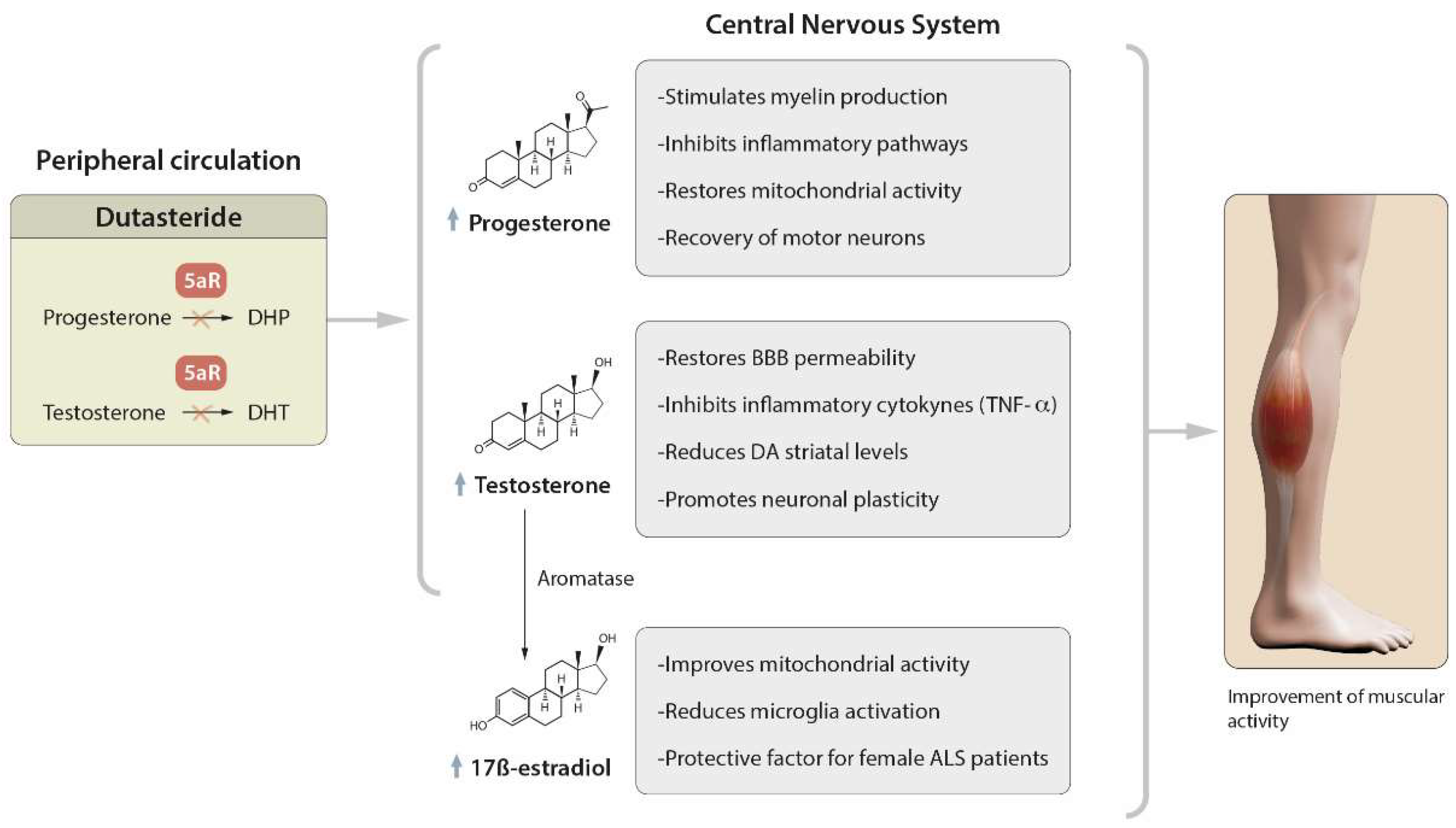
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that is characterized by the loss of upper and lower motor neurons (MNs) in the cerebral cortex, brainstem and spinal cord, with consequent weakness, atrophy and the progressive paralysis of all muscles. There is currently no medical cure, and riluzole and edaravone are the only two known approved drugs for treating this condition. However, they have limited efficacy, and hence there is a need to find new molecules. Dutasteride, a dual inhibitor of type 1 and type 2 5α-reductase (5AR) enzymes, the therapeutic purposes of which, to date, are the treatment of benign prostatic hyperplasia and androgenic alopecia, shows great anti-ALS properties by the molecular-topology methodology.
