Biocompatible hyaluronic acid (HA, hyaluronan) gel implants have altered the therapeutic landscape of surgery and medicine, fostering an array of innovative products that include viscosurgical aids, synovial supplements, and drug-eluting nanomaterials. However, it is perhaps the explosive growth in the cosmetic applications of injectable dermal fillers that has captured the brightest spotlight, emerging as the dominant modality in plastic surgery and aesthetic medicine. The popularity surge with which injectable HA fillers have risen to in vogue status has also brought a concomitant increase in the incidence of once-rare iatrogenic vaso-occlusive injuries ranging from disfiguring facial skin necrosis to disabling neuro-ophthalmological sequelae. As ourresearchers' understanding of the pathophysiology of these injuries has evolved, supplemented by more than a century of astute observations, the formulation of novel therapeutic and preventative strategies has permitted the amelioration of this burdensome complication.
- hyaluronic acid
- dermal fillers
- hyaluronidase
- urokinase
- fibrinolytic therapy
- skin necrosis
- retinal injury
- cerebrovascular stroke
- pulmonary embolism
- angiosome
1. Introduction
2. Hyaluronan Biophysiology and Aesthetic Applications
The popularity of HA as an injectable material has placed it at the forefront of FIVO thanks to its widespread use in aesthetic medicine. First isolated from the bovine ocular vitreous and described by Meyer and Palmer in 1934, HA is a biomacromolecule with important physiological functions, remarkable versatility, and numerous applications in medicine [40,41][6][7]. Since the elucidation of its chemical structure in 1954, HA has been routinely employed in wound dressings, ophthalmological viscosurgical aids, and synovial viscosupplementation products [42,43][8][9]. Following the advent of genetically modified bacterial fermentation processes that enabled the large-scale manufacturing of HA derivatives, the number of available HA products has grown substantially and now includes a wide array of applications that include the correction of facial rhytids, volume depletion, and contour loss [44,45,46,47][10][11][12][13].2.1. Basic Biochemical Properties and Function
Hyaluronan is a ubiquitous heteropolysaccharide consisting of alternating disaccharide units composed of β-1,4-D-glucuronic acid and β-1,3-N-acetyl-D-glucosamine [48][14]. Biochemically, the HA macromolecule is a stable non-sulfated, non-branched glycosaminoglycan with important structural, hygroscopic, and signaling roles within the extracellular matrix (ECM) [49,50][15][16]. The human body contains approximately 15 g of hyaluronan—capable of binding 1000× its weight in water—with nearly half of all HA present in the skin and featuring a resident half-life of only 24 h [51][17]. In vivo, HA exists as a highly hydrated, polyanionic molecule ranging in weight from 100 kDa in serum to 7000 kDa in the vitreous humor [52,53][18][19]. Because of their charged nature, hyaluronan molecules display significant intra- and inter-molecular hydrogen bonds, providing secondary and tertiary structural rigidity, forming anti-parallel helical duplexes that can assemble into infinitely large meshworks, imparting this molecule its viscoelastic properties [54,55,56][20][21][22]. The biological functions performed by hyaluronan in living tissues are numerous. Structurally, HA’s vast hygroscopic meshwork imparts a hydrating, space-filling quality to the extracellular milieu that facilitates the cellular and biochemical activities of tissues. The osmotic and viscoelastic characteristics of HA also enhance the soft tissue turgor, pliability, and resiliency of the dermis, conferring a youthful quality to the skin [57][23]. Physiologically, hyaluronan serves as an important mediator and signaling molecule in wound healing, interacting directly with CD44 and modulating epidermal growth factor receptor (EGFR) and transforming growth factor-β1 (TGFβ1) receptor activity [58,59,60,61][24][25][26][27]. Furthermore, individual HA molecules also possess signaling properties that are dependent on molecular weight. Short (<20 kDa) and medium (20–500 kDa) molecules demonstrate pro-angiogenic, immunostimulatory actions, while high-molecular weight (HMW) HA (>500 kDa) shows the opposite tendency toward an immunodepressive effect [62][28].2.2. Physiological HA Biosynthesis and Degradation
In eukaryotes, the synthesis of hyaluronan is governed by a family of glycosyl-transferases named HA synthases (HASs), which convert two distinct uridine diphosphate (UDP)-sugar precursors (UDP-glucuronic acid and UDP-N-acetylglucosamine) into HMW HA [63][29]. In humans, the production of hyaluronan relies on three different isoenzymes—HAS1, HAS2, and HAS3—all of which are transmembrane proteins that polymerize HA from cytosolic substrates directly into the extra-cellular environment. HAS2 carries the most significant functional role, based on the lethality of HAS2 knockouts, and is responsible for most HA production in tissues [64,65][30][31]. The regulation of HA synthesis appears to be dependent on a variety of signaling factors associated with inflammatory and wound healing mediators, including