Reconstruction of defects in the maxillofacial region following traumatic injuries, craniofacial deformities, defects from tumor removal, or infections in the maxillofacial area represents a major challenge for surgeons. Various materials have been studied for the reconstruction of defects in the maxillofacial area. Biodegradable metals have been widely researched due to their excellent biological properties. Magnesium (Mg) and Mg-based materials have been extensively studied for tissue regeneration procedures due to biodegradability, mechanical characteristics, osteogenic capacity, biocompatibility, and antibacterial properties. The aim of this review was to analyze and discuss the applications of Mg and Mg-based materials in reconstructive oral and maxillofacial surgery in the fields of guided bone regeneration, dental implantology, fixation of facial bone fractures and soft tissue regeneration.
- magnesium
- biodegradable metals
- maxillofacial surgery
1. Introduction
[1][2]
. Reconstruction or augmentation of craniofacial bones is one of the most frequent surgical procedures in maxillofacial surgery. After blood transfusion, bone grafting is the second-most common tissue transplantation procedure worldwide[3]
. Extensive clinical research on bone grafting and augmentation with autografts, allografts and xenografts has been performed. Autografts taken from the same patient are considered the gold standard for bone reconstruction, since no immune reaction is expected. However, the need for additional surgical intervention, donor site morbidity, limited bone availability and significant graft resorption emphasized the need for different bone substituents[4][5]
. Allografts taken from genetically non-identical members of the same species carry the risk of pathogen transfer and immune system rejection[6]
. Xenografts, usually bovine-derived, are most often used to augment intraoral bone defects[7]
. However, the application of animal-derived materials to humans has certain limitations concerning patients’ religion, dietary restrictions and ethical controversy[7]
. To overcome these drawbacks, extensive research on bone tissue engineering using bio-mimicking, resorbable and biocompatible bone substitutes has been performed in the past years. These synthetic bone substitutes serve as an artificial extracellular matrix to promote bone healing until they are partially or completely replaced by newly formed bone [8][9]. Biodegradable polymers are extensively studied as bone scaffolds and have proven osteoconductive properties as well as excellent biocompatibility[8][9]
. However, low mechanical strength, unstable rates of degradability and immune reaction to products of polymer degradation limit their use in clinical practice [10]. Titanium (Ti) is the most commonly used non-biodegradable metal in maxillofacial surgery for stabilization of fractures or osteotomies, dental implantation procedures and guided bone regeneration (GBR). However, Ti-based materials are bioinert, and secondary surgical intervention is often needed to remove the Ti materials from the organism mainly due to discomfort or surgical site infection, which may occur in up to 33% of cases[11]
.[12]
. Magnesium (Mg)-based materials have been used in medicine since the 19th century. Mg is an essential metal for the human organism, and it is involved in more than 300 cell enzymatic reactions, mitochondrial activity, protein translation, DNA synthesis and cell proliferation[13]
. About 60% of Mg in a healthy adult is deposited in bones[13]
. Mg is resorbed from the intestines, and its homeostasis in the organism depends on renal function [13]. Due to its mechanical properties, an elastic modulus similar to human bones and its biosafety, Mg had been used in orthopedic surgery in the early 20th century until it was replaced with Ti materials [14][15]. The elastic modulus of Mg is about 45 GPa which is much closer to that of cortical bone (10–23 GPa) compared to Ti[16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126][127][128][129][130]
1. Introduction
2. Biological Properties of Mg-Based Materials
The biodegradability of magnesium-based materials is the major advantage of Mg materials. Mg corrodes in the physiological environment and releases species such as Mg ions (Mg2+), alloying elements, H2 gas, and OH− [9]. In an alkaline environment, magnesium hydroxide Mg(OH)2 is deposited on the Mg matrix and forms a protective layer [10]. In the case of fast degradation and corrosion of Mg-based materials, a locally high concentration of Mg ions disturbs calcium-mediated bone reparation and regeneration processes. Products of corrosion of Mg-based materials such as magnesium hydroxide and hydrogen gas may impair tissue healing due to the formation of gas cavities and compression to surrounding soft tissues [17,18][17][18]. Due to the roles of Mg in major cellular functions, magnesium-based materials in the forms of bone cement, bone scaffolds, and implant coatings were evaluated as promising candidates for bone regeneration therapies. Various in vitro studies reported Mg ions to have positive effects on bone cells, including enhanced proliferation, migration and alkaline phosphatase activity, increased differentiation capacity of human osteoblast cells, and increased proliferation of bone marrow-derived stromal cells (BMSCs) [17,18,19][17][18][19]. Having in mind that Mg-based materials are biodegradable, the osteogenic effect of Mg ions is dose-dependent. Concentrations of Mg ions in tissue ranging from 2.5–10 mM have a positive effect on the proliferation and differentiation of human BMSCs [19,20][19][20]. However, higher concentrations of Mg ions in the tissue were connected with decreased mineralization capacity and matrix deposition of BMSCs [21,22][21][22]. The inhibitory effect on osteogenesis of a high local Mg concentration in tissue was linked with alteration in calcium metabolism in cells due to competition between calcium and magnesium ions for the same ion transporters and the inhibition of expression of the calcium-sensing receptor [22,23][22][23]. This resulted in a decreased intracellular calcium concentration and decreased calcium influx in cells [22,23][22][23]. Investigations on the implementation of Mg-based materials found no adverse effects on health. The resorption of Mg results in elevated local concentrations of ions, which is rarely harmful to cells because cells can handle concentrations of Mg about 16-times higher than the physiological ones [10]. Upon implantation in the organism, degradation of Mg does not result in increased Mg deposition in lymph nodes [24]. In vivo studies reported that there were no health risks following Mg implantation in rats with chronic renal failure [25]. The results of an in vivo study indicated that Mg absorption, after implantation of Mg alloy rods, at the degradation rate of 2.32 mm/yr did not lead to dysfunction of the heart, liver, kidney, and spleen of the rabbits [26]. Moreover, Mg alloy rods inserted in the femoral bone of the New Zealand rabbits did not cause changes in the Mg serum levels, kidney and liver function, and histological structure of the vital organs, like the heart and spleen [27]. Clinical trials following the implantation of Mg screws for the treatment of orthopedic fractures found no signs of hypermagnesemia and demonstrated normal levels of Mg blood concentration [3]. Also, no complications, such as allergic reactions, liver/renal dysfunction, or an increase in Mg serum levels, were observed after the application of Mg alloy compressive screws in patients undergoing corrective orthopedic surgeries [28].3. Bioresorbable Mg-Based Materials for Guided Bone Regeneration (GBR)
3.1. GBR Membranes
GBR in the maxillofacial region has been extensively studied over the past decades. Loss of jaw bones due to periodontitis, tooth extractions, operation on tumors and cysts, systemic diseases or infections results in different jaw abnormalities and changes to the occlusion. GBR comprises the use of bone scaffolds or substituents and biomembranes in order to augment bone defects and induce osteogenesis [29]. Biomembranes act as a barrier between hard and soft tissues. They prevent the soft tissues from interfering with osteogenesis, thus providing enough space for the differentiation of osteoprogenitor cells. Biomembranes used in clinical practice could be resorbable based on synthetic (poly(lactic-co-glycolic acid) (PLGA), polyethyleneimine (PEI), poly(L-lactic acid) (PLLA)) or natural (collagen, chitosan) polymers, non-resorbable (Ti mesh, or polytetrafluoroethylene (e-PTFE)) (Figure 1).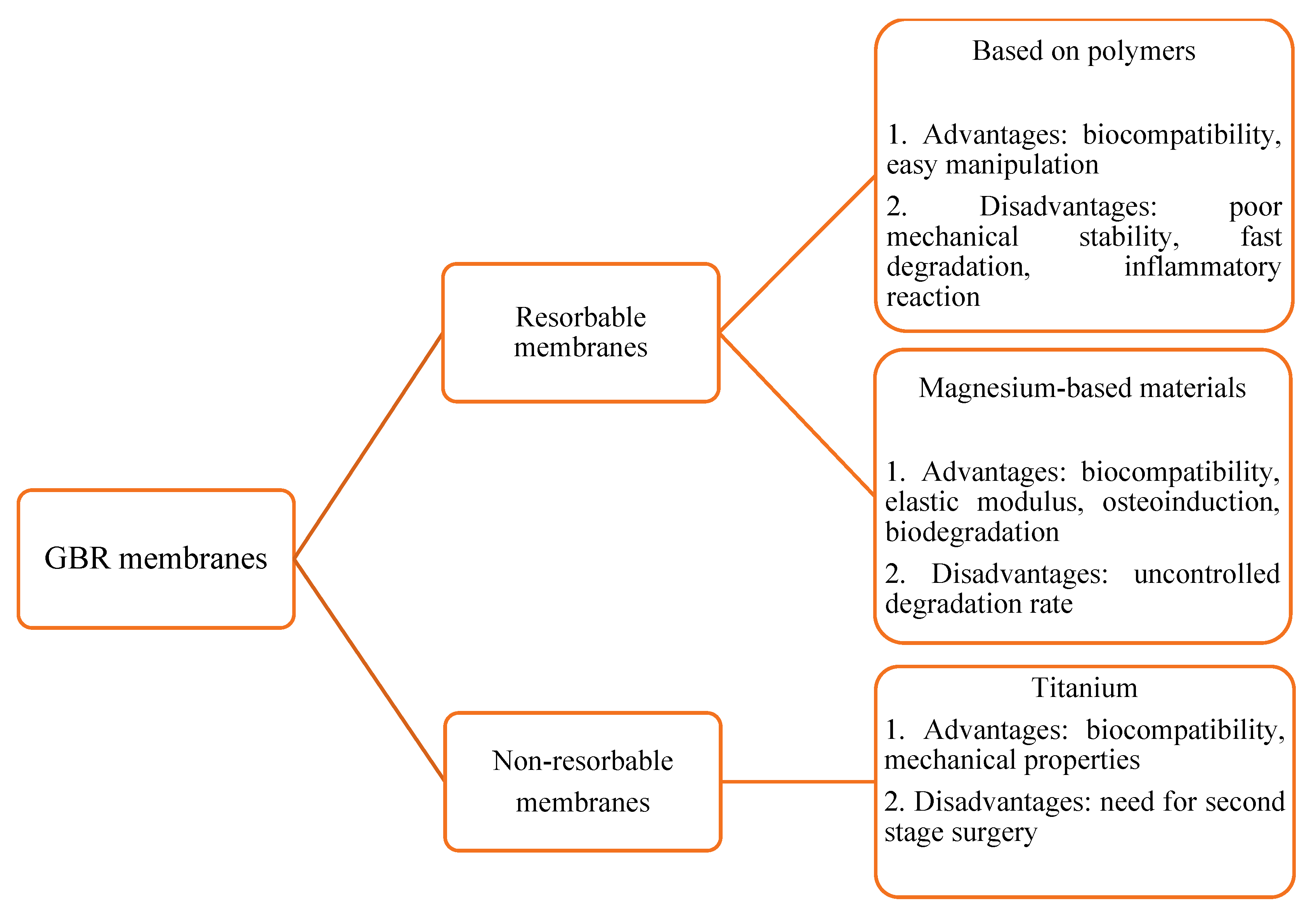