- amyotrophic lateral sclerosis
- dutasteride
- neuroprotection
- oxidative stress
- inflammation
- TDP43
- neurosteroids
1. Role of Dutasteride in the Activity of Steroid Hormones
2. Regulatory Mechanisms of Protein Aggregation
3. Neuroprotective and Antioxidant Effects of Dutasteride
4. Efficacy of Dutasteride against Neuroinflammation
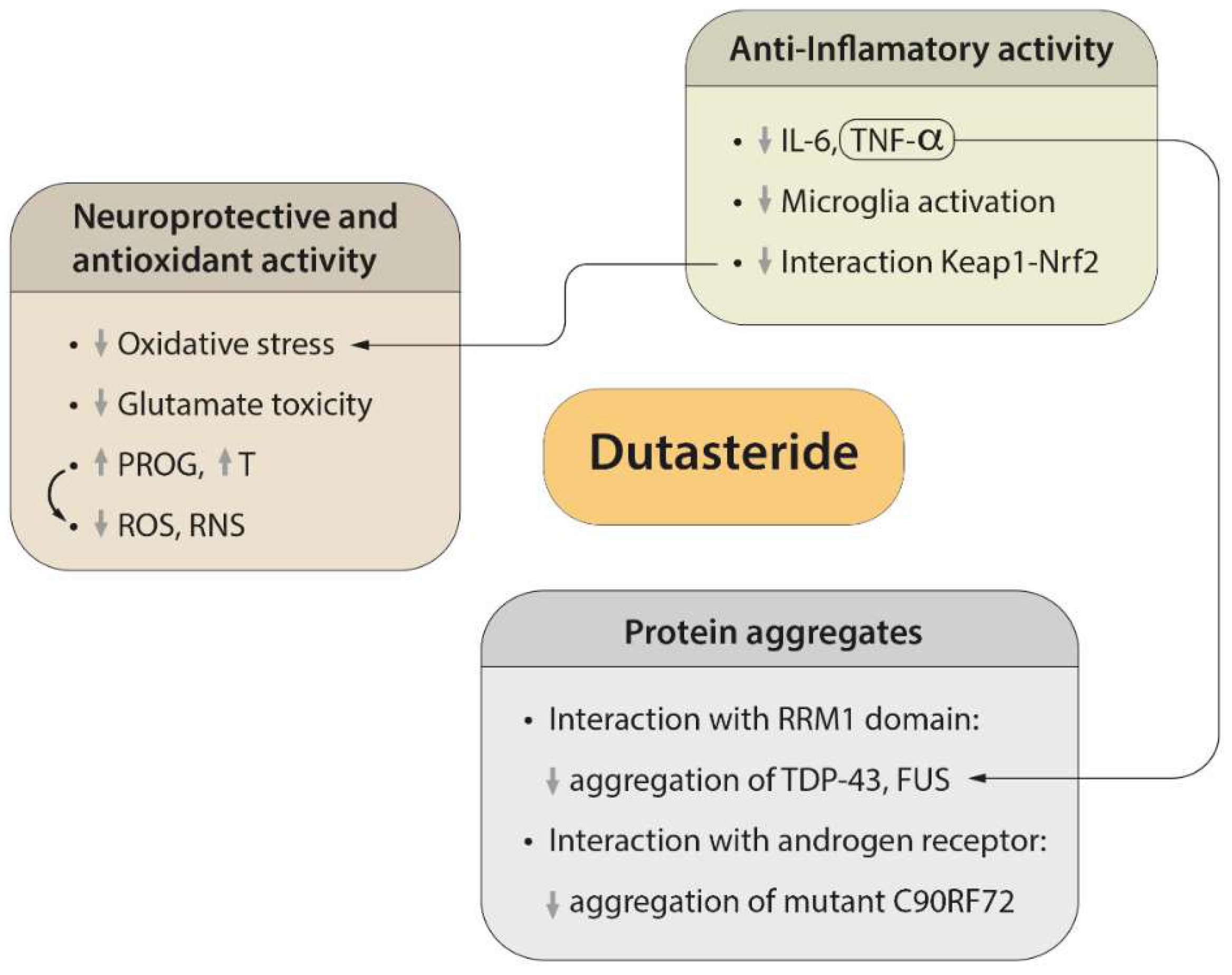
5. Possible Side Effects of Dutasteride
References
- Carroll, J.C.; Rosario, E.R. The Potential Use of Hormone-Based Therapeutics for the Treatment of Alzheimer’s Disease. Curr. Alzheimer Res. 2012, 9, 18–34.