transforming growth (TGF-β), insulin-like growth factor (IGF), fibroblast growth factor (FGF), and prostaglandins [66,67][32][33]. The degradation of HA is executed by endogenous hyaluronidases, endo-beta-N-acetylhexosaminidases that hydrolyze endogenous and exogenous HA. In humans, six homologous genes encoding for hyaluronidases have historically been described: HYAL-1 through HYAL-4, PHYAL-1, and SPAM-1 [68][34]. Of these, HYAL-1 and 2 are by far the most active in human tissues, with HYAL-2 existing as a free, unbound enzyme responsible for the breakdown of most extracellular HA into intermediate-sized polysaccharides and HYAL-1 serving as the membrane-bound form in lysosomes capable of further degrading endocytosed HA into its constituent monosaccharides [69][35]. The intravascular half-life of hyaluronidases is short, spanning only 2–3 min because of the presence of plasma glycoprotein inhibitors [70][36]. In tissues, hyaluronidases have a more prolonged activity, remaining active for 24–48 h; nevertheless, some murine studies have shown a complete drop in activity after 3–6 h [71,72][37][38]. For this reason, hyaluronidase re-dosing every 1–6 h has been recommended in cases of FIVO [73][39]. Additionally, other HA degradation pathways have been described recently that also bear significance. Specifically, the clearance of intravascular HA appears to rely on endocytosis via HARE (HA receptor for endocytosis)-mediated binding by the sinusoidal endothelial cells in the liver and spleen [74][40]. In addition, a new membrane-bound protein, HYBID (hyaluronan binding protein involved in hyaluronan depolymerization), has been identified and shown to play a significant role in HA turnover in the skin. HYBID appears to participate in an endocytosis-mediated breakdown of extracellular HA and its senescence-related dysfunction has been implicated in the pathophysiology of skin elastosis and aging [75][41].2.3. Commercial HA Synthesis and Reversal Agents
The commercial manufacturing of raw HA precursors constitutes a multi-billion-dollar industry subserving a wide range of cosmetic, dietary, and pharmaceutical-grade products [76][42]. The production of injectable HA gels requires HMW (>1000 kDa) raw HA that must adhere to established safety and purity standards [77][43]. Bacterial fermentation is currently the largest source of HMW HA, having largely supplanted the more-costly, lower-yield, animal-derived preparations from rooster combs and bovine vitreous. Specifically, the bacterium S. equis, subspecies zoopidemicus—a gram-positive, capsule-forming Lancefield type C streptococcus—is the most-commonly employed strain used in HA production [78][44]. Compared with animal-derived sources, bacterial-based methods demonstrate enhanced-purity, higher molecular weight, lower immunogenicity, and greater product yield [79][45]. Nonetheless, new heterologous recombinant expression systems harnessing B. subtilis and even cell-free in vitro production systems employing a soluble form of Class II HA synthases show promise in further achieving enhanced yield rates, higher molecular weight chains, and diminished polydispersity [80,81,82][46][47][48]. Prior to serving as the raw ingredient in the fabrication of dermal fillers, HMW HA (500–2500 kDa) derived from bacterial cultures is precipitated in isopropanol before undergoing a robust purification process that removes endogenous toxin remnants from the streptococcal source [83][49]. The rising demand for HA gel fillers has spurred a proportionate growth in the manufacturing of hyaluronidase mixtures to be used in the rapid degradation and reversal of injected hyaluronan products. In the United States, several brands have received FDA approval for the enhancement in tissue hydration and drug diffusion, though their use as HA reversal agents currently still remains off-label. The human recombinant form (Hylenex®, Halozyme Therapeutics Inc., San Diego, CA, USA) is currently the most favored among aesthetic clinicians because of its low risk of hypersensitivity reactions and higher potency, though bovine (Amphadase®, Amphastar Pharmaceuticals Inc., Rancho Cucamonga, CA, USA) and ovine (Vitrase®, ISTA Pharmaceuticals Inc., Irvine, CA, USA) varieties are available [84,85][50][51]. These products are supplied as small, single-use 1–2 mL vials containing limited quantities (150–200 USP units/mL) that are significantly smaller than the high doses (500–1500 U, q 1–6 h) currently advocated in the management of FIVO injuries [86,87,88][52][53][54].3. Pathophysiology of HA-Mediated Vascular Occlusion
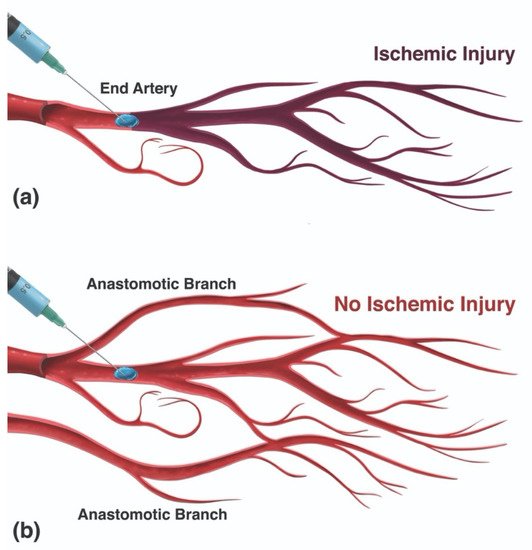
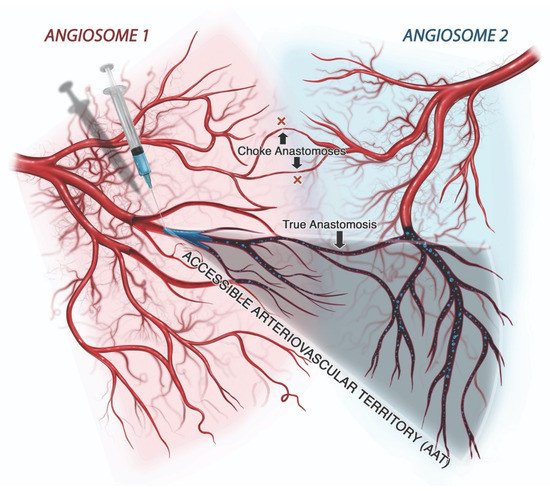