3.2. Mg-Based Scaffolds for GBR
Bone tissue is a natural composite mixture of organic (collagen fibers) and inorganic substances (hydroxyapatite crystals) [2]. Composite scaffolds combining the advantages of biodegradable polymers such as PLGA and PEI with hydroxyapatite (HA) ceramics have been extensively studied because they resemble the natural bone structure, and its mechanical and osteoconductive properties are enhanced by a thin biodegradable polymer coating [4]. In vivo studies with composite HA–polymer scaffolds resulted in complete repair of a critical-sized defect in rabbit’s calvaria, a large defect of rabbit’s ulna, as well a critical size mandibular defect in swine [2,4,40][2][4][40]. However, due to the insufficient mechanical properties of composite bone scaffolds, deformation and brittle fracture may occur [41]. For this reason, Mg-based materials with excellent biocompatibility and mechanical properties were incorporated into HA to enhance their biological and physicochemical properties. In vitro and in vivo experiments demonstrated significantly improved HA properties with the addition of Mg [42]. The addition of Mg to HA resulted in improved chemical properties compared to stoichiometric HA, such as reduced crystallinity, high specific surface area, and enhanced solubility in natural tissues. These factors lead to improved cell adhesion, proliferation, and metabolic activity [43]. A mixture of HA and β-TCP doped with Mg (magnesium-doped biphasic calcium phosphate) mimics the natural inorganic bone matrix with excellent physicochemical properties [44]. Furthermore, the presence of Mg ions during synthesis also improves the thermal stability of HA and produces a more stable phase composition after heat treatment, which enables the production of porous or granulated scaffolds for biomedical applications, including oral and maxillofacial surgery and orthopedics [44,45][44][45]. Magnesium Hydroxyapatite (MgHA) scaffold was analyzed for bone regeneration in alveolar critical-sized bone defects in several animal and human trials. The results suggest that the MgHA scaffold could be a very effective bone substitute [46]. Various in vitro studies reported excellent biocompatibility for several cell lines [47,48,49][47][48][49]. Sartori et al. demonstrated in an in vivo study conducted on sheep that MgHA provides osteoconductive structural support during the process of bone regeneration [50]. Santos et al. concluded in an in vivo experiment that MgHA, when implanted in a critical bone defect in rat calvaria, is a biocompatible and osteoconductive biomaterial [51]. A clinical study by Grigolato et al. showed that MgHA, used as a bone substitute in a mandibular defect due to ameloblastoma, exhibits excellent biological behavior and high osseointegration potential [52]. MgHA is a relatively well-studied Mg-based bone substitute material, and there are several commercial products researched for the reconstruction of maxillofacial bone defects. Teeth extractions cause significant changes in the dimensions of the alveolar ridge due to resorption of the alveolar socket, which may impair dental implantation and prosthetic reconstruction [53]. Resorption of the alveolar socket is rapid following tooth extraction due to loss of function, and about 40–60% of bone is resorbed in the first two years [54]. The preservation of the alveolar socket volume following tooth extractions and alveolar ridge preservation or augmentation could be achieved using MgHA scaffolds. In a clinical study by Crespo et al. a split-mouth design was used to compare histologic and histomorphometric results of MgHA and porcine bone grafts for the preservation of fresh dental sockets [55]. The results of this restudy earch showed similar biologic behavior in bone formation and resorption processes. A similar clinical study that compared radiographic and histomorphometric results of MgHA and calcium sulfate grafts in fresh sockets after tooth extractions found a lower reduction of the alveolar ridge, more bone formation and more residual implant material in the MgHA group [56]. A prospective 2-year clinical study evaluated the survival of dental implants loaded 14 weeks after vertical alveolar ridge augmentation with nano-structured MgHA covered with Ti-polytetrafluoroethylene (e-PTFE) membrane [54]. The results of this restudyearch suggested that vertical ridge augmentation around Ti implants using MgHA can be successful in cases with early implant loading. However, an in vivo animal study with canines did not find a significant effect of MgHA on alveolar socket preservation and osseointegration of implants placed immediately into extraction sockets [57]. Recently, a clinical study investigated the effectiveness of a biomimetic MgHA/collagen-based bone substitute for alveolar socket preservation compared to deproteinized bovine bone matrix [58]. The results after 6 months showed similar vertical and horizontal alveolar ridge resorption, similar new bone formation between the groups and a significantly higher residual material for deproteinized bovine bone matrix. Crespo et al. compared the use of MgHA and autologous bone graft for maxillary sinus lift procedures [59]. The results of this clinical study suggested MgHA as a possible alternative to autologous bone graft for sinus lift operations. There are promising results from using bovine bone grafts enriched with Mg for bone regeneration. An in vivo study on the biological properties of bovine xenogeneic biomaterial enriched with Mg on the healing of critical-sized defects on rat calvaria showed Mg biomaterial demonstrated osteoinductive properties and biodegradability during healing [60]. Similar results were reported for the rabbit calvaria defect repair in an in vivo study [61]. Mg-based bone types of cement have been used in orthopedics for bone and tendon repair [62]. The results of canine in vivo study that evaluated Mg-based bone cement for bone grafting of immediate implantation of extraction sockets showed success at filling in the bone defects without implant loss during the observation period [63]. However, the use of Mg-based bone types of cement may be doubtful due to their 3D structure and lack of porosity, which enables osteoconductive properties [10].4. Mg and Mg-Based Materials for Ti Implant Coating
Surface characteristics of Ti implants have a major impact on the process of implant osseointegration, and research in the field of implant surface modification is important despite good and predictable rates of implant success [65][64]. Various surface coatings on dental implants were investigated in order to improve implant surface for stronger micromechanical retention and improved biological processes for osteogenesis [65,66][64][65]. Surface coating with bioceramics such as hydroxyapatite, calcium phosphate, and bioactive glass resulted in improved osseointegration. However, the practice has significant complications due to poor mechanical strength, brittleness and bacterial infections around implants [67][66]. Mg and Mg-based materials were studied as possible implant surface coatings due to elastic modulus of the material, osteogenic effect, biocompatibility and biodegradation of these materials. Results of in vitro and in vivo studies found positive effects of Mg coatings such as Mg carbonate, Mg fluoride, Mg oxide, Mg silicate, and HA incorporated with Mg and Zinc (Zn) [65][64]. The results of in vitro studies demonstrated that Ti implants coated with Mg-based coatings showed enhanced BMSCs proliferation and increased expression of osteogenic markers (alkaline phosphatase, osteocalcin, osteopontin, bone sialoprotein, RUNX-2), increased collagen type I deposition and antibacterial activity [68,69,70,71,72,73,74][67][68][69][70][71][72][73]. In an in vivo animal study comparing antibacterial properties of Mg and Mg-Zn co-implanted, Yu et al. showed both surfaces to have an excellent antibacterial effect against specific periodontal pathogens, such as Porphyromonas gingivalis, Streptococcus mutans and Fusobacterium nucleatum [68][67]. Additionally, another study found that MgO-HA and MgF2-HA coatings had a significantly better antibacterial effect against Enterococcus spp., Micrococcus spp. and Candida albicans than HA coatings [72][71]. New bone formation is quantitatively measured with the metrics of bone–implant contact and bone area. The results of in vivo studies revealed improved osseointegration, better new bone architecture, higher bone volume/total volume and bone-to-implant ratio with Mg coatings than conventional Ti surfaces [75,76,77,78][74][75][76][77]. Cho et al. found in a study on rabbits that the concentration of Mg ions had a significant effect on osseointegration since implants coated with 9.24% Mg had remarkably better removal torque value, bone–implant contact, bone fill area and new bone formation [75][74].5. Bioresorbable Mg-Based Materials for Osteosynthesis of the Facial Bone Fractures
