisation of Candida rugosa lipase on a highly hydrophobic support: A stable immobilised lipase suitable for non-aqueous synthesis. Biotechnol. Rep. 2020, 28, e00535.
- Trbojević, J.; Veličković, D.; Dimitrijević, A.; Bezbradica, D.; Dragačević, V.; Gavrović, M.; Milosavić, N. Design of biocompatible immobilized Candida rugosa lipase with potential application in food industry. J. Sci. Food Agric. 2016, 96, 4281–4287.
- Monoj, G. Chapter 13-Trans Fat Alternatives and Challenges. In Practical Guide to Vegetable Oil Processing, 2nd ed.; Gupta, M.K., Ed.; AOCS Press: Ubana, IL, USA, 2017; pp. 341–374.
- Dayton, C. 11-Enzymatic Interesterification. In Green Vegetable Oil Processing; Farr, W.E., Proctor, A., Eds.; AOCS Press: Ubana, IL, USA, 2014; pp. 205–224.
- Gricajeva, A.; Kazlauskas, S.; Kalėdienė, L.; Bendikienė, V. Analysis of Aspergillus sp. lipase immobilization for the application in organic synthesis. Int. J. Biol. Macromol. 2018, 108, 1165–1175.
- Muniandy, M.; Lasekan, O.; Ghazali, M.H.; Rahman Mohd, B. Lipase-Catalyzed formation of pentyl nonanoate using screened immobilized from Rhizomucor meihei. Braz. J. Chem. Eng. Life Sci. 2019, 36, 1089–1097.
- Zhang, X.; Ai, N.; Wang, J.; Tong, L.; Zheng, F.; Sun, B. Lipase-catalyzed modification of the flavor profiles in recombined skim milk products by enriching the volatile components. J. Dairy Sci. 2016, 99, 8665–8679.
- Shaimaa, H.; Shaaban, H.; Mahmoud, H.; Abbas, K.; Farouk, A. Preparation of Ras Cheese Flavour Concentrate using Lipolyzed Cream and Skim Milk Curd. Int. J. Dairy Sci. 2017, 12, 275–281.
- Tecelão, C.; Rivera, I.; Sandoval, G.; Ferreira-Dias, S. Carica papaya latex: A low-cost biocatalyst for human milk fat substitutes production. Eur. J. Lipid Sci. Technol. 2012, 114, 266–276.
- Konkit, M.; Kim, W. Activities of amylase, proteinase, and lipase enzymes from Lactococcus chungangensis and its application in dairy products. J. Dairy Sci. 2016, 99, 4999–5007.
- Esteban, M.; Mancheño, J.; de las Rivas, B.; Muñoz, R. Characterization of a halotolerant lipase from the lactic acid bacteria Lactobacillus plantarum useful in food fermentations. LWT-Food Sci. Technol. 2015, 60, 246–252.
- Alvarez, Y.; Esteban, M.; Cortés, Á.; Gago, F.; Acebrón, I.; Benavente, R.; Mardo, K.; de las Rivas, B.; Muñoz, R.; Mancheño, J. Esterase LpEst1 from Lactobacillus plantarum: A Novel and Atypical Member of the αβ Hydrolase Superfamily of Enzymes. PLoS ONE 2014, 9, e92257.
- Liu, C.; Chen, Y.; Hou, M.; Hu, N.; Chen, C.; Shaw, J. Crystallographic analysis of the Staphylococcus epidermidis lipase involved in esterification in aqueous solution. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018, 74, 351–354.
- Molina, M.; Hakalin, N.; Rodríguez, L.; Alcaraz, L.; López, F.; Martínez, M.; Prieto, A. Effect of the Immobilization Strategy on the Efficiency and Recyclability of the Versatile Lipase from Ophiostoma piceae. Molecules 2019, 24, 1313.
- Knob, A.; Izidoro, S.; Lacerda, L.; Rodrigues, A.; de Lima, V. A novel lipolytic yeast Meyerozyma guilliermondii: Efficient and low-cost production of acid and promising feed lipase using cheese whey. Biocatal. Agric. Biotechnol. 2020, 24, 101565.
- Chaves, A.F.L.; Castro, J.M.P.; Medeiros, T.B.; Soares, F.M.B. Trends in lipase immobilization: Bibliometric review and patent analysis. Process Biochem. 2021, 110, 37–51.