The mechanism of injury in FIVO represents a multifactorial cascade of events that includes vasocannulation, vasoinoculation, vasodissemination, and vasoocclusion, resulting in tissue underperfusion and ischemia (Figure 71). Although arterial compression by extravascular filler boluses is recognized as a valid alternative etiology of FIVO, such a mechanism is rare outside of instances involving surgerized tissues with a tenuous blood supply or those nourished by end-arteries with absent cross-perfusion from adjacent angiosomes. In normal tissues, compression is likely to result in diminished blood flow, inducing pallor and decreased capillary refill, but without complete loss of perfusion or tissue necrosis [109][55]. In mouse and rabbit FIVO models, arterial compression by filler could not be induced and did not result in any prolonged period of significant underperfusion or ischemia [110,111][56][57]. In humans, single-point obstructions of facial vessels are easily overcome by a vastly redundant blood supply from adjacent angiosomes, via true anastomoses, which dilate post filler-placement [112][58]. Even in instances of bilateral external carotid artery (ECA) ligation, historically performed for intractable epistaxis, compensatory flow from the internal carotid artery (ICA) system has been sufficient to avoid tissue necrosis [113,114,115][59][60][61]. Therefore, outside of compression of distal arterial branches in patients with abnormally underperfused tissues, the intravascular model of FIVO is likely to be the most prevalent. In this section, wresearchers review the pathophysiology of this intravascular model and outline preventative and therapeutic opportunities that exist along each phase of injury.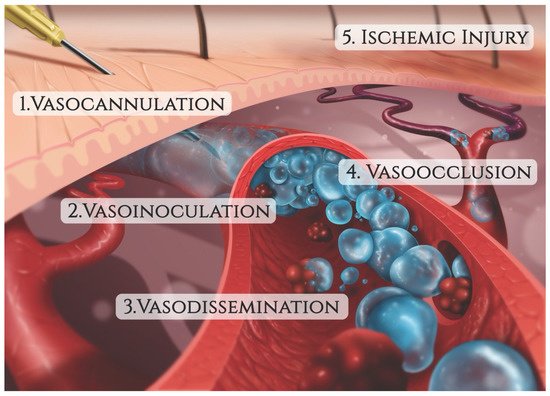
3.1. Vaso-Cannulation
The accidental penetration of a blood vessel during active filler injection represents the triggering event that immediately precedes FIVO [116,117][62][63]. Blunt-tipped microcannulas and sharp hypodermic needles, both routinely employed in aesthetic injectable treatments, can potentially perforate vessel walls and initiate intraluminal inoculation of filler. Microcannulas, because of their flexible shaft and closed tip configuration, have generally been regarded as more prudent and are endorsed by multiple consensus panels on injection safety [118,119][64][65]. Compared with needles, microcannulas cause less tissue trauma, pain, edema, and bruising, and can achieve a more reliable plane-specific placement of filler [120,121,122,123][66][67][68][69]. When employed by specialized practitioners with knowledge of vascular anatomy, microcannula use has demonstrated a significantly lower risk of FIVO compared with needle injections [124][70]. Nevertheless, higher gauge microcannulas (27 g and up) have vasopenetration forces equivalent to those of needles (1–2 kg⋅m/s2), suggesting that their safety advantage is limited or nonexistent in many treatment applications [125,126][71][72]. The likelihood of vessel cannulation also depends on the force necessary to advance a needle/cannula subcutaneously. Histologically, the central facial tissues of the perioral and perinasal regions feature thicker, more fibrous interconnections between the muscular aponeurosis and the skin (Figure 82) [127][73]. In those regions, the superficial musculoaponeurotic system (SMAS) displays a type II morphology, which lacks the soft intervening adipose layer and smooth gliding plane that characterize the type I tissues of the lateral face. The more restrained nature of vessels within type II SMAS and the tissue’s greater resistance to cannulation mean that even blunt devices, such as microcannulas, may easily perforate through vascular structures, as evidenced by multiple illustrative case reports [128,129,130,131,132][74][75][76][77][78].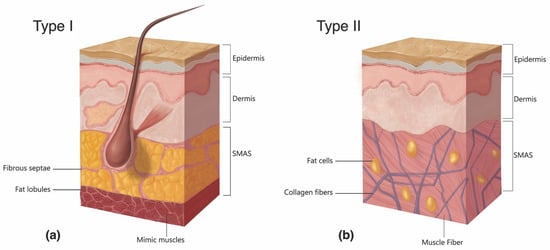
3.2. Vaso-Inoculation
In FIVO, the intraluminal inoculation of a blood vessel represents a brief, but significant event in which a variable amount of filler undergoes a pressurized delivery into the intravascular milieu. Conceptually, different mechanisms of inoculation exist depending on the positioning of the needle/cannula tip relative to the lumen of the vessel: intraluminal versus extraluminal (Figure 93) [146][92]. Intraluminal positioning results in a more efficient delivery of inoculum than extraluminal placement, in which the material must work back into a vessel along the track created by the needle/cannula. Given the blind nature of filler injections and the substantial needle movements that occur during filling, especially in light of the small diameters of most facial vessels, a combination of extraluminal and intraluminal mechanisms is likely to occur.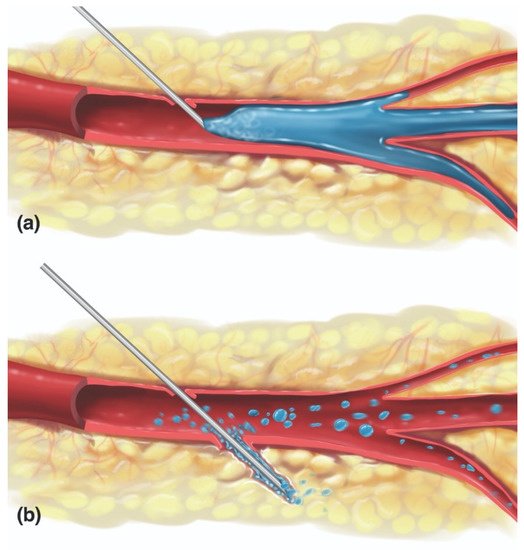
3.3. Vaso-Dissemination
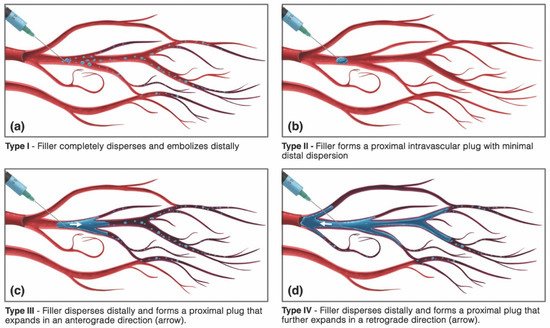
The intravascular behavior of HA gels is incompletely understood, but several animal studies and post hoc histopathological analyses have offered insight into the patterns of dissemination of dermal fillers [160,161,162][107][108][109]. The type of blockade induced by HA gels seemingly depends on the volume of inoculum as well as the rheological properties and particle size profile of the offending product. Small amounts of a low-viscosity filler injected into a large-caliber artery likely result in complete distal dispersion, while large volumes of a high-viscosity filler are more likely to produce an obstructive plug. Nie et al. proved this to be true by showing a higher rate of total, irreversible vessel occlusion with HA (Restylane®) than compared with injections of a lower viscosity, small-particle polymethylmethacrylate (PMMA) filler (Artecoll®; Hafod BV, Rotterdam, The Netherlands) in the rabbit-ear model [151][97]. However, a comparative rabbit ear study employing equivalent amounts of different HA (Restylane®, Juvederm® Ultra, and Belotero®) and non-HA (Radiesse®; Merz USA, Greensboro, NC, USA) products did not identify differences in the extent of ischemic injury or necrosis, suggesting that most HA fillers are more alike than they are dissimilar in their intravascular dispersal [111][57]. The vaso-disseminating behavior of a gel inoculum is also dictated by whether the affected vessel is arterial or venous in nature. Arterial inoculations are probably responsible for most of the local injuries occurring in the head and neck, while venous cannulations likely account for the majority of distant injuries (Table 2), although exceptions may occur owing to the presence of arteriovenous shunts, which have been described within the head and neck [163][110]. Intravenously placed facial filler can remain stationary—inciting a locoregional thrombophlebitic reaction that may intensify up to cerebral sinus thrombosis—or may undergo cardiopetal embolization to ultimately lodge within the pulmonary arterial tree, resulting in a pulmonary embolism [164,165][111][112]. Furthermore, venous occlusions can contribute to tissue ischemia even in cases stemming from arterial-cannulation because of the presence of arteriovenous shunting, contributing to superimposed congestive venous failure [166][113]. Ultimately, however, whether they are local, distant, venous, or arterial, the ischemic injury mechanism incited by FIVO bears an arterio-occlusive etiology. As a result, in this section, the intra-arterial mechanisms of filler dispersal will be the predominant focus of discussion.| Arterial * | Venous |
|---|---|
| Muco-Cutaneous/Soft Tissue Necrosis | Local Venous Thrombophlebitis |
| Vision Loss +/− Ophthalmoplegia | Cerebral Sinus Thrombosis |
| Ischemic Cerebral Stroke | Pulmonary Embolism |
| Facial Paralysis/Peripheral Nerve Injury | Myocardial Infarction (PFO **) |
References
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical modification of hyaluronan and their biomedical applications. Front. Chem. 2022, 10, 830671.
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56.
- American Society of Plastic Surgeons. 2020 Plastic Surgery Statistics Report. 2021. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf (accessed on 15 June 2022).
- Facial Injectables Market. Available online: https://www.fortunebusinessinsights.com/industry-reports/facial-injectables-market-100603 (accessed on 15 June 2022).
- Global Facial Injectable Market Size Report, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/facial-injectables-industry (accessed on 15 June 2022).
- Meyer, K.; Palmer, J. The polysaccharide of the vitreous humor. J. Biol. Chem. 1934, 107, 629–634.
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol. 2016, 40, 35–40.
- Weissman, B.; Meyer, K. The structure of hyalobiuronic acid and of hyaluronic acid from umbilical cord. J. Am. Chem. Soc. 1954, 76, 1753–1757.
- Chong, B.F.; Blank, L.M.; Mclaughlin, R.; Nielsen, L.K. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005, 66, 341–351.
- de Oliveira, J.D.; Carvalho, L.S.; Gomes, A.M.; Queiroz, L.R.; Magalhães, B.S.; Parachin, N.S. Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms. Microb. Cell Fact. 2016, 15, 119.
- Selyanin, M.A.; Boykov, P.Y.; Khabarov, V.N.; Polyak, F. The history of hyaluronic acid discovery, foundational research and initial use. In Hyaluronic Acid; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015.
- Greco, T.M.; Antunes, M.B.; Yellin, S.A. Injectable fillers for volume replacement in the aging face. Facial Plast. Surg. 2012, 28, 8–20.
- Ugarte, A.; Blevins, L.W.; Wills-Kofford, J.C.; Soares, D.J. Dermal filler re-contouring of the aged jawline: A useful non-surgical modality in facial rejuvenation. J. Aesthetic Nurs. 2022, 11, 70–78.
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701.
- Volpi, N.; Schiller, J.; Stern, R.; Soltés, L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr. Med. Chem. 2009, 16, 1718–1745.
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. 1992, 6, 2397–2404.
- O’Regan, M.; Martini, I.; Crescenzi, F.; De Luca, C.; Lansing, M. Molecular mechanisms and genetics of hyaluronan biosynthesis. Int. J. Biol. Macromol. 1994, 16, 283–286.
- Laurent, U.B.G.; Reed, R.K. Turnover of hyaluronan in the tissues. Adv. Drug Del. Rev. 1991, 7, 237–256.
- Cowman, M.K.; Lee, H.G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The content and size of hyaluronan in biological fluids and tissues. Front. Immunol. 2015, 2, 261.
- Scott, J.E.; Heatley, F. Hyaluronan forms specific stable tertiary structures in aqueous solution: A 13C NMR study. Proc. Natl. Acad. Sci. USA 1999, 27, 4850–4855.
- Liao, Y.-H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan: Pharmaceutical characterization and drug delivery. Drug Deliv. 2005, 12, 327–342.
- Dea, I.C.; Moorhouse, R.; Rees, D.A.; Arnott, S.; Guss, J.M.; Balazs, E.A. Hyaluronic acid: A novel, double helical molecule. Science 1973, 179, 560–562.
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinology 2012, 4, 253–258.
- Maytin, E.V. Hyaluronan: More than just a wrinkle filler. Glycobiology 2016, 26, 553–559.