Resorbable Mg-based materials have been extensively studied for use in orthopedic surgery since the beginning of the 20th century due to their biological properties and the elastic modulus similar to natural bone [79][78]. However, they were replaced by bioinert Ti materials due to superior mechanical properties for the treatment of complicated and load-bearing fractures. Since the implantation of Ti plates and screws for fracture immobilization requires a secondary surgical intervention and removal of Ti material due to infection, discomfort or plate exposure, bioresorbable Mg-based materials were de novo analyzed for the treatment of traumatic bone injuries [11]. Pre-clinical and clinical studies were mostly performed for the stabilization of orthopedic fractures, while limited data are available for the treatment of maxillofacial injuries. For orthopedic injuries, both pure Mg and its alloys were evaluated. The biological and mechanical properties of Mg and its alloys are mainly influenced by material behavior in the tissue following implantation. In the natural conditions in the tissue, Mg corrodes and releases Mg ions and H2 gas into surrounding tissues and may cause significant emphysema in the rapid corrosion process [79][78]. There are pieces of evidence that H2 may induce osteogenesis and reduce osteoclastogenesis and thus benefit bone reparation [80][79]. The corrosion of Mg and Mg alloys depends on material structure (heterogeneity, metal purity and microstructure of the alloy), mechanical loads, pH of the surrounding tissues and vascularization [81,82,83][80][81][82]. The corrosion rate and degradation of Mg and its alloys are the main factors influencing their clinical application [81,82,83][80][81][82]. Pure Mg (99.99%) has a low corrosion rate and low mechanical strength [84][83]. However, pre-clinical studies found pure Mg promotes osteogenesis and fracture healing on rabbit femoral condyle fractures using Mg screws [84][83]. Mg alloys with rare earth elements (RE) such as scandium (Sc), yttrium (Y), gadolinium (Gd), zirconium (Zr) and neodymium (Nd) were synthesized in order to decrease corrosion and reduce degradation rate of 99.99% pure Mg. The most widely studied Mg-based alloys comprise AZ (Mg-Al-Zn system) and WE alloys (Mg-RE-Zr system) [85][84]. AZ alloys such as AZ31 (Mg-3Al-1Zn) and AZ91 (Mg9Al-1Zn) have excellent mechanical properties, but the high degradation rate and local toxicity of aluminum limit their clinical use [86][85]. On the other hand, WE43 alloy (Mg-4Y-3RE) is coated with a RE-oxide layer, improving corrosion resistance and biocompatibility [87][86]. WE43 alloy (Mg-3.5% Y-2.3% Nd-0.5% Zr, wt.%), MgYREZr alloy and Mg-Nd-Zn-Zr alloy were assessed in in vitro and in in vivo studies for bone repair, which resulted in good bone repair when used as pins or screws for bone fixation in orthopedic patients [88,89][87][88]. ZX00 (Mg-Zn-Ca alloy) is another resorbable alloy that revealed good results in pre-clinical studies on bone regeneration [90][89]. Recent clinical trials investigating the use of Mg and its alloys for stabilization of orthopedic fractures revealed excellent results in fracture reduction of displaced femoral neck fractures, hallux valgus and medial malleolar fractures, with the bone regeneration rates comparable to Ti screws [91,92,93,94,95][90][91][92][93][94]. Most of these trials investigated the use of MgYREZr alloy screws to stabilize unstable fractures. Due to the release of H2 ions due to corrosion, a radiolucent zone around screws was observed in the majority of postoperative radiological exams. However, no severe complications were observed [96][95]. Reports on the use of Mg and its alloys in the treatment of facial bone fractures are s carce. Traumatic injuries to the facial bones are among the most common injuries to the body, mostly reported in traffic accidents and interpersonal violence. Fractures in the maxillofacial area have a significant impact on patients’ appearance, speech and mastica-tion [1,2][1][2]. The treatment of facial bone fractures requires the repositioning of fractured bone fragments to the anatomical state and osteosynthesis with Ti plates and screws. Ti plates and screws are used due to the excellent biocompatibility and biomechanical properties of Ti and usually are left for life [11]. However, they sometimes need to be extracted due to an infection or discomfort [66][65]. Biodegradable plates and screws may be beneficial in avoiding second-stage surgery. Biodegradable materials for use in the maxillofacial area must overcome some factors specific to this region. These include factors such as significant masticatory muscle forces, presence of saliva and intraoral pathogens. This is because most of the surgical interventions are performed through an intraoral approach, with different elastic modules of facial bones as well as various shapes of bones [80,97][79][96]. Biodegradable polymer fixation plates made from PLLA and PLGA have poor mechanical properties and may cause an inflammatory reaction [98][97]. Mg-based materials possess good mechanical strength and biocompatibility with proven clinical applications. However, the compressive yield strength of Mg-based alloys is lower than Ti alloys which questions their use for load-bearing fractures such as mandible fractures [99][98]. Pre-clinical studies revealed Mg-based materials as promising candidates for maxillofacial bone osteosynthesis [99–110][98][99][100][101][102][103][104][105][106][107][108][109]. Mandibular fractures are the most common fractures in the maxillofacial area, and their treatment consists of thick Ti plates and locking screws to restore the bone’s anatomical shape along with occlusion, and avoid postoperative movement of the fragments by heavy masticatory forces [102][101]. Only one animal study by Nujokat et al. used MgYZrRee (WE43) custom-made fixation plates and screws for the stabilization of mandibular osteotomy at the mandibular angle [103][102]. The results of this in vivo study proved good mechanical stability at the osteotomy site. However, the performed osteotomy was monocortical and did not represent a full bicortical fracture line. Mg screws were investigated in several studies and reported better mechanical properties compared to the polymeric material but lower mechanical and torsional strength than Ti controls [98–100][97][98][99]. Interesting are the results of Mg screws for stabilization of osteotomy lines for bilateral ramus sagittal split osteotomy (BSSO) performed for orthognathic surgery procedures where the mandibular setback or advance is performed to correct maxillofacial deformities. The results of two studies based on finite element modeling found the use of Mg or Mg-Ca-Zn screws could stabilize the osteotomy lines even with masticatory loading [100,101][99][100]. Further pre-clinical and clinical trials are needed in order to obtain an Mg-based fixation system for mandible fractures and osteotomies to overcome current disadvantages regarding mechanical stress and