- Clark, R.V.; Hermann, D.J.; Cunningham, G.R.; Wilson, T.H.; Morrill, B.B.; Hobbs, S. Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual 5α-Reductase Inhibitor. J. Clin. Endocrinol. Metab. 2004, 89, 2179–2184.
- Jin, Y.; Penning, T.M. Steroid 5alpha-Reductases and 3alpha-Hydroxysteroid Dehydrogenases: Key Enzymes in Androgen Metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2001, 15, 79–94.
- Monachelli, G.G.; Meyer, M.; Rodríguez, G.E.; Garay, L.I.; Sica, R.E.P.; De Nicola, A.F.; Deniselle, M.C.G. Endogenous Progesterone Is Associated to Amyotrophic Lateral Sclerosis Prognostic Factors. Acta Neurol. Scand. 2011, 123, 60–67.
- Schumacher, M.; Denier, C.; Oudinet, J.P.; Adams, D.; Guennoun, R. Progesterone Neuroprotection: The Background of Clinical Trial Failure. J. Steroid Biochem. Mol. Biol. 2016, 160, 53–56.
- Wright, D.W.; Kellermann, A.L.; Hertzberg, V.S.; Clark, P.L.; Frankel, M.; Goldstein, F.C.; Salomone, J.P.; Dent, L.L.; Harris, O.A.; Ander, D.S.; et al. ProTECT: A Randomized Clinical Trial of Progesterone for Acute Traumatic Brain Injury. Ann. Emerg. Med. 2007, 49, 391–402.
- De Jong, S.; Huisman, M.; Sutedja, N.; Van Der Kooi, A.; De Visser, M.; Schelhaas, J.; Van Der Schouw, Y.; Veldink, J.; Van Den Berg, L.; De Jong, S.; et al. Endogenous Female Reproductive Hormones and the Risk of Amyotrophic Lateral Sclerosis. J. Neurol. 2013, 260, 507–512.
- Hancevic, M.; Bilic, H.; Sitas, B.; Pavlisa, G.; Borovecki, F.; Munitic, I.; Bilic, E. Attenuation of ALS Progression during Pregnancy—Lessons to Be Learned or Just a Coincidence? Neurol. Sci. 2019, 40, 1275–1278.
- Mhaouty-Kodja, S. Role of the Androgen Receptor in the Central Nervous System. Mol. Cell. Endocrinol. 2018, 465, 103–112.
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-Mediated Neuroinflammation in Neurodegenerative Diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120.
- Mouihate, A. TLR4-Mediated Brain Inflammation Halts Neurogenesis: Impact of Hormonal Replacement Therapy. Front. Cell. Neurosci. 2014, 8, 146.
- Novak, C.M.; Ozen, M.; McLane, M.; Alqutub, S.; Lee, J.Y.; Lei, J.; Burd, I. Progesterone Improves Perinatal Neuromotor Outcomes in a Mouse Model of Intrauterine Inflammation via Immunomodulation of the Placenta. Am. J. Reprod. Immunol. 2018, 79, e12842.
- Atallah, A.; Mhaouty-Kodja, S.; Grange-Messent, V. Chronic Depletion of Gonadal Testosterone Leads to Blood-Brain Barrier Dysfunction and Inflammation in Male Mice. J. Cereb. Blood Flow Metab. 2017, 37, 3161–3175.