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.; Lokeshwar, V.B. The Role of CD44 in Disease Pathophysiology and Targeted Treatment. Front. Immunol. 2015, 6, 182.
- Wang, S.J.; Bourguignon, L.Y. Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am. J. Pathol. 2011, 178, 956–963.
- Webber, J.; Jenkins, R.H.; Meran, S.; Phillips, A.; Steadman, R. Modulation of TGFbeta1-dependent myofibroblast differentiation by hyaluronan. Am. J. Pathol. 2009, 175, 148–160.
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715.
- Caon, I.; Parnigoni, A.; Viola, M.; Karousou, E.; Passi, A.; Vigetti, D. Cell Energy metabolism and hyaluronan synthesis. J. Histochem. Cytochem. 2021, 69, 35–47.
- Itano, N.; Kimata, K. Mammalian hyaluronan synthases. IUBMB Life 2002, 54, 195–199.
- Viola, M.; Vigetti, D.; Karousou, E.; D’Angelo, M.L.; Caon, I.; Moretto, P.; De Luca, G.; Passi, A. Biology and biotechnology of hyaluronan. Glycoconj. J. 2015, 32, 93–103.
- Tammi, R.H.; Passi, A.G.; Rilla, K.; Karousou, E.; Vigetti, D.; Makkonen, K.; Tammi, M.I. Transcriptional and posttranslational regulation of hyaluronan synthesis. FEBS J. 2011, 278, 1419–1428.
- Viola, M.; Bartolini, B.; Vigetti, D.; Karousou, E.; Moretto, P.; Deleonibus, S.; Sawamura, T.; Wight, T.N.; Hascall, V.C.; De Luca, G.; et al. Oxidized low density lipoprotein (LDL) affects hyaluronan synthesis in human aortic smooth muscle cells. J. Biol. Chem. 2013, 288, 29595–29603.
- Bastow, E.R.; Byers, S.; Golub, S.B.; Clarkin, C.E.; Pitsillides, A.; Fosang, A.J. Hyaluronan synthesis and degradation in cartilage and bone. Cell. Mol. Life Sci. 2008, 65, 395–413.
- Jung, H. Hyaluronidase: An overview of its properties, applications, and side effects. Arch. Plast. Surg. 2020, 47, 297–300.
- Girish, K.S.; Kemparaju, K.; Nagaraju, S.; Vishwanath, B.S. Hyaluronidase inhibitors: A biological and therapeutic perspective. Curr. Med. Chem. 2009, 16, 2261–2288.
- Kim, H.J.; Kwon, S.B.; Whang, K.U.; Lee, J.S.; Park, Y.L.; Lee, S.Y. The duration of hyaluronidase and optimal timing of hyaluronic acid (HA) filler reinjection after hyaluronidase injection. J. Cosmet. Laser Ther. 2018, 20, 52–57.
- Kwak, S.S.; Yoon, K.H.; Kwon, J.H.; Kang, W.H.; Rhee, C.H.; Yang, G.H.; Cruz, D.J.M.; Son, W.C. Comparative analysis of hyaluronidase-mediated degradation among seven hyaluronic acid fillers in hairless mice. Clin. Cosmet. Investig. Dermatol. 2021, 8, 241–248.
- Kim, D.-W.; Yoon, E.-S.; Ji, Y.-H.; Park, S.-H.; Lee, B.-I.; Dhong, E.-S. Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 1590–1595.
- Weigel, J.A.; Weigel, P.H. Characterization of the recombinant rat 175-kDa hyaluronan receptor for endocytosis (HARE). J. Biol. Chem. 2003, 278, 42802–42811.
- Yoshida, H.; Okada, Y. Role of HYBID (hyaluronan binding protein involved in hyaluronan depolymerization), Alias KIAA1199/CEMIP, in hyaluronan degradation in normal and photoaged skin. Int. J. Mol. Sci. 2019, 20, 5804.
- Mikulic, M.U.S. Hyaluronic acid raw material market size 2014–2024, by application Hyaluronic acid raw material market size in the U.S from 2014 to 2024, by application. Statista 2018, 212, 1–2.
- Huerta-Ángeles, G.; Nešporová, K.; Ambrožová, G.; Kubala, L.; Velebný, V. An effective translation: The development of hyaluronan-based medical products from the physicochemical, and preclinical aspects. Front. Bioeng. Biotechnol. 2018, 6, 62.
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell Fact. 2011, 10, 99.
- Ucm, R.; Aem, M.; Lhb, Z.; Kumar, V.; Taherzadeh, M.J.; Garlapati, V.K.; Chandel, A.K. Comprehensive review on biotechnological production of hyaluronic acid: Status, innovation, market and applications. Bioengineered 2022, 13, 9645–9661.
- Widner, B.; Behr, R.; von Dollen, S.; Tang, M.; Heu, T.; Sloma, A.; Sternberg, D.; DeAngelis, P.L.; Weigel, P.H.; Brown, S. Hyaluronic acid production in Bacillus subtilis. Appl. Environ. Microbiol. 2005, 71, 3747–3752.
- Jing, W.; Deangelis, P.L. Dissection of the two transferase activities of the Pasteurella multocida hyaluronan synthase: Two active sites exist in one polypeptide. Glycobiology 2000, 10, 883–889.
- Jing, W.; Deangelis, P.L. Synchronized chemoenzymatic synthesis of monodisperse hyaluronan polymers. J. Biol. Chem. 2004, 279, 42345–42349.
- Ferreira, R.G.; Azzoni, A.R.; Santana, M.H.A.; Petrides, D. Technoeconomic analysis of a hyaluronic acid production process utilizing streptococcal fermentation. Processes 2021, 9, 241.
- Silverstein, S.M.; Greenbaum, S.; Stern, R. Hyaluronidase in ophthalmology. J. Appl. Res. 2012, 1, 12–13.
- Murray, G.; Convery, C.; Walker, L.; Davies, E. Guideline for the safe use of hyaluronidase in aesthetic medicine, including modified high-dose protocol. J. Clin. Aesthet. Dermatol. 2021, 14, 69–75.