low torsional strength. On the other hand, the results of animal studies on the fixation of the midface complex fractures are more promising. Midface fractures, mainly fractures of the maxilla and zygomatic bone, are load-shearing types of fractures where no significant masticatory forces are implied to reduce the stability of the fracture line. The use of WE43 plates and screws for the fixation of fractures in the midface resulted in good osteotomy lines stability, biocompatibility, and osseointegration [103–106][102][103][104][105]. The gas formation was observed for 12 weeks postoperatively without side effects on bone regeneration and wound healing, proposing that the material’s degradation rate is adequate. The use of PLLA-coated ZK60 plates and screws for fixation of Le Fort I osteotomy in beagles resulted in significant gas formation and local inflammation due to the fast biodegradation of the material [107][106]. Although ZK60 plates showed good mechanical properties, it seemed that PLLA coating failed to prevent the rapid absorption of the alloy due to micro-cracks on the surface [107][106]. Further research is needed to obtain alloys with more predictable rates of biodegradation and mechanical properties for these types of fractures. Fixation systems based on Mg materials used in these studies were thicker and had a bigger volume compared to Ti fixation systems, although there was no significant discomfort to the subjects. Promising results of pre-clinical studies have been published regarding the use of WE43 plates and screws for the fixation of fractures in the frontal bone [108,109][107][108]. The stability of the plates and biocompatibility were comparable to the Ti fixation system. The repair of orbital fractures represents a significant challenge to the surgeons due to the proximity of intracranial structures, paranasal sinuses, the poor blood supply of the bones and osteoprogenitor cell insufficiency [110][109]. The thin bony walls of the orbit, especially the inferior and medial walls, are the most prominent locations for fractures. Blow-out fractures of the orbital floor are the most common fracture of the orbit. Current materials used for fracture reduction and reconstruction of the orbital volume are bioinert Ti meshes, plates, and polyethylene meshes. Zhang et al. developed Ca-P coated Mg-Zn-Gd scaffold to reconstruct a large defect of the medial orbital wall in a canine model [110][109]. The results showed excellent osteoconductivity, angiogenesis and bone regeneration with the scaffold. The reseauthorchers observed no gas formation and orbital emphysema. Only two clinical studies by Leonhardt et al. reported the effectiveness of Mg-based materials for the treatment of fractures in maxillofacial surgery [111,112][110][111]. These studies reported repositioning and fixation of mandibular condyle fracture with Magnezix® CS 2.7 mm screw (MgYREZr alloy). The authoresearchers reported excellent stabilization of fragments and complete restoration of temporomandibular joint (TMJ) function. Gas formation around screws was reported and seen as radiolucent areas on control CBCT exams. One year follow-up was uneventful, and there was no need for screw removal.6. Mg-Based Materials for Soft Tissue Regeneration
7. Conclusions
7. Conclusions
References
- Probst, F.A.; Fliefel, R.; Burian, E.; Probst, M.; Eddicks, M.; Cornelsen, M.; Riedl, C.; Seitz, H.; Aszódi, A.; Schieker, M.; et al.Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate-poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep. 2020, 10, 2062. [CrossRef] [PubMed]Probst, F.A.; Fliefel, R.; Burian, E.; Probst, M.; Eddicks, M.; Cornelsen, M.; Riedl, C.; Seitz, H.; Aszódi, A.; Schieker, M.; et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep. 2020, 10, 2062.
- Stevanovic, M.; Selakovic, D.; Vasovic, M.; Ljujic, B.; Zivanovic, S.; Papic, M.; Zivanovic, M.; Milivojevic, N.; Mijovic, M.;Tabakovic, S.; et al. Comparison of hydroxyapatite/poly(lactide-co-glycolide) and hydroxyapatite/polyethyleneimine compositescaffolds in bone regeneration of swine mandibular critical size defects: In vivo study. Molecules 2022, 16, 1694. [CrossRef][PubMed]Stevanovic, M.; Selakovic, D.; Vasovic, M.; Ljujic, B.; Zivanovic, S.; Papic, M.; Zivanovic, M.; Milivojevic, N.; Mijovic, M.; Tabakovic, S.; et al. Comparison of hydroxyapatite/poly(lactide-co-glycolide) and hydroxyapatite/polyethyleneimine composite scaffolds in bone regeneration of swine mandibular critical size defects: In vivo study. Molecules 2022, 16, 1694.
- Herber, V.; Okutan, B.; Antonoglou, G.; Sommer, N.G.; Payer, M. Bioresorbable magnesium-based alloys as novel biomaterials inoral bone regeneration: General review and clinical perspectives. J. Clin. Med. 2021, 10, 1842. [CrossRef] [PubMed]Herber, V.; Okutan, B.; Antonoglou, G.; Sommer, N.G.; Payer, M. Bioresorbable magnesium-based alloys as novel biomaterials in oral bone regeneration: General review and clinical perspectives. J. Clin. Med. 2021, 10, 1842.
- Jokanovi ́c, V.; ˇColovi ́c, B.; Markovi ́c, D.; Petrovi ́c, M.; Soldatovi ́c, I.; Antonijevi ́c, D.; Milosavljevi ́c, P.; Sjerobabin, N.; Sopta, J.Extraordinary biological properties of a new calcium hydroxyapatite/poly(lactide-co-glycolide)-based scaffold confirmed byin vivo investigation. Biomed. Eng. Biomed. Tech. 2017, 62, 295–306. [CrossRef]Jokanović, V.; Čolović, B.; Marković, D.; Petrović, M.; Soldatović, I.; Antonijević, D.; Milosavljević, P.; Sjerobabin, N.; Sopta, J. Extraordinary biological properties of a new calcium hydroxyapatite/poly(lactide-co-glycolide)-based scaffold confirmed by in vivo investigation. Biomed. Eng. Biomed. Tech. 2017, 62, 295–306.
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of iliac crest bone graft harvesting.Clin. Orthop. Relat. Res. 1996, 329, 300–309. [CrossRef]Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of iliac crest bone graft harvesting. Clin. Orthop. Relat. Res. 1996, 329, 300–309.
- Boyce, T.; Edwards, J.; Scarborough, N. Allograft bone. The influence of processing on safety and performance. Orthop. Clin. 1999,30, 571–581. [CrossRef]Boyce, T.; Edwards, J.; Scarborough, N. Allograft bone. The influence of processing on safety and performance. Orthop. Clin. 1999, 30, 571–581.
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials forperiodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [CrossRef]Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9.
- Karadži ́c, I.; Vuˇci ́c, V.; Jokanovi ́c, V.; Debeljak-Martaˇci ́c, J.; Markovi ́c, D.; Petrovi ́c, S.; Glibeti ́c, M. Effects of novel hydroxyapatite-based 3D biomaterials on proliferation and osteoblastic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A2015, 103, 350–357. [CrossRef]Karadžić, I.; Vučić, V.; Jokanović, V.; Debeljak-Martačić, J.; Marković, D.; Petrović, S.; Glibetić, M. Effects of novel hydroxyapatite-based 3D biomaterials on proliferation and osteoblastic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2015, 103, 350–357.
- Liu, L.; Shi, G.; Cui, Y.; Li, H.; Li, Z.; Zeng, Q.; Guo, Y. Individual construction of freeform-fabricated polycaprolactone scaffoldsfor osteogenesis. Biomed. Eng. Biomed. Tech. 2017, 62, 467–479. [CrossRef]Liu, L.; Shi, G.; Cui, Y.; Li, H.; Li, Z.; Zeng, Q.; Guo, Y. Individual construction of freeform-fabricated polycaprolactone scaffolds for osteogenesis. Biomed. Eng. Biomed. Tech. 2017, 62, 467–479.
- Xia, D.; Yang, F.; Zheng, Y.; Liu, Y.; Zhou, Y. Research status of biodegradable metals designed for oral and maxillofacialapplications: A review. Bioact. Mater. 2021, 6, 4186–4208. [CrossRef]Xia, D.; Yang, F.; Zheng, Y.; Liu, Y.; Zhou, Y. Research status of biodegradable metals designed for oral and maxillofacial applications: A review. Bioact. Mater. 2021, 6, 4186–4208.
- Cousin, A.S.; Bouletreau, P.; Giai, J.; Ibrahim, B.; Louvrier, A.; Sigaux, N. Severity and long-term complications of surgical siteinfections after orthognathic surgery: A retrospective study. Sci. Rep. 2020, 10, 12015. [CrossRef] [PubMed]Cousin, A.S.; Bouletreau, P.; Giai, J.; Ibrahim, B.; Louvrier, A.; Sigaux, N. Severity and long-term complications of surgical site infections after orthognathic surgery: A retrospective study. Sci. Rep. 2020, 10, 12015.