- Huang, X.; Roet, K.C.D.; Zhang, L.; Brault, A.; Berg, A.P.; Jefferson, A.B.; Klug-McLeod, J.; Leach, K.L.; Vincent, F.; Yang, H.; et al. Human Amyotrophic Lateral Sclerosis Excitability Phenotype Screen: Target Discovery and Validation. Cell Rep. 2021, 35, 109224.
- Litim, N.; Bourque, M.; Al Sweidi, S.; Morissette, M.; Di Paolo, T. The 5α-Reductase Inhibitor Dutasteride but Not Finasteride Protects Dopamine Neurons in the MPTP Mouse Model of Parkinson’s Disease. Neuropharmacology 2015, 97, 86–94.
- Litim, N.; Morissette, M.; Caruso, D.; Melcangi, R.C.; Di Paolo, T. Effect of the 5α-Reductase Enzyme Inhibitor Dutasteride in the Brain of Intact and Parkinsonian Mice. J. Steroid Biochem. Mol. Biol. 2017, 174, 242–256.
- Vest, R.S.; Pike, C.J. Gender, Sex Steroid Hormones, and Alzheimer’s Disease. Horm. Behav. 2013, 63, 301–307.
- Bianchi, V.E.; Rizzi, L.; Bresciani, E.; Omeljaniuk, R.J.; Torsello, A. Androgen Therapy in Neurodegenerative Diseases. J. Endocr. Soc. 2020, 4, bvaa120.
- Fargo, K.N.; Foecking, E.M.; Jones, K.J.; Sengelaub, D.R.; Fargo, K.N.; Galbiati, M.; Foecking, E.M.; Poletti, A.; Jones, K.J. Neuroprotective Actions of Androgens on Motoneurons. Front. Neuroendocr. 2009, 30, 130–141.
- Wilborn, C.; Taylor, L.; Poole, C.; Foster, C.; Willoughby, D.; Kreider, R. Effects of a Purported Aromatase and 5α-Reductase Inhibitor on Hormone Profiles in College-Age Men. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 457–465.
- Kim, Y.J.; Soto, M.; Branigan, G.L.; Rodgers, K.; Brinton, R.D. Association between Menopausal Hormone Therapy and Risk of Neurodegenerative Diseases: Implications for Precision Hormone Therapy. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12174.
- Galvez-Llompart, M.; Zanni, R.; Garcia-Domenech, R.; Galvez, J. How Molecular Topology Can Help in Amyotrophic Lateral Sclerosis (ALS) Drug Development: A Revolutionary Paradigm for a Merciless Disease. Pharmaceuticals 2022, 15, 94.
- Lukavsky, P.J.; Daujotyte, D.; Tollervey, J.R.; Ule, J.; Stuani, C.; Buratti, E.; Baralle, F.E.; Damberger, F.F.; Allain, F.H.T. Molecular Basis of UG-Rich RNA Recognition by the Human Splicing Factor TDP-43. Nat. Struct. Mol. Biol. 2013, 20, 1443–1449.
- Kuo, P.H.; Chiang, C.H.; Wang, Y.T.; Doudeva, L.G.; Yuan, H.S. The Crystal Structure of TDP-43 RRM1-DNA Complex Reveals the Specific Recognition for UG- and TG-Rich Nucleic Acids. Nucleic Acids Res. 2014, 42, 4712–4722.
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419.
- Chang, C.K.; Chiang, M.H.; Toh, E.K.W.; Chang, C.F.; Huang, T.H. Molecular Mechanism of Oxidation-Induced TDP-43 RRM1 Aggregation and Loss of Function. FEBS Lett. 2013, 587, 575–582.
- Liu, W.; Li, C.; Shan, J.; Wang, Y.; Chen, G. Insights into the Aggregation Mechanism of RNA Recognition Motif Domains in TDP-43: A Theoretical Exploration. R. Soc. Open Sci. 2021, 8, 210160.
- McLeod, V.M.; Chiam, M.D.F.; Perera, N.D.; Lau, C.L.; Boon, W.C.; Turner, B.J. Mapping Motor Neuron Vulnerability in the Neuraxis of Male SOD1G93A Mice Reveals Widespread Loss of Androgen Receptor Occurring Early in Spinal Motor Neurons. Front. Endocrinol. 2022, 13, 122.