- Murray, G.; Convery, C.; Walker, L.; Davies, E. Guideline for the management of hyaluronic acid filler-induced vascular occlusion. J. Clin. Aesthet. Dermatol. 2021, 14, 61–69.
- Walker, L.; Convery, C.; Davies, E.; Murray, G.; Croasdell, B. Consensus opinion for the management of soft tissue filler induced vision loss. J. Clin. Aesthet. Dermatol. 2021, 14, 84–94.
- Urdiales-Gálvez, F.; Delgado, N.E.; Figueiredo, V.; Lajo-Plaza, J.V.; Mira, M.; Moreno, A.; Ortíz-Martí, F.; Del Rio-Reyes, R.; Romero-Álvarez, N.; Del Cueto, S.R.; et al. Treatment of soft tissue filler complications: Expert consensus recommendations. Aesthet. Plast. Surg. 2018, 42, 498–510.
- Lima, V.G.F.; Regattieri, N.A.T.; Pompeu, M.F.; Costa, I.M.C. External vascular compression by hyaluronic acid filler documented with high-frequency ultrasound. J. Cosmet. Dermatol. 2019, 18, 1629–1631.
- Chang, S.-H.; Yousefi, S.; Qin, J.; Tarbet, K.; Dziennis, S.; Wang, R.; Chappell, M.C. External compression versus intravascular injection: A mechanistic animal model of filler-induced tissue ischemia. Ophthalmic Plast. Reconstr. Surg. 2016, 32, 261–266.
- Hwang, C.J.; Morgan, P.V.; Pimentel, A.; Sayre, J.W.; Goldberg, R.A.; Duckwiler, G. Rethinking the role of nitroglycerin ointment in ischemic vascular filler complications: An animal model with ICG imaging. Ophthalmic Plast. Reconstr. Surg. 2016, 32, 118–122.
- Rocha, P.S.; Guerra, T.A.; Teixeira, D.A. Description of a safe doppler ultrasound-guided technique for hyaluronic acid filler in the face-A method to avoid adverse vascular events. J. Cosmet. Dermatol. 2021, 21, 2783–2787.
- Wolferman, A.; Dwyer, F.P., Jr. Unilateral and bilateral ligation of the external carotid for epistaxis. AMA Arch. Otolaryngol. 1955, 62, 310–315.
- Hassard, A.D.; Kirkpatrick, D.A.; Wong, F.S. Ligation of the external carotid and anterior ethmoidal arteries for severe or unusual epistaxis resulting from facial fractures. Can. J. Surg. 1986, 29, 447–449.
- Cooke, E.T. An evaluation and clinical study of severe epistaxis treated by arterial ligation. J. Laryngol. Otol. 1985, 99, 745–749.
- Schelke, L.; Decates, T.; Kadouch, J.; Velthuis, P. Incidence of vascular obstruction after filler injections. Aesthet. Surg. J. 2020, 40, 457–460.
- Wollina, U.; Goldman, A. Facial vascular danger zones for filler injections. Dermatol. Ther. 2020, 3, 4285.
- Humzah, M.D.; Ataullah, S.; Chiang, C.; Malhotra, R.; Goldberg, R. The treatment of hyaluronic acid aesthetic interventional induced visual loss (AIIVL): A consensus on practical guidance. J. Cosmet. Dermatol. 2019, 18, 71–76.
- Cohen, J.L.; Biesman, B.S.; Dayan, S.H.; de Lorenzi, C.; Lambros, V.L.; Nestor, M.S.; Sadick, N.; Sykes, J. Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: Consensus recommendations. Aesthet. Surg. J. 2015, 35, 844–849.
- Hexsel, D.; Soirefmann, M.; Porto, M.D.; Siega, C.; Schilling-Souza, J.; Brum, C. Double-blind, randomized, controlled clinical trial to compare safety and efficacy of a metallic cannula with that of a standard needle for soft tissue augmentation of the nasolabial folds. Dermatol. Surg. 2012, 38, 207–214.
- van Loghem, J.A.J.; Humzah, D.; Kerscher, M. Cannula versus sharp needle for placement of soft tissue fillers: An observational cadaver study. Aesthet. Surg. J. 2017, 38, 73–88.
- Pavicic, T.; Frank, K.; Erlbacher, K.; Neuner, R.; Targosinski, S.; Schenck, T.; Gotkin, H.; Cotofana, S. Precision in dermal filling: A comparison between needle and cannula when using soft tissue fillers. J. Drugs. Dermatol. 2017, 16, 866–872.
- Jones, D.; Palm, M.; Cox, S.E.; McDermott, M.; Sartor, M.; Chawla, S. Safety and effectiveness of hyaluronic acid filler, VYC-20L, via cannula for cheek augmentation: A randomized, single-blind, controlled study. Dermatol. Surg. 2021, 47, 1590–1594.
- Alam, M.; Kakar, R.; Dover, J.S.; Harikumar, V.; Kang, B.Y.; Wan, H.T.; Poon, E.; Jones, D.H. Rates of vascular occlusion associated with using needles vs cannulas for filler injection. JAMA Dermatol. 2021, 157, 174–180.
- Ugradar, S.; Hoenig, J. Measurement of the force required by blunt-tipped microcannulas to perforate the facial artery. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 444–446.
- Pavicic, T.; Webb, K.L.; Frank, K.; Gotkin, R.H.; Tamura, B.; Cotofana, S. Arterial wall penetration forces in needles versus cannulas. Plast. Reconstr. Surg. 2019, 143, 504–512.
- Ghassemi, A.; Prescher, A.; Riediger, D.; Axer, H. Anatomy of the SMAS revisited. Aesthet. Plast. Surg. 2003, 27, 258–264.
- Yang, Q.; Lu, B.; Guo, N.; Li, L.; Wang, Y.; Ma, X.; Su, Y. Fatal cerebral infarction and ophthalmic artery occlusion after nasal augmentation with hyaluronic acid—A case report and review of literature. Aesthet. Plast. Surg. 2020, 44, 543–548.