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. 2014, 77, 1–34. [CrossRef]Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. 2014, 77, 1–34.
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An update on physiological, clinical andanalytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [CrossRef]Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26.
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [CrossRef] [PubMed]Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692.
- Hang, R.; Wang, C.; Yu, Z.; Li, Z.; Xiao, Y. Biodegradable metallic wires in dental and orthopedic applications: A review. Metals2018, 8, 212. [CrossRef]Hang, R.; Wang, C.; Yu, Z.; Li, Z.; Xiao, Y. Biodegradable metallic wires in dental and orthopedic applications: A review. Metals 2018, 8, 212.
- Rider, P.; Kaˇcarevi ́c, Ž.P.; Elad, A.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.; Molnar, B.; Hesse, B.; et al.Biodegradation of a magnesium alloy fixation screw used in a guided bone regeneration model in Beagle dogs. Materials 2022,15, 4111. [CrossRef] [PubMed]Rider, P.; Kačarević, Ž.P.; Elad, A.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.; Molnar, B.; Hesse, B.; et al. Biodegradation of a magnesium alloy fixation screw used in a guided bone regeneration model in Beagle dogs. Materials 2022, 15, 4111.
- Weng, L.; Webster, T.J. Nanostructured magnesium increases bone cell density. Nanotechnology 2012, 23, 485105. [CrossRef]Weng, L.; Webster, T.J. Nanostructured magnesium increases bone cell density. Nanotechnology 2012, 23, 485105.
- Weng, L.; Webster, T.J. Nanostructured magnesium has fewer detrimental effects on osteoblast function. Int. J. Nanomed. 2013,8, 1773. [CrossRef]Weng, L.; Webster, T.J. Nanostructured magnesium has fewer detrimental effects on osteoblast function. Int. J. Nanomed. 2013, 8, 1773.
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.;Schnettler, R.; et al. Applications of metals for bone regeneration. Int. J. Mol. Sci. 2018, 19, 826. [CrossRef]Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of metals for bone regeneration. Int. J. Mol. Sci. 2018, 19, 826.
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenicactivity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [CrossRef]Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842.
- Li, Y.; Wang, J.; Yue, J.; Wang, Y.; Yang, C.; Cui, Q. High magnesium prevents matrix vesicle-mediated mineralization in humanbone marrow-derived mesenchymal stem cells via mitochondrial pathway and autophagy. Cell Biol. Int. 2018, 42, 205–215.[CrossRef] [PubMed]Li, Y.; Wang, J.; Yue, J.; Wang, Y.; Yang, C.; Cui, Q. High magnesium prevents matrix vesicle-mediated mineralization in human bone marrow-derived mesenchymal stem cells via mitochondrial pathway and autophagy. Cell Biol. Int. 2018, 42, 205–215.
- Tsao, Y.T.; Shih, Y.Y.; Liu, Y.A.; Liu, Y.S.; Lee, O.K. Knockdown of SLC41A1 magnesium transporter promotes mineralization andattenuates magnesium inhibition during osteogenesis of mesenchymal stromal cells. Stem Cell Res. Ther. 2017, 8, 39. [CrossRef][PubMed]Tsao, Y.T.; Shih, Y.Y.; Liu, Y.A.; Liu, Y.S.; Lee, O.K. Knockdown of SLC41A1 magnesium transporter promotes mineralization and attenuates magnesium inhibition during osteogenesis of mesenchymal stromal cells. Stem Cell Res. Ther. 2017, 8, 39.
- Nakatani, S.; Mano, H.; Ryanghyok, I.; Shimizu, J.; Wada, M. Excess magnesium inhibits excess calcium-induced matrix-mineralization and production of matrix gla protein (MGP) by ATDC5 cells. Biochem. Biophys. Res. Commun. 2006, 348, 1157–1162.[CrossRef] [PubMed]Nakatani, S.; Mano, H.; Ryanghyok, I.; Shimizu, J.; Wada, M. Excess magnesium inhibits excess calcium-induced matrix-mineralization and production of matrix gla protein (MGP) by ATDC5 cells. Biochem. Biophys. Res. Commun. 2006, 348, 1157–1162.
- Torroni, A.; Xiang, C.; Witek, L.; Rodriguez, E.D.; Coelho, P.G.; Gupta, N. Biocompatibility and degradation properties of WE43Mg alloys with and without heat treatment: In vivo evaluation and comparison in a cranial bone sheep model. J. Cranio-Maxillofac.Surg. 2017, 45, 2075–2083. [CrossRef]Torroni, A.; Xiang, C.; Witek, L.; Rodriguez, E.D.; Coelho, P.G.; Gupta, N. Biocompatibility and degradation properties of WE43 Mg alloys with and without heat treatment: In vivo evaluation and comparison in a cranial bone sheep model. J. Cranio-Maxillofac. Surg. 2017, 45, 2075–2083.
- Wang, J.; Xu, J.; Liu, W.; Li, Y.; Qin, L. Biodegradable magnesium (Mg) implantation does not impose related metabolic disordersin rats with chronic renal failure. Sci. Rep. 2016, 6, 26341. [CrossRef] [PubMed]Wang, J.; Xu, J.; Liu, W.; Li, Y.; Qin, L. Biodegradable magnesium (Mg) implantation does not impose related metabolic disorders in rats with chronic renal failure. Sci. Rep. 2016, 6, 26341.
- Amukarimi, S.; Mozafari, M. Biodegradable magnesium biomaterials-road to the clinic. Bioengineering 2022, 5, 107. [CrossRef]Amukarimi, S.; Mozafari, M. Biodegradable magnesium biomaterials-road to the clinic. Bioengineering 2022, 5, 107.
- He, Y.; Tao, H.; Zhang, Y.; Jiang, Y.; Zhang, S.; Zhao, C.; Li, J.; Zhang, B.; Song, Y.; Zhang, X. Biocompatibility of bio-Mg-Zn alloywithin bone with heart, liver, kidney and spleen. Chin. Sci. Bull. 2009, 54, 484–491. [CrossRef]He, Y.; Tao, H.; Zhang, Y.; Jiang, Y.; Zhang, S.; Zhao, C.; Li, J.; Zhang, B.; Song, Y.; Zhang, X. Biocompatibility of bio-Mg-Zn alloy within bone with heart, liver, kidney and spleen. Chin. Sci. Bull. 2009, 54, 484–491.
- Luthringer, B.J.; Feyerabend, F.; Willumeit-Römer, R. Magnesium-based implants: A mini-review. Magnes. Res. 2014, 27, 142–154.[CrossRef]Luthringer, B.J.; Feyerabend, F.; Willumeit-Römer, R. Magnesium-based implants: A mini-review. Magnes. Res. 2014, 27, 142–154.
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue engineering for bone regenerationand osseointegration in the oral cavity. Dent. Mater. 2015, 31, 317–338. [CrossRef]Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent. Mater. 2015, 31, 317–338.
- Ge, J.; Yang, C.; Wang, Y.; Zheng, J.; Hua, H.; Zhu, J. Comparison of different grafting materials for treatment of bone defect distalto the molar in canine. Clin. Implant Dent. Relat. Res. 2018, 20, 444–454. [CrossRef]Ge, J.; Yang, C.; Wang, Y.; Zheng, J.; Hua, H.; Zhu, J. Comparison of different grafting materials for treatment of bone defect distal to the molar in canine. Clin. Implant Dent. Relat. Res. 2018, 20, 444–454.
- Barbeck, M.; Kuhnel, L.; Witte, F.; Pissarek, J. Degradation, bone regeneration and tissue response of an innovative volume stablemagnesium-supported GBR/GTR barrier membrane. Int. J. Mol. Sci. 2020, 9, 3098. [CrossRef] [PubMed]Barbeck, M.; Kuhnel, L.; Witte, F.; Pissarek, J. Degradation, bone regeneration and tissue response of an innovative volume stable magnesium-supported GBR/GTR barrier membrane. Int. J. Mol. Sci. 2020, 9, 3098.
- Zhao, C.; Hou, P.; Ni, J.; Han, P.; Chai, Y.; Zhang, X. Ag- incorporated FHA coating on pure Mg: Degradation and in vitroantibacterial properties. ACS Appl. Mater. Interfaces 2016, 8, 5093–5103. [CrossRef] [PubMed]Zhao, C.; Hou, P.; Ni, J.; Han, P.; Chai, Y.; Zhang, X. Ag- incorporated FHA coating on pure Mg: Degradation and in vitro antibacterial properties. ACS Appl. Mater. Interfaces 2016, 8, 5093–5103.
