Triple-negative breast cancer (TNBC) refers to a type of breast cancer in which the immunohistochemistry of the cancer tissue is negative for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2), and it accounts for 15–20% of all breast cancer patients. Because of its rapid progression, most patients with TNBC have progressed to the more malignant and aggressive metastatic TNBC (mTNBC), with a shorter survival period by the time they seek medical attention. The majority of breast cancer deaths are caused by mTNBC. According to pathological characteristics, it lacks specific therapeutic targets, and it cannot be completely removed surgically due to unclear distant micro-metastases. Therefore, treatment of mTNBC is usually based on chemotherapy. However, according to clinical statistics, the overall response rate (ORR‘) of mTNBC with single-agent chemotherapy is only 10–30%, and with the best multi-drug combination chemotherapy regimen it is only 63%. The average pathologically complete response (pCR) to mTNBC with multi-drug combination chemotherapy regimen is about 30–40%. In summary, the benefit of chemotherapy for patients with mTNBC is not promising. The search for treatments with high clearance, good targeting, and few side effects has become a major focus of medical research.
- cancer therapy
- metastatic triple-negative breast cancer
- immunotherapy
- immune checkpoint blockade therapy
- PD-1/PD-L1
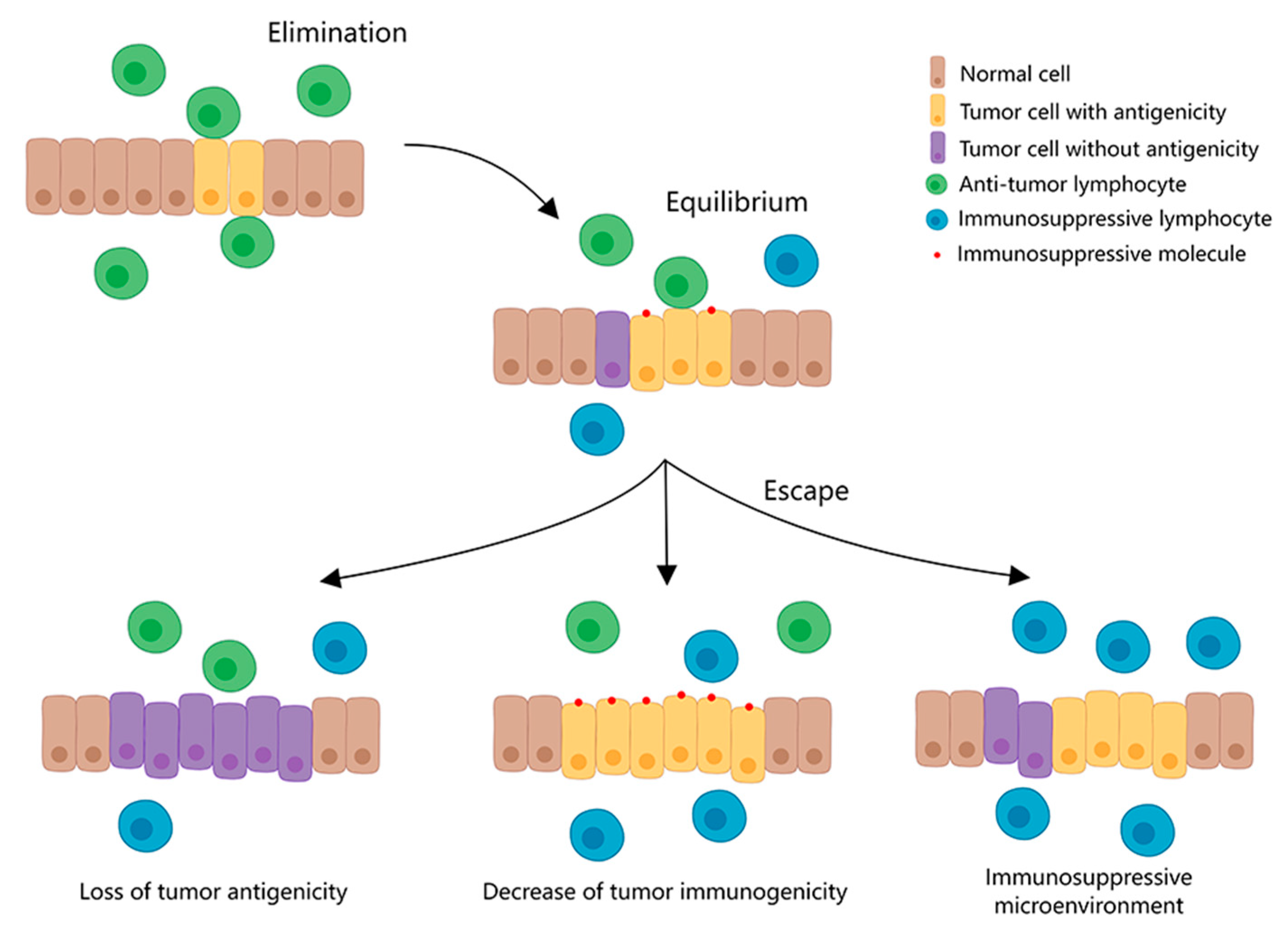
1. Immune Checkpoint and Immune Checkpoint Blockade Therapy
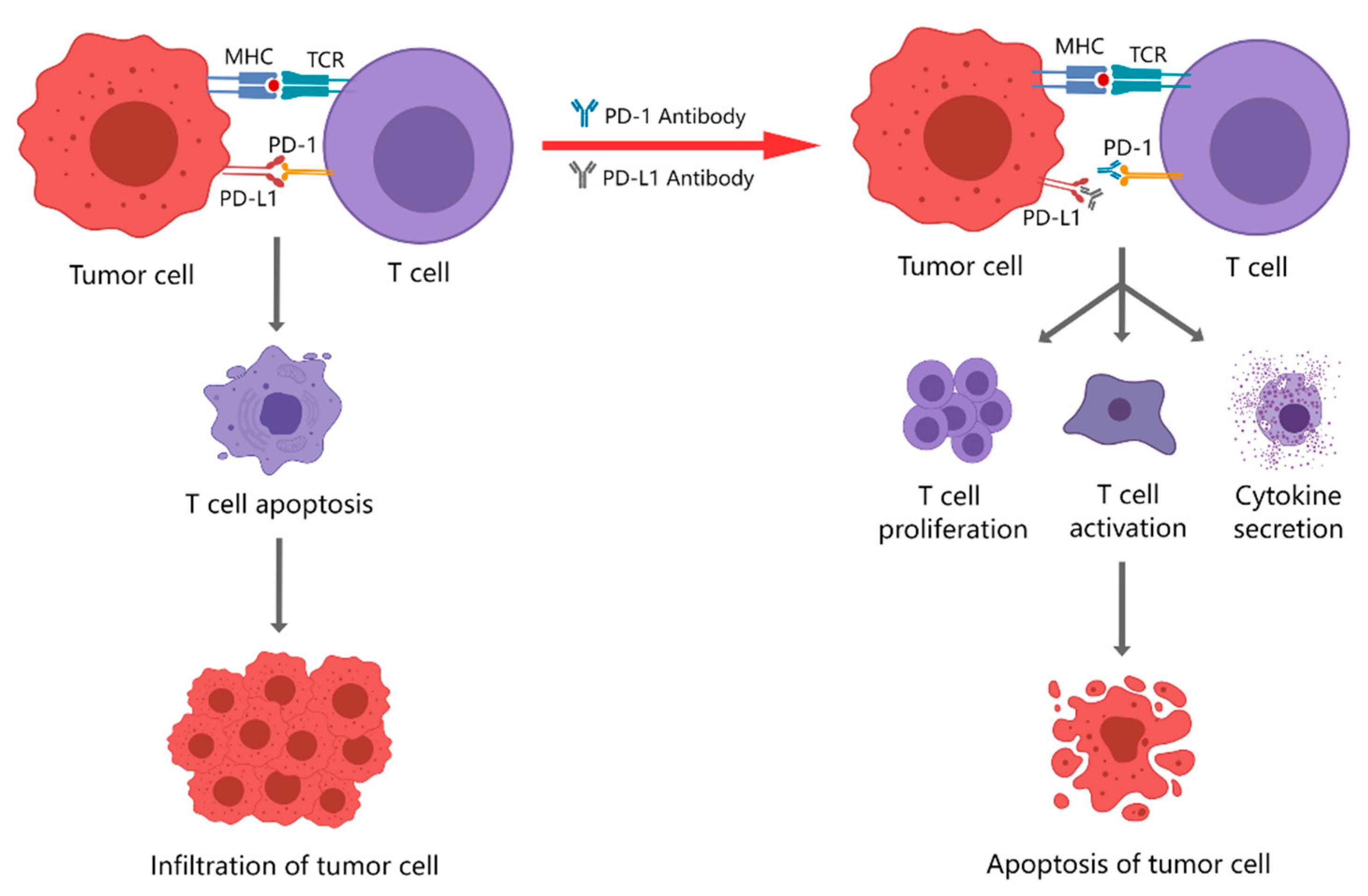
2. PD-1/PD-L1 Inhibitors Currently Used for Clinical Treatment
2.1. PD-1 Inhibitors
2.2. PD-L1 Inhibitors
3. Monotherapy with PD-1/PD-L1 Inhibitors
References
- Van Parijs, L.; Abbas, A.K. Homeostasis and self-tolerance in the immune system: Turning lymphocytes off. Science 1998, 280, 243–248.