- Castillo, E.; Casas, L.; Sandoval, G. Medium-engineering: A useful tool for modulating lipase activity and selectivity. Biocatalysis 2016, 1, 178–188.
- Itoh, T. Activation of Lipase-Catalyzed Reactions Using Ionic Liquids for Organic Synthesis. In Application of Ionic Liquids in Biotechnology; Itoh, T., Koo, Y.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 79–104.
- Sandoval, G.; Quintana, P.; Baldessari, A.; Ballesteros, A.; Plou, F. Lipase-catalyzed preparation of mono- and diesters of ferulic acid. Biocatal. Biotransformation 2015, 33, 89–97.
- Xu, P.; Zheng, G.; Zong, M.; Li, N.; Lou, W. Recent progress on deep eutectic solvents in biocatalysis. Bioresour. Bioprocess. 2017, 4, 34.
- Coelho, A.L.S.; Orlandelli, R.C. Immobilized microbial lipases in the food industry: A systematic literature review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1689–1703.
- Li, X.; Zhao, Y.; Lai, X.; Nong, J.; Zhao, G.; Xiao, X. One-pot biocatalytic synthesis and antioxidant activities of highly lipophilic naringin derivatives by using bi-functional whole-cells. Food Res. Int. 2020, 136, 109291.
- Sharma, R.; Sharma, N. Microbial Lipase Mediated by Health Beneficial Modification of Cholesterol and Flavors in Food Products: A Review. Recent Pat. Biotechnol. 2018, 12, 81–91.
- Khanniri, E.; Bagheripoor, N.; Sohrabvandi, S.; Mortazavian, M.; Khosravi, K.; Mohammad, R. Application of Liposomes in Some Dairy Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 484–493.
- Shojaei, M.; Golmakani, M.; Eskandari, M.; Toh, M.; Liu, S. Natural flavor biosynthesis by lipase in fermented milk using in situ produced ethanol. J. Food Sci. Technol. 2021, 58, 1858–1868.
- Ferreira, S.; Osório, N.; Tecelão, C. 9-Bioprocess technologies for production of structured lipids as nutraceuticals. In Current Developments in Biotechnology and Bioengineering; Rai, A.K., Singh, S.P., Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 209–237.
- Guo, Y.; Cai, Z.; Xie, Y.; Ma, A.; Zhang, H.; Rao, P.; Wang, Q. Synthesis, physicochemical properties, and health aspects of structured lipids: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 759–800.
- Burdge, G.; Calder, P. Introduction to fatty acids and lipids. World Rev. Nutr. Diet. 2015, 112, 1–16.
- Astrup, A.; Magkos, F.; Bier, D.; Brenna, J.; de Oliveira Otto, M.; Hill, J.; King, J.; Mente, A.; Ordovas, J.; Volek, J.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857.
- Temkov, M.; Mureșan, V. Tailoring the Structure of Lipids, Oleogels and Fat Replacers by Different Approaches for Solving the Trans-Fat Issue-A Review. Foods 2021, 10, 1376.
- Bahari, A.; Akoh, C. Synthesis of a Cocoa Butter Equivalent by Enzymatic Interesterification of Illipe Butter and Palm Midfraction. J. Am. Oil Chem. Soc. 2018, 95, 547–555.
- Bahari, A.; Akoh, C. Texture, rheology and fat bloom study of ‘chocolates’ made from cocoa butter equivalent synthesized from illipe butter and palm mid-fraction. LWT 2018, 97, 349–354.
- Casas, L.; Gasteazoro, F.; Duquesne, S.; Bordes, F.; Marty, A.; Sandoval, G. Lipases: An Overview. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Springer New York: New York, NY, USA, 2018; pp. 3–38.
- Tecelão, C.; Perrier, V.; Dubreucq, E.; Ferreira, S. Production of Human Milk Fat Substitutes by Interesterification of Tripalmitin with Ethyl Oleate Catalyzed by Candida parapsilosis Lipase/Acyltransferase. J. Am. Oil Chem. Soc. 2019, 96, 777–787.
- Hasibuan, H.A.; Sitanggang, A.B.; Andarwulan, N.; Hariyadi, P. Enzymatic Synthesis of Human Milk Fat Substitute—A Review on Technological Approaches. Food Technol. Biotechnol. 2021, 59, 475–495.