- Yan, Z.Y.; Zhu, J.H.; Liu, G.Q.; Liu, Z.C.; Guo, C.B.; Cui, N.H.; Han, J.M. Feasibility and efficacy of a degradable magnesium-alloyGBR membrane for bone augmentation in a distal bone-defect model in Beagle dogs. Bioinorg. Chem. Appl. 2022, 2022, 4941635.[CrossRef] [PubMed]Yan, Z.Y.; Zhu, J.H.; Liu, G.Q.; Liu, Z.C.; Guo, C.B.; Cui, N.H.; Han, J.M. Feasibility and efficacy of a degradable magnesium-alloy GBR membrane for bone augmentation in a distal bone-defect model in Beagle dogs. Bioinorg. Chem. Appl. 2022, 2022, 4941635.
- Guo, C.W.; Yu, Q.; Sun, B.Z.; Wang, C.Y.; Yang, J.X. Evaluation of alveolar bone repair with mineralized collagen block reinforcedwith Mg–Ca alloy rods. J. Biomater. Tissue Eng. 2018, 8, 1–10. [CrossRef]Guo, C.W.; Yu, Q.; Sun, B.Z.; Wang, C.Y.; Yang, J.X. Evaluation of alveolar bone repair with mineralized collagen block reinforced with Mg–Ca alloy rods. J. Biomater. Tissue Eng. 2018, 8, 1–10.
- Si, J.; Shen, H.; Miao, H.; Tian, Y.; Huang, H.; Shi, J.; Shen, G. In vitro and in vivo evaluations of Mg-Zn-Gd alloy membrane onguided bone regeneration for rabbit calvarial defect. J. Magnes. Alloy. 2021, 9, 281–291. [CrossRef]Si, J.; Shen, H.; Miao, H.; Tian, Y.; Huang, H.; Shi, J.; Shen, G. In vitro and in vivo evaluations of Mg-Zn-Gd alloy membrane on guided bone regeneration for rabbit calvarial defect. J. Magnes. Alloy. 2021, 9, 281–291.
- Wu, S.; Jang, Y.S.; Kim, Y.K.; Kim, S.Y.; Ko, S.O.; Lee, M.H. Surface modification of pure magnesium mesh for guided boneregeneration: In vivo evaluation of rat calvarial defect. Materials 2019, 12, 2684. [CrossRef]Wu, S.; Jang, Y.S.; Kim, Y.K.; Kim, S.Y.; Ko, S.O.; Lee, M.H. Surface modification of pure magnesium mesh for guided bone regeneration: In vivo evaluation of rat calvarial defect. Materials 2019, 12, 2684.
- Chen, Y.; Ye, S.H.; Sato, H.; Zhu, Y.; Shanov, V.; Tiasha, T.; Wagner, W.R. Hybrid scaffolds of Mg alloy mesh reinforcedpolymer/extracellular matrix composite for critical-sized calvarial defect reconstruction. J. Tissue Eng. Regen. Med. 2018, 12,1374–1388. [CrossRef]Chen, Y.; Ye, S.H.; Sato, H.; Zhu, Y.; Shanov, V.; Tiasha, T.; Wagner, W.R. Hybrid scaffolds of Mg alloy mesh reinforced polymer/extracellular matrix composite for critical-sized calvarial defect reconstruction. J. Tissue Eng. Regen. Med. 2018, 12, 1374–1388.
- Brown, A.; Zaky, S.; Ray, H., Jr.; Sfeir, C. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration followingtooth extraction. Acta Biomater. 2015, 11, 543–553. [CrossRef]Brown, A.; Zaky, S.; Ray, H., Jr.; Sfeir, C. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015, 11, 543–553.
- Wang, F.; Xia, D.; Wang, S.; Gu, R.; Yang, F.; Zhao, X.; Liu, X.; Zhu, Y.; Liu, H.; Xu, Y.; et al. Photocrosslinkable Col/PCL/Mgcomposite membrane providing spatiotemporal maintenance and positive osteogenetic effects during guided bone regeneration.Bioact. Mater. 2022, 13, 53–63. [CrossRef]Wang, F.; Xia, D.; Wang, S.; Gu, R.; Yang, F.; Zhao, X.; Liu, X.; Zhu, Y.; Liu, H.; Xu, Y.; et al. Photocrosslinkable Col/PCL/Mg composite membrane providing spatiotemporal maintenance and positive osteogenetic effects during guided bone regeneration. Bioact. Mater. 2022, 13, 53–63.
- Micic, M.; Antonijevic, D.; Milutinovic-Smiljanic, S.; Trisic, D.; Colovic, B.; Kosanovic, D.; Prokic, B.; Vasic, J.; Zivkovic, S.;Milasin, J.; et al. Developing a novel resorptive hydroxyapatite-based bone substitute for over-critical size defect reconstruction:Physicochemical and biological characterization and proof of concept in segmental rabbit’s ulna reconstruction. Biomed. Eng.Biomed. Tech. 2020, 65, 491–505. [CrossRef]Micic, M.; Antonijevic, D.; Milutinovic-Smiljanic, S.; Trisic, D.; Colovic, B.; Kosanovic, D.; Prokic, B.; Vasic, J.; Zivkovic, S.; Milasin, J.; et al. Developing a novel resorptive hydroxyapatite-based bone substitute for over-critical size defect reconstruction: Physicochemical and biological characterization and proof of concept in segmental rabbit’s ulna reconstruction. Biomed. Eng. Biomed. Tech. 2020, 65, 491–505.
- Zhang, Y.; Lin, T.; Meng, H.; Wang, X.; Peng, H.; Liu, G.; Wei, S.; Lu, Q.; Wang, Y.; Wang, A.; et al. 3D gel-printed porousmagnesium scaffold coated with dibasic calcium phosphate dihydrate for bone repair in vivo. J. Orthop. Transl. 2022, 33, 13–23.[CrossRef] [PubMed]Zhang, Y.; Lin, T.; Meng, H.; Wang, X.; Peng, H.; Liu, G.; Wei, S.; Lu, Q.; Wang, Y.; Wang, A.; et al. 3D gel-printed porous magnesium scaffold coated with dibasic calcium phosphate dihydrate for bone repair in vivo. J. Orthop. Transl. 2022, 33, 13–23.
- Xu, T.; He, X.; Chen, Z.; He, L.; Lu, M.; Ge, J.; Weng, J.; Mu, Y.; Duan, K. Effect of magnesium particle fraction on osteoinductionof hydroxyapatite sphere-based scaffolds. J. Mater. Chem. B 2019, 7, 5648–5660. [CrossRef] [PubMed]Xu, T.; He, X.; Chen, Z.; He, L.; Lu, M.; Ge, J.; Weng, J.; Mu, Y.; Duan, K. Effect of magnesium particle fraction on osteoinduction of hydroxyapatite sphere-based scaffolds. J. Mater. Chem. B 2019, 7, 5648–5660.
- Landi, E.; Tampieri, A.; Mattioli-Belmonte, M.; Celotti, G.; Sandri, M.; Gigante, A.; Fava, P.; Biagini, G. Biomimetic Mg- andMgCO3-substituted hydroxyapatites: Synthesis characterization and in vitro behaviour. J. Eur. Ceram. Soc. 2006, 26, 2593–2601.[CrossRef]Landi, E.; Tampieri, A.; Mattioli-Belmonte, M.; Celotti, G.; Sandri, M.; Gigante, A.; Fava, P.; Biagini, G. Biomimetic Mg- and MgCO3-substituted hydroxyapatites: Synthesis characterization and in vitro behaviour. J. Eur. Ceram. Soc. 2006, 26, 2593–2601.
- Ballouze, R.; Marahat, M.H.; Mohamad, S.; Saidin, N.A.; Kasim, S.R.; Ooi, J.P. Biocompatible magnesium-doped biphasic calciumphosphate for bone regeneration. J. Biomed. Mater. Res.-B Appl. Biomater. 2021, 109, 1426–1435. [CrossRef]Ballouze, R.; Marahat, M.H.; Mohamad, S.; Saidin, N.A.; Kasim, S.R.; Ooi, J.P. Biocompatible magnesium-doped biphasic calcium phosphate for bone regeneration. J. Biomed. Mater. Res.-B Appl. Biomater. 2021, 109, 1426–1435.