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167.
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742.
- Klein, C.; Jamois, C.; Nielsen, T. Anti-CD20 treatment for B-cell malignancies: Current status and future directions. Expert Opin. Biol. Ther. 2021, 21, 161–181.
- Zhu, F.-C.; Hu, S.-Y.; Hong, Y.; Hu, Y.-M.; Zhang, X.; Zhang, Y.-J.; Pan, Q.-J.; Zhang, W.-H.; Zhao, F.-H.; Zhang, C.-F.; et al. Efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine in Chinese women aged 18–25 years: Event-triggered analysis of a randomized controlled trial. Cancer Med. 2017, 6, 12–25.
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365.
- Terness, P.; Chuang, J.J.; Bauer, T.; Jiga, L.; Opelz, G. Regulation of human auto- and alloreactive T cells by indoleamine 2,3-dioxygenase (IDO)-producing dendritic cells: Too much ado about IDO? Blood 2005, 105, 2480–2486.
- Jiang, W.J.; Deng, X.-Y.; Li, X.-L.; Li, X.-Y.; Zeng, Z.-Y.; Xiong, W.; Li, G.-Y.; Xiong, F.; Guo, C. Immunotherapy targeted to immune checkpoint—A revolutionary breakthrough in cancer therapy written on the occasion of the 2018 Nobel Prize in Physiology or Medicine. Prog. Biochem. Biophys. 2018, 45, 1178–1186.
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778.
- Qiu, X.Y.; Hu, D.X.; Chen, W.-Q.; Chen, R.Q.; Qian, S.R.; Li, C.Y.; Li, Y.J.; Xiong, X.X.; Liu, D.; Pan, F.; et al. PD-L1 confers glioblastoma multiforme malignancy via Ras binding and Ras/Erk/EMT activation. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1754–1769.
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214.
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Reviews. Cancer 2016, 16, 275.
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.; LeBoeuf, N.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immun.Therapy. Cancer 2017, 5, 95.
- Kwok, G.; Yau, T.C.C.; Chiu, J.W.; Tse, E.; Kwong, Y.L. Pembrolizumab (Keytruda). Hum. Vaccines 2016, 12, 2777–2789.
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265.
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76.
- McDermott, D.F.; Sosman, J.A.; Sznol, M.; Massard, C.; Gordon, M.S.; Hamid, O.; Powderly, J.D.; Infante, J.R.; Fassò, M.; Wang, Y.V.; et al. Atezolizumab, an Anti-Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. J. Clin. Oncol. 2016, 34, 833–842.
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.-P.; et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019, 5, 74–82.
- Seiwert, T.Y.; Burtness, B.; Weiss, J. A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV-associated head and neck (H/N) cancer. Mccarthy 2014, 32, 6011.
- Muro, K.; Bang, Y.; Shankaran, V.; Geva, R.; Catenacci, D.V.T.; Eder, S.G.P.; Berger, R.; Gonzalez, E.J.; Ray, A.; Dolled-Filhart, M. Relationship between PD-L1 expression and clinical outcomes in patients (Pts) with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab (Pembro; MK-3475) in KEYNOTE-012. J. Clin. Oncol. 2015, 33, 3.
- Chow, L.Q.; Burtness, B.; Weiss, J.; Berger, R.; Eder, J.P.; Gonzalez, E.J.; Pulini, J.; Johnson, J.; Dolled-Filhart, M.; Emancipator, K. A phase Ib study of pembrolizumab (Pembro; MK-3475) in patients (Pts) with human papiilloma virus (HPV)-positive and negative head and neck cancer. Ann. Oncol. 2014, 25 (Suppl. S4), 1–41.
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404.
- Winer, E.P.; Lipatov, O.; Im, S.-A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511.
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686.