- Thanasarnaksorn, W.; Cotofana, S.; Rudolph, C.; Kraisak, P.; Chanasumon, N.; Suwanchinda, A. Severe vision loss caused by cosmetic filler augmentation: Case series with review of cause and therapy. J. Cosmet. Dermatol. 2018, 17, 712–718.
- Hu, X.Z.; Hu, J.Y.; Wu, P.S.; Yu, S.B.; Kikkawa, D.O.; Lu, W. Posterior ciliary artery occlusion caused by hyaluronic acid injections into the forehead: A case report. Medicine 2016, 95, e3124.
- Darling, M.D.; Peterson, J.D.; Fabi, S.G. Impending necrosis after injection of hyaluronic acid and calcium hydroxylapatite fillers: Report of 2 cases treated with hyperbaric oxygen therapy. Dermatol. Surg. 2014, 40, 1049–1052.
- Wibowo, A.; Kapoor, K.M.; Philipp-Dormston, W.G. Reversal of post-filler vision loss and skin ischaemia with high-dose pulsed hyaluronidase injections. Aesthet. Plast. Surg. 2019, 43, 1337–1344.
- Goodman, G.J.; Magnusson, M.R.; Callan, P.; Roberts, S.; Hart, S.; McDonald, C.B.; Liew, S.; Porter, C.; Corduff, N.; Clague, M. Neither positive nor negative aspiration before filler injection should be relied upon as a safety maneuver. Aesthet. Surg. J. 2021, 41, 134–136.
- Casabona, G. Blood aspiration test for cosmetic fillers to prevent accidental intravascular injection in the face. Dermatol. Surg. 2015, 41, 841–847.
- Goodman, G.J.; Magnusson, M.R.; Callan, P.; Roberts, S.; Hart, S.; Lin, F.; Rahman, E.; McDonald, C.B.; Liew, S.; Porter, C.; et al. Aspiration before tissue filler-an exercise in futility and unsafe practice. Aesthet. Surg. J. 2022, 42, 89–101.
- Kapoor, K.M.; Murthy, R.; Hart, S.L.A.; Cattin, T.A.; Nola, P.F.; Rossiter, A.P.; Singh, R.; Singh, S. Factors influencing pre-injection aspiration for hyaluronic acid fillers: A systematic literature review and meta-analysis. Dermatol. Ther. 2021, 34, e14360.
- Moon, H.-J.; Lee, W.; Kim, J.-S.; Yang, E.-J.; Sundaram, H. Aspiration revisited: Prospective evaluation of a physiologically pressurized model with animal correlation and broader applicability to filler complications. Aesthet. Surg. J. 2021, 41, 1073–1083.
- Schelke, L.W.; Decates, T.S.; Velthuis, P.J. Ultrasound to improve the safety of hyaluronic acid filler treatments. J. Cosmet. Dermatol. 2018, 17, 1019–1024.
- Lee, G.S.K. Use of handheld ultrasound Doppler to prevent complications from intra-arterial injection of dermal fillers: Clinical experience. J. Cosmet. Dermatol. 2019, 18, 1267–1270.
- Jagus, D.; Skrzypek, E.; Migda, B.; Wozniak, W.; Mlosek, R.K. Usefulness of Doppler sonography in aesthetic medicine. J. Ultrason. 2021, 20, 268–272.
- Lee, W.; Kim, J.-S.; Moon, H.-J.; Yang, E.-J. A safe doppler ultrasound-guided method for nasolabial fold correction with hyaluronic acid filler. Aesthet. Surg. J. 2021, 41, 486–492.
- Iwayama, T.; Hashikawa, K.; Osaki, T.; Yamashiro, K.; Horita, N.; Fukumoto, T. Ultrasonography-guided cannula method for hyaluronic acid filler injection with evaluation using laser speckle flowgraphy. Plast. Reconstr. Surg. Glob. Open 2018, 6, 1776.
- Quezada-Gaón, N.; Wortsman, X. Ultrasound-guided hyaluronidase injection in cosmetic complications. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 39–40.
- Munia, M.A.; Munia, C.G.; Parada, M.B.; Ben-Hurferraz Parente, J.; Wolosker, N. Doppler ultrasound in the management of vascular complications associated with hyaluronic acid dermal fillers. J. Clin. Aesthet. Dermatol. 2022, 15, 40–43.
- Levy, J.; Barrett, D.L.; Harris, N.; Jeong, J.J.; Yang, X.; Chen, S.C. High-frequency ultrasound in clinical dermatology: A review. Ultrasound J. 2021, 13, 24.
- DeLorenzi, C. New high dose pulsed hyaluronidase protocol for hyaluronic acid filler vascular adverse events. Aesthet. Surg. J. 2017, 37, 814–825.
- Paul, S.; Hoey, M.; Egbert, J. Pressure measurements during injection of corticosteroids: In vivo studies. Med. Biol. Eng. Comput. 1999, 37, 645–651.
- Cho, K.-H.; Pozza, E.D.; Toth, G.; Gharb, B.B.; Zins, J.E. Pathophysiology study of filler-induced blindness. Aesthet. Surg. J. 2019, 39, 96–106.
- Townsend, J.M.; Beck, E.C.; Gehrke, S.H.; Berkland, C.J.; Detamore, M.S. Flow behavior prior to crosslinking: The need for precursor rheology for placement of hydrogels in medical applications and for 3D bioprinting. Prog. Polym. Sci. 2019, 91, 126–140.
- Lee, Y.; Oh, S.M.; Lee, W.; Yang, E.J. Comparison of hyaluronic acid filler ejection pressure with injection force for safe filler injection. J. Cosmet. Dermatol. 2021, 20, 1551–1556.
- Nie, F.; Xie, H.; Wang, G.; An, Y. Risk Comparison of filler embolism between polymethyl methacrylate (PMMA) and hyaluronic acid (HA). Aesthet. Plast. Surg. 2019, 43, 853–860.