- McLeod, V.M.; Chiam, M.D.F.; Lau, C.L.; Rupasinghe, T.W.; Boon, W.C.; Turner, B.J. Dysregulation of Steroid Hormone Receptors in Motor Neurons and Glia Associates with Disease Progression in ALS Mice. Endocrinology 2020, 161, bqaa113.
- Fujita, K.; Nakamura, Y.; Oka, T.; Ito, H.; Tamura, T.; Tagawa, K.; Sasabe, T.; Katsuta, A.; Motoki, K.; Shiwaku, H.; et al. A Functional Deficiency of TERA/VCP/P97 Contributes to Impaired DNA Repair in Multiple Polyglutamine Diseases. Nat. Commun. 2013, 4, 1816.
- Cook, C.N.; Wu, Y.; Odeh, H.M.; Gendron, T.F.; Jansen-West, K.; del Rosso, G.; Yue, M.; Jiang, P.; Gomes, E.; Tong, J.; et al. C9orf72 Poly(GR) Aggregation Induces TDP-43 Proteinopathy. Sci. Transl. Med. 2020, 12, eabb3774.
- Zhou, F.; Dong, H.; Liu, Y.; Yan, L.; Sun, C.; Hao, P.; Liu, Y.; Zhai, J.; Liu, Y. Raloxifene, a Promising Estrogen Replacement, Limits TDP-25 Cell Death by Enhancing Autophagy and Suppressing Apoptosis. Brain Res. Bull. 2018, 140, 281–290.
- Boddy, S.; Islam, M.; Moll, T.; Kurz, J.; Burrows, D.; McGown, A.; Bhargava, A.; Julian, T.H.; Harvey, C.; Marshall, J.N.; et al. Unbiased Metabolome Screen Leads to Personalized Medicine Strategy for Amyotrophic Lateral Sclerosis. Brain Commun. 2022, 4, fcac069.
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180.
- D’Ambrosi, N.; Cozzolino, M.; Carrì, M.T. Neuroinflammation in Amyotrophic Lateral Sclerosis: Role of Redox (Dys)Regulation. Antioxid. Redox Signal. 2018, 29, 15–36.
- Liu, J.; Wang, F. Role of Neuroinflammation in Amyotrophic Lateral Sclerosis: Cellular Mechanisms and Therapeutic Implications. Front. Immunol. 2017, 8, 1005.
- Merz, S.F.; Bengtson, C.P.; Tepohl, C.; Hagenston, A.M.; Bading, H.; Bas-Orth, C. A Microscopy-Based Small Molecule Screen in Primary Neurons Reveals Neuroprotective Properties of the FDA-Approved Anti-Viral Drug Elvitegravir. Mol. Brain 2020, 13, 124.
- Soskic, V.; Schrattenholz, A. WO2006058781A2-Finasteride, Dutasteride and Related Compounds for Preventing/Treating Neurologically-Associated Disorders-Google Patents. Available online: https://patents.google.com/patent/WO2006058781A2/en (accessed on 17 August 2022).
- Meydan, S.; Kus, I.; Tas, U. Effects of Testosterone on Orchiectomy-Induced Oxidative Damage in the Rat Hippocampus. J. Chem. Neuroanat. 2010, 40, 281–285.
- Son, S.W.; Lee, J.S.; Kim, H.G.; Kim, D.W.; Ahn, Y.C.; Son, C.G. Testosterone Depletion Increases the Susceptibility of Brain Tissue to Oxidative Damage in a Restraint Stress Mouse Model. J. Neurochem. 2016, 136, 106–117.
- Toro-Urrego, N.; Garcia-Segura, L.M.; Echeverria, V.; Barreto, G.E. Testosterone Protects Mitochondrial Function and Regulates Neuroglobin Expression in Astrocytic Cells Exposed to Glucose Deprivation. Front. Aging Neurosci. 2016, 8, 152.