- Sopyan, I.; Rahim, T.A. Porous magnesium-doped biphasic calcium phosphate ceramics prepared via polymeric sponge method.Mater. Manuf. Process. 2012, 27, 702–706. [CrossRef]Sopyan, I.; Rahim, T.A. Porous magnesium-doped biphasic calcium phosphate ceramics prepared via polymeric sponge method. Mater. Manuf. Process. 2012, 27, 702–706.
- Barallat, L.; Ruiz-Magaz, V.; Levi, P.A., Jr.; Mareque-Bueno, S.; Galindo-Moreno, P.; Nart, J. Histomorphometric results in ridgepreservation procedures comparing various graft materials in extraction sockets with nongrafted sockets in humans: A systematicreview. Implant Dent. 2014, 23, 539–554. [CrossRef]Barallat, L.; Ruiz-Magaz, V.; Levi, P.A., Jr.; Mareque-Bueno, S.; Galindo-Moreno, P.; Nart, J. Histomorphometric results in ridge preservation procedures comparing various graft materials in extraction sockets with nongrafted sockets in humans: A systematic review. Implant Dent. 2014, 23, 539–554.
- Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Rebelo, A.; Valério, P.; Ferreira, J. Rietveld structure and in vitro analysis on theinfluence of magnesium in biphasic (hydroxyapatite and β-tricalcium phosphate) mixtures. J. Biomed. Mater. Res. B 2009, 90,404–411. [CrossRef]Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Rebelo, A.; Valério, P.; Ferreira, J. Rietveld structure and in vitro analysis on the influence of magnesium in biphasic (hydroxyapatite and β-tricalcium phosphate) mixtures. J. Biomed. Mater. Res. B 2009, 90, 404–411.
- Kim, D.; Shin, K.; Jung, J.S.; Chun, H.H.; Park, S.S.; Lee, J.K.; Park, H.; Yoon, S. The role of magnesium ion substituted biphasiccalcium phosphate spherical micro-scaffolds in osteogenic differentiation of human adipose tissue-derived mesenchymal stemcells. J. Nanosci. Nanotechnol. 2015, 15, 5520–5523. [CrossRef]Kim, D.; Shin, K.; Jung, J.S.; Chun, H.H.; Park, S.S.; Lee, J.K.; Park, H.; Yoon, S. The role of magnesium ion substituted biphasic calcium phosphate spherical micro-scaffolds in osteogenic differentiation of human adipose tissue-derived mesenchymal stem cells. J. Nanosci. Nanotechnol. 2015, 15, 5520–5523.
- Kim, D.J.; Kim, T.; Lee, J.D.; Shin, K.; Jung, J.S.; Hwang, K.; Lee, J.K.; Park, H.; Yoon, S. Preparation and in vitro and in vivoperformance of magnesium ion substituted biphasic calcium phosphate spherical microscaffolds as human adipose tissue-derivedmesenchymal stem cell microcarriers. J. Nanomater. 2013, 2013, 762381. [CrossRef]Kim, D.J.; Kim, T.; Lee, J.D.; Shin, K.; Jung, J.S.; Hwang, K.; Lee, J.K.; Park, H.; Yoon, S. Preparation and in vitro and in vivo performance of magnesium ion substituted biphasic calcium phosphate spherical microscaffolds as human adipose tissue-derived mesenchymal stem cell microcarriers. J. Nanomater. 2013, 2013, 762381.
- Sartori, M.; Giavaresi, G.; Tschon, M.; Martini, L.; Dolcini, L.; Fiorini, M.; Pressato, D.; Fini, M. Long-term in vivo experimentalinvestigations on magnesium doped hydroxyapatite bone substitutes. J. Mater. Sci. Mater. Med. 2014, 25, 1495–1504. [CrossRef]Sartori, M.; Giavaresi, G.; Tschon, M.; Martini, L.; Dolcini, L.; Fiorini, M.; Pressato, D.; Fini, M. Long-term in vivo experimental investigations on magnesium doped hydroxyapatite bone substitutes. J. Mater. Sci. Mater. Med. 2014, 25, 1495–1504.
- Santos, G.G.; Nunes, V.L.C.; Marinho, S.M.O.C.; Santos, S.R.A.; Rossi, A.M.; Miguel, F.B. Biological behavior of magnesium-substituted hydroxyapatite during bone repair. Braz. J. Biol. 2021, 81, 53–61. [CrossRef] [PubMed]Santos, G.G.; Nunes, V.L.C.; Marinho, S.M.O.C.; Santos, S.R.A.; Rossi, A.M.; Miguel, F.B. Biological behavior of magnesium-substituted hydroxyapatite during bone repair. Braz. J. Biol. 2021, 81, 53–61.
- Grigolato, R.; Pizzi, N.; Brotto, M.C.; Corrocher, G.; Desando, G.; Grigolo, B. Magnesium-enriched hydroxyapatite as bone filler inan ameloblastoma mandibular defect. Int. J. Clin. Exp. Med. 2015, 8, 281–288. [PubMed]Grigolato, R.; Pizzi, N.; Brotto, M.C.; Corrocher, G.; Desando, G.; Grigolo, B. Magnesium-enriched hydroxyapatite as bone filler in an ameloblastoma mandibular defect. Int. J. Clin. Exp. Med. 2015, 8, 281–288.
- Horowitz, R.; Holtzclaw, D.; Rosen, P.S. A review on alveolar ridge preservation following tooth extraction. J. Evid. Based Dent.Pract. 2012, 12, 149–160. [CrossRef]Horowitz, R.; Holtzclaw, D.; Rosen, P.S. A review on alveolar ridge preservation following tooth extraction. J. Evid. Based Dent. Pract. 2012, 12, 149–160.
- Canullo, L.; Sisti, A. Early implant loading after vertical ridge augmentation (VRA) using e-PTFE titanium-reinforced membraneand nano-structured hydroxyapatite: 2-year prospective study. Eur. J. Oral Implantol. 2010, 3, 59–69. [PubMed]Canullo, L.; Sisti, A. Early implant loading after vertical ridge augmentation (VRA) using e-PTFE titanium-reinforced membrane and nano-structured hydroxyapatite: 2-year prospective study. Eur. J. Oral Implantol. 2010, 3, 59–69.
- Crespi, R.; Capparè, P.; Gherlone, E. Comparison of magnesium-enriched hydroxyapatite and porcine bone in human extractionsocket healing: A histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implants 2011, 26, 1057–1062. [PubMed]Crespi, R.; Capparè, P.; Gherlone, E. Comparison of magnesium-enriched hydroxyapatite and porcine bone in human extraction socket healing: A histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implants 2011, 26, 1057–1062.
- Crespi, R.; Capparè, P.; Gherlone, E. Magnesium-enriched hydroxyapatite compared to calcium sulfate in the healing of humanextraction sockets: Radiographic and histomorphometric evaluation at 3 months. J. Periodontol. 2009, 80, 210–218. [CrossRef]Crespi, R.; Capparè, P.; Gherlone, E. Magnesium-enriched hydroxyapatite compared to calcium sulfate in the healing of human extraction sockets: Radiographic and histomorphometric evaluation at 3 months. J. Periodontol. 2009, 80, 210–218.
- Caneva, M.; Botticelli, D.; Stellini, E.; Souza, S.L.S.; Salata, L.A.; Lang, N.P. Magnesium-enriched hydroxyapatite at immediateimplants: A histomorphometric study in dogs. Clin. Oral Implants Res. 2010, 22, 512–517. [CrossRef]Caneva, M.; Botticelli, D.; Stellini, E.; Souza, S.L.S.; Salata, L.A.; Lang, N.P. Magnesium-enriched hydroxyapatite at immediate implants: A histomorphometric study in dogs. Clin. Oral Implants Res. 2010, 22, 512–517.
- Taschieri, S.; Del Fabbro, M.; Panda, S.; Goker, F.; Babina, K.S.; Tampieri, A.; Mortellaro, C. Prospective clinical and histologicevaluation of alveolar socket healing following ridge preservation using a combination of hydroxyapatite and collagen biomimeticxenograft versus demineralized bovine bone. J. Craniofac. Surg. 2019, 30, 1089–1094. [CrossRef]Taschieri, S.; Del Fabbro, M.; Panda, S.; Goker, F.; Babina, K.S.; Tampieri, A.; Mortellaro, C. Prospective clinical and histologic evaluation of alveolar socket healing following ridge preservation using a combination of hydroxyapatite and collagen biomimetic xenograft versus demineralized bovine bone. J. Craniofac. Surg. 2019, 30, 1089–1094.