- Koziej, M.; Polak, J.; Wnuk, J.; Trybus, M.; Walocha, J.; Chrapusta, A.; Brzegowy, P.; Mizia, E.; Popiela, T.; Hołda, M. The transverse facial artery anatomy: Implications for plastic surgery procedures. PLoS ONE 2019, 14, 0211974.
- Lee, S.-H.; Ha, T.-J.; Koh, K.-S.; Song, W.-C. External and internal diameters of the facial artery relevant to intravascular filler injection. Plast. Reconstr. Surg. 2019, 143, 1031–1037.
- Kim, B.S.; Jung, Y.J.; Chang, C.H.; Choi, B.Y. The anatomy of the superficial temporal artery in adult koreans using 3-dimensional computed tomographic angiogram: Clinical research. J. Cerebrovasc. Endovasc. Neurosurg. 2013, 15, 145–151.
- Phumyoo, T.; Jiirasutat, N.; Jitaree, B.; Rungsawang, C.; Uruwan, S.; Tansatit, T. Anatomical and ultrasonography-based investigation to localize the arteries on the central forehead region during the glabellar augmentation procedure. Clin. Anat. 2020, 33, 370–382.
- Money, S.M.; Wall, W.B.; Davis, L.S.; Edmondson, A.C. Lumen diameter and associated anatomy of the superior labial artery with a clinical application to dermal filler injection. Dermatol. Surg. 2020, 46, 678–684.
- Khan, T.T.; Colon-Acevedo, B.; Mettu, P.; DeLorenzi, C.; Woodward, J.A. An anatomical analysis of the supratrochlear artery: Considerations in facial filler injections and preventing vision loss. Aesthet. Surg. J. 2017, 37, 203–208.
- Edsman, K.L.M.; Öhrlund, Å. Cohesion of hyaluronic acid fillers: Correlation between cohesion and other physicochemical properties. Dermatol. Surg. 2018, 44, 557–562.
- van Loghem, J.; Sattler, S.; Casabona, G.; Cotofana, S.; Fabi, S.G.; Goldie, K.; Gout, U.; Kerscher, M.; Lim, T.S.; de Sanctis Pecora, C.; et al. Consensus on the use of hyaluronic acid fillers from the cohesive polydensified matrix range: Best practice in specific facial indications. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1175–1199.
- Goodman, G.J.; Liew, S.; Callan, P.; Hart, S. Facial aesthetic injections in clinical practice: Pretreatment and posttreatment consensus recommendations to minimise adverse outcomes. Australas. J. Dermatol. 2020, 61, 217–225.
- Chen, Y.; Zhang, Y.-L.; Luo, S.-K. Experimentally induced arterial embolism by hyaluronic acid injection: Clinicopathologic observations and treatment. Plast. Reconstr. Surg. 2019, 143, 1088–1097.
- Zhang, L.; Feng, X.; Shi, H.; Wu, W.T.L.; Wu, S. Blindness after facial filler injections: The role of extravascular hyaluronidase on intravascular hyaluronic acid embolism in the rabbit experimental model. Aesthet. Surg. J. 2020, 40, 319–326.
- Nie, F.; Xie, H. Effect of local massage on prevention and treatment of intra-arterial polymethyl methacrylate embolism complications: An experimental animal study. Chin. J. Plast. Reconstr. Surg. 2022, 4, 6–12.
- Taylor, G.I.; Shoukath, S.; Gascoigne, A.; Corlett, R.J.; Ashton, M.W. The functional anatomy of the ophthalmic angiosome and its implications in blindness as a complication of cosmetic facial filler procedures. Plast. Reconstr. Surg. 2020, 146, 745.
- Abduljabbar, M.H.; Basendwh, M.A. Complications of hyaluronic acid fillers and their managements. J. Derm. Derm. Surg. 2016, 20, 100–106.
- Park, H.J.; Jung, K.H.; Kim, S.Y.; Lee, J.H.; Jeong, J.Y.; Kim, J.H. Hyaluronic acid pulmonary embolism: A critical consequence of an illegal cosmetic vaginal procedure. Thorax 2010, 65, 360–361.
- Zhuang, Y.; Yang, M.; Liu, C. An islanded rabbit auricular skin flap model of hyaluronic acid injection-induced embolism. Aesthet. Plast. Surg. 2016, 40, 421–427.
- Taylor, G.I.; Palmer, J.H. The vascular territories (angiosomes) of the body: Experimental study and clinical applications. Br. J. Plast. Surg. 1987, 40, 113–141.
- Taylor, G.I.; Corlett, R.J.; Ashton, M.W. The functional angiosome: Clinical implications of the anatomical concept. Plast. Reconstr. Surg. 2017, 140, 721–733.
- Jajoria, H.; Venkataram, A.; Mysore, V. Importance of choke vessels in injectable fillers. J. Cutan. Aesthet. Surg. 2020, 13, 185–190.
- Gascoigne, A.C.; Taylor, G.I.; Corlett, R.J.; Briggs, C.; Ashton, M.W. Increasing perfusion pressure does not distend perforators or anastomoses but reveals arteriovenous shuntings. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2857.
- Ashton, M.W.; Taylor, G.I.; Corlett, R.J. The role of anastomotic vessels in controlling tissue viability and defining tissue necrosis with special reference to complications following injection of hyaluronic acid fillers. Plast. Reconstr. Surg. 2018, 141, 818–830.
- Ogawa, R.; Oki, K.; Hyakusoku, H. Skin perforator freeways and pathways: Understanding the role of true and choke anastomoses between perforator angiosomes and their impact on skin flap planning and outcomes. Plast. Reconstr. Surg. 2014, 133, 719–720.
- Mao, Y.; Li, H.; Ding, M.; Hao, X.; Pan, J.; Tang, M.; Chen, S. Comparative study of choke vessel reconstruction with single and multiple perforator-based flaps on the murine back using delayed surgery. Ann. Plast. Surg. 2019, 82, 93–98.