- Trifković, K.; Benković, M. Chapter 1-Introduction to Nutraceuticals and Pharmaceuticals. In Nutraceuticals and Natural Product Pharmaceuticals; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–31.
- Sun, L.; Xin, F.; Alper, H. Bio-synthesis of food additives and colorants-a growing trend in future food. Biotechnol. Adv. 2021, 47, 107694.
- Tomasz, P.; Maciej, B. The study on the use of flavonodi- prhosphatidylcholilne coating in extending the oxidative stability of flaxseed oil during storage. Food Packag. Sheld Life 2021, 28, 5.
- Farooq, S.; Abdullah; Zhang, H.; Weiss, J. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil-water interface of food emulsions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4250–4277.
- Boutillier, S.; Fourmentin, S.; Laperche, B. Food additives and the future of health: An analysis of the ongoing controversy on titanium dioxide. Futures 2020, 122, 102598.
- de Carvalho, C.; Caramujo, M. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583.
- Carocho, M.; Barreiro, M.; Morales, P.; Ferreira, I. Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399.
- Revilla, I.; González, M.; Vivar, A.; Blanco, M.; Lobos, I.; Hernández, J. Antioxidant capacity of different cheeses: Affectinf factors and prediction by near infrared spectroscopy. J. Dairy 2015, 99, 5074–5082.
- Gammone, M.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46.
- Pop, P.A.M.a.A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74.
- Zieniuk, B.; Wołoszynowska, M.; Białecka, E.; Fabiszewska, A. Application of freeze-dried Yarrowia lipolytica biomass in the synthesis of lipophilic antioxidants. Biotechnol. Lett. 2021, 43, 601–612.
- Zieniuk, B.; Białecka, E.; Wierzchowska, K.; Fabiszewska, A. Recent advances in the enzymatic synthesis of lipophilic antioxidant and antimicrobial compounds. World J. Microbiol. Biotechnol. 2021, 38, 11.
- Oteng, A.; Kersten, S. Mechanisms of Action of trans Fatty Acids. Adv. Nutr. 2020, 11, 697–708.
- Sies, H.; Jones, D. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Reviews. Mol. Cell Biol. 2020, 21, 363–383.
- Godic, A.; Poljšak, B.; Adamic, M.; Dahmane, R. The Role of Antioxidants in Skin Cancer Prevention and Treatment. Oxidative Med. Cell. Longev. 2014, 2014, 860479.
- Khalaf, A.T.; Wei, Y.; Alneamah, S.J.A.; Al-Shawi, S.G.; Kadir, S.Y.A.; Zainol, J.; Liu, X. What Is New in the Preventive and Therapeutic Role of Dairy Products as Nutraceuticals and Functional Foods? BioMed Res. Int. 2021, 2021, 8823222.
- Ceriello, A.; Esposito, K.; La Sala, L.; Pujadas, G.; De Nigris, V.; Testa, R.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. The protective effect of the Mediterranean diet on endothelial resistance to GLP-1 in type 2 diabetes: A preliminary report. Cardiovasc. Diabetol. 2014, 13, 140.
- Tan, B.; Norhaizan, M.; Liew, W. Nutrients and Oxidative Stress: Friend or Foe? Oxidative Med. Cell Longev. 2018, 2018, 9719584.
- Diamanti, E.; Papalou, O.; Kandaraki, E.; Kassi, G. Mechanisms in endocrinology: Nutrition as a mediator of oxidative stress in metabolic and reproductive disorders in women. Eur. J. Endocrinol. 2017, 176, R79–R99.
- Klaunig, J. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778.
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544.
- Lee, J.D.; Cai, Q.; Shu, X.O.; Nechuta, S.J. The Role of Biomarkers of Oxidative Stress in Breast Cancer Risk and Prognosis: A Systematic Review of the Epidemiologic Literature. J. Women’s Health (2002) 2017, 26, 467–482.
- Zhang, L.; Li, L.; Gao, G.; Wei, G.; Zheng, Y.; Wang, C.; Gao, N.; Zhao, Y.; Deng, J.; Chen, H.; et al. Elevation of GPRC5A expression in colorectal cancer promotes tumor progression through VNN-1 induced oxidative stress. Int. J. Cancer 2017, 140, 2734–2747.