- Ota, H.; Akishita, M.; Akiyoshi, T. Testosterone Deficiency Accelerates Neuronal and Vascular Aging of SAMP8 Mice: Protective Role of ENOS and SIRT1. PLoS ONE 2012, 7, e29598.
- Matsumoto, A. Hormonally Induced Neuronal Plasticity in the Adult Motoneurons. Brain Res. Bull. 1997, 44, 539–547.
- Leranth, C.; Petnehazy, O.; MacLusky, N.J. Gonadal Hormones Affect Spine Synaptic Density in the CA1 Hippocampal Subfield of Male Rats. J. Neurosci. 2003, 23, 1588–1592.
- Beyer, C.; Hutchison, J.B. Androgens Stimulate the Morphological Maturation of Embryonic Hypothalamic Aromatase-Immunoreactive Neurons in the Mouse. Brain Res. Dev. Brain Res. 1997, 98, 74–81.
- Marron, T.U.; Guerini, V.; Rusmini, P. Androgen-Induced Neurite Outgrowth Is Mediated by Neuritin in Motor Neurones. J. Neurochem. 2005, 92, 10–20.
- Byers, J.S.; Huguenard, A.L.; Kuruppu, D.; Liu, N.K.; Xu, X.M.; Sengelaub, D.R. Neuroprotective Effects of Testosterone on Motoneuron and Muscle Morphology Following Spinal Cord Injury. J. Comp. Neurol. 2012, 520, 2683–2696.
- Hong, Y.; Liu, Y.; Yu, D.; Wang, M.; Hou, Y. The Neuroprotection of Progesterone against Aβ-Induced NLRP3-Caspase-1 Inflammasome Activation via Enhancing Autophagy in Astrocytes. Int. Immunopharmacol. 2019, 74, 105669.
- De Nicola, A.F.; Meyer, M.; Garay, L.; Kruse, M.S.; Schumacher, M.; Guennoun, R.; Gonzalez Deniselle, M.C. Progesterone and Allopregnanolone Neuroprotective Effects in the Wobbler Mouse Model of Amyotrophic Lateral Sclerosis. Cell. Mol. Neurobiol. 2022, 42, 23–40.
- Fernández-Rhodes, L.E.; Kokkinis, A.D.; White, M.J.; Watts, C.A.; Auh, S.; Jeffries, N.O.; Shrader, J.A.; Lehky, T.J.; Li, L.; Ryder, J.E.; et al. Efficacy and Safety of Dutasteride in Patients with Spinal and Bulbar Muscular Atrophy: A Randomised Placebo-Controlled Trial. Lancet. Neurol. 2011, 10, 140–147.
- Mizoguchi, S.; Mori, K.; Shin, T.; Wang, Z.; DeFranco, D.B.; Yoshimura, N.; Mimata, H. Effects of Dutasteride in a Rat Model of Chemically Induced Prostatic Inflammation—Potential Role of Estrogen Receptor β. Prostate 2020, 80, 1413.
- Luo, D.; Han, L.; Gao, S.; Xiao, Z.; Zhou, Q.; Cheng, X.; Zhang, Y.; Zhou, W. Lincs Dataset-Based Repositioning of Dutasteride as an Anti-Neuroinflammation Agent. Brain Sci. 2021, 11, 1411.
- Wang, Y.; Xiao, C.Y.; Lin, H.Q.; Hu, J.S.; Ip, T.M.; Chi-Cheong Wan, D. Development of an Enzyme-Linked Immunosorbent Assay for Keap1-Nrf2 Interaction Inhibitors Identification. Redox Biol. 2020, 34, 101573.
- Hirshburg, J.M.; Kelsey, P.A.; Therrien, C.A.; Gavino, A.C.; Reichenberg, J.S. Adverse Effects and Safety of 5-Alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review-PubMed. J. Clin. Aesthet Dermatol 2016, 9, 56–62.
- Roehrborn, C.G.; Boyle, P.; Nickel, J.C.; Hoefner, K.; Andriole, G. Efficacy and Safety of a Dual Inhibitor of 5-Alpha-Reductase Types 1 and 2 (Dutasteride) in Men with Benign Prostatic Hyperplasia. Urology 2002, 60, 434–441.