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485.
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480.
- Durazzo, A.; Lucarini, M.; Santini, A. Nutraceuticals in Human Health. Foods 2020, 9, 370.
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896.
- Aydin, S.; Dalgic, S.; Karaman, M.; Kirlangic, F.; Yildirim, H. Effects of Fulvic Acid on Different Cancer Cell Lines. Proceedings 2017, 1, 1031.
- Swat, M.; Rybicka, I.; Gliszczyńska, A. Characterization of Fulvic Acid Beverages by Mineral Profile and Antioxidant Capacity. Foods 2019, 8, 605.
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021.
- Melis, S.; Delcour, J. Impact of wheat endogenous lipids on the quality of fresh bread: Key terms, concepts, and underlying mechanisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3715–3754.
- Dai, Y.; Tyl, C. A review on mechanistic aspects of individual versus combined uses of enzymes as clean label-friendly dough conditioners in breads. J. Food Sci. 2021, 86, 1583–1598.
- Lien, G.; Bram, P.; Karolien, D.; Jan, D. Lipases and Their Functionality in the Production of Wheat-Based Food Systems. Compr. Rev. Food Sci. Food Saf. 2014, 13, 978–989.
- Min, B.; Salt, L.; Wilde, P.; Kosik, O.; Hassall, K.; Przewieslik, A.; Burridge, A.; Poole, M.; Snape, J.; Wingen, L.; et al. Genetic variation in wheat grain quality is associated with differences in the galactolipid content of flour and the gas bubble properties of dough liquor. Food Chem. X 2020, 6, 100093.
- Lien, R.G.; Bram, P.; Hanne, G.M.; Jan, A.D. A lipase based approach to understand the role of wheat endogenous lipids in bread crumb firmness evolution during storage. LWT-Food Sci. Technol. 2015, 64, 874–880.
- Gomes, A.; Meneses, A.; Araújo, P.; Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105.
- López, J.; Benaiges, M.; Valero, F. Rhizopus oryzae Lipase, a Promising Industrial Enzyme: Biochemical Characteristics, Production and Biocatalytic Applications. Catalysts 2020, 10, 1277.
- Nitesh, C.; Himanshu, V.; Roshan, D. Flavors and Fragrance Market by Type (Flavors and Fragrance), Nature (Natural and Synthetic), and Application (Food & Beverages, Cosmetics & Personal Care, Home Care and Fabric Care): Global Opportunity Analysis and Industry Forecast, 2021–2027. Available online: https://www.researchandmarkets.com/reports/5341604/flavors-and-fragrance-market-by-type-nature-and (accessed on 11 August 2021).
- Heinsman, N.; Franssen, M.; van der Padt, A.; Boom, R.; van’t Riet, K. Lipase-mediated Resolution of Branched Chain Fatty Acids. Biocatal. Biotransformation 2002, 20, 297–309.
- Leffingwell, J. Chirality & Odour Perception. Available online: www.leffingwell.com/chirality/chirality.htm (accessed on 23 April 2022).
- Akacha, N.B.; Gargouri, M. Microbial and enzymatic technologies used for the production of natural aroma compounds: Synthesis, recovery modeling, and bioprocesses. Food Bioprod. Process. 2015, 94, 675–706.
- Bruno Nicolau, P.; Adones, S.; Lorena, F.; Gláucia Maria, P.; Gustavo, M.; Juliano Lemos, B. Recent advances in the microbial and enzymatic production of aroma compounds. Curr. Opin. Food Sci. 2021, 37, 98–106.
- Bansode, S.; Rathod, V. Enzymatic sythesis of Isoamyl butyrate under microwave irradiation. Chem. Eng. Process.-Process Intensif. 2018, 129, 71–76.
- Bansode, S.; Hardikar, M.; Rathod, V. Evaluation of reaction parameters and kinetic modelling for Novozym 435 catalysed synthesis of isoamyl butyrate. J. Chem. Technol. Biotechnol. 2017, 92, 1306–1314.
- Ferreira, S.; Sandoval, G.; Plou, F.; Valero, F. The potential use of lipases in the production of fatty acid derivatives for the food and nutraceutical industries. Electron. J. Biotechnol. 2013, 16, 1–38.
More