- Crespi, R.; Mariani, E.; Benasciutti, E.; Capparè, P.; Cenci, S.; Gherlone, E. Magnesium-enriched hydroxyapatite versus autologousbone in maxillary sinus grafting: Combining histomorphometry with osteoblast gene expression profiles ex vivo. J. Periodontol.2009, 80, 586–593. [CrossRef]Crespi, R.; Mariani, E.; Benasciutti, E.; Capparè, P.; Cenci, S.; Gherlone, E. Magnesium-enriched hydroxyapatite versus autologous bone in maxillary sinus grafting: Combining histomorphometry with osteoblast gene expression profiles ex vivo. J. Periodontol. 2009, 80, 586–593.
- Radeti ́c, A.T.J.; Cvek, S.Z.; Tomas, M.; Erjavec, I.; Ogui ́c, M.; Kaˇcarevi ́c, Ž.P.; Peloza, O.C. CSBD healing in rats after application ofbovine xenogeneic biomaterial enriched with magnesium alloy. Int. J. Mol. Sci. 2021, 22, 9089. [CrossRef]Radetić, A.T.J.; Cvek, S.Z.; Tomas, M.; Erjavec, I.; Oguić, M.; Kačarević, Ž.P.; Peloza, O.C. CSBD healing in rats after application of bovine xenogeneic biomaterial enriched with magnesium alloy. Int. J. Mol. Sci. 2021, 22, 9089.
- Park, J.W.; Ko, H.J.; Jang, J.H.; Kang, H.; Suh, J.Y. Increased new bone formation with a surface magnesium-incorporateddeproteinized porcine bone substitute in rabbit calvarial defects. J. Biomed. Mater. Res. 2012, 100, 834–840. [CrossRef] [PubMed]Park, J.W.; Ko, H.J.; Jang, J.H.; Kang, H.; Suh, J.Y. Increased new bone formation with a surface magnesium-incorporated deproteinized porcine bone substitute in rabbit calvarial defects. J. Biomed. Mater. Res. 2012, 100, 834–840.
- Roller, B.L.; Kuroki, K.; Bozynski, C.C.; Pfeiffer, F.M.; Cook, J.L. Use of a novel magnesium-based resorbable bone cement foraugmenting anchor and tendon fixation. Am. J. Orthoped. 2018, 47. [CrossRef] [PubMed]Roller, B.L.; Kuroki, K.; Bozynski, C.C.; Pfeiffer, F.M.; Cook, J.L. Use of a novel magnesium-based resorbable bone cement for augmenting anchor and tendon fixation. Am. J. Orthoped. 2018, 47.
- Sehlke, B.M.; Wilson, T.G.; Jones, A.A.; Yamashita, M.; Cochran, D.L. The use of a magnesium-based bone cement to secureimmediate dental implants. Int. J. Oral Maxillofac. Implants 2013, 28, e357–e367. [CrossRef] [PubMed]Sehlke, B.M.; Wilson, T.G.; Jones, A.A.; Yamashita, M.; Cochran, D.L. The use of a magnesium-based bone cement to secure immediate dental implants. Int. J. Oral Maxillofac. Implants 2013, 28, e357–e367.
- Guan, X.; Xiong, M.; Zeng, F.; Xu, B.; Yang, L.; Guo, H.; Niu, J.; Zhang, J.; Chen, C.; Pei, J.; et al. Enhancement of osteogenesis andbiodegradation control by brushite coating on Mg-Nd-Zn-Zr alloy for mandibular bone repair. ACS Appl. Mater. Interfaces 2014, 6,21525–21533. [CrossRef]Almehmadi, A. Effect of magnesium-based coatings on titanium or zirconia substrates on bone regeneration and implant—A systematic review. Front. Mater. 2021, 8, 754697.
- Almehmadi, A. Effect of magnesium-based coatings on titanium or zirconia substrates on bone regeneration and implant—Asystematic review. Front. Mater. 2021, 8, 754697. [CrossRef]Stanford, C. Surface modifications of dental implants. Aust. Dent. J. 2008, 53, S26–S33.
- Stanford, C. Surface modifications of dental implants. Aust. Dent. J. 2008, 53, S26–S33. [CrossRef]Lung, C.Y.K.; Matinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2012, 28, 467–477.
- Lung, C.Y.K.; Matinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater.2012, 28, 467–477. [CrossRef]Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603.
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis,angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [CrossRef]Xie, Y.; Zhai, W.; Chen, L.; Chang, J.; Zheng, X.; Ding, C. Preparation and in vitro evaluation of plasma-sprayed Mg2SiO4 coating on titanium alloy. Acta Biomater. 2009, 5, 2331–2337.
- Xie, Y.; Zhai, W.; Chen, L.; Chang, J.; Zheng, X.; Ding, C. Preparation and in vitro evaluation of plasma-sprayed Mg2SiO4 coatingon titanium alloy. Acta Biomater. 2009, 5, 2331–2337. [CrossRef]Won, S.; Huh, Y.H.; Cho, L.R.; Lee, H.S.; Byon, E.S.; Park, C.J. Cellular response of human bone marrow derived mesenchymal stem cells to titanium surfaces implanted with calcium and magnesium ions. Tissue Eng. Regen. Med. 2017, 14, 123–131.
- Won, S.; Huh, Y.H.; Cho, L.R.; Lee, H.S.; Byon, E.S.; Park, C.J. Cellular response of human bone marrow derived mesenchymalstem cells to titanium surfaces implanted with calcium and magnesium ions. Tissue Eng. Regen. Med. 2017, 14, 123–131. [CrossRef]Onder, S.; Calikoglu-Koyuncu, A.C.; Kazmanli, K.; Urgen, M.; Kok, F.N.; Torun-Kose, G. Magnesium doping on TiN coatings affects mesenchymal stem cell differentiation and proliferation positively in a dose-dependent manner. Biomed. Mater. Eng. 2018, 29, 427–438.
- Onder, S.; Calikoglu-Koyuncu, A.C.; Kazmanli, K.; Urgen, M.; Kok, F.N.; Torun-Kose, G. Magnesium doping on TiN coatingsaffects mesenchymal stem cell differentiation and proliferation positively in a dose-dependent manner. Biomed. Mater. Eng. 2018,29, 427–438. [CrossRef] [PubMed]Mihailescu, N.; Stan, G.E.; Duta, L.; Chifiriuc, M.C.; Bleotu, C.; Sopronyi, M.; Luculescu, C.; Oktar, F.N.; Mihailescu, I.N. Structural, compositional, mechanical characterization and biological assessment of bovine-derived hydroxyapatite coatings reinforced with MgF2 or MgO for implants functionalization. Mater. Sci. Eng. C 2016, 59, 863–874.
- Mihailescu, N.; Stan, G.E.; Duta, L.; Chifiriuc, M.C.; Bleotu, C.; Sopronyi, M.; Luculescu, C.; Oktar, F.N.; Mihailescu, I.N. Structural,compositional, mechanical characterization and biological assessment of bovine-derived hydroxyapatite coatings reinforced withMgF2 or MgO for implants functionalization. Mater. Sci. Eng. C 2016, 59, 863–874. [CrossRef] [PubMed]Jiang, X.; Wang, G.; Li, J.; Zhang, W.; Xu, L.; Pan, H.; Wen, J.; Wu, Q.; She, W.; Jiao, T.; et al. Magnesium ion implantation on a micro/nanostructured titanium surface promotes its bioactivity and osteogenic differentiation function. Int. J. Nanomed. 2014, 8, 2387–2398.
- Jiang, X.; Wang, G.; Li, J.; Zhang, W.; Xu, L.; Pan, H.; Wen, J.; Wu, Q.; She, W.; Jiao, T.; et al. Magnesium ion implantation on amicro/nanostructured titanium surface promotes its bioactivity and osteogenic differentiation function. Int. J. Nanomed. 2014, 8,2387–2398. [CrossRef] [PubMed]Park, K.D.; Lee, B.A.; Piao, X.H.; Lee, K.K.; Park, S.W.; Oh, H.K.; Young, J.K.; Hong, J.P. Effect of magnesium and calcium phosphate coatings on osteoblastic responses to the titanium surface. J. Adv. Prosthodont. 2013, 5, 402–408.
- Park, K.D.; Lee, B.A.; Piao, X.H.; Lee, K.K.; Park, S.W.; Oh, H.K.; Young, J.K.; Hong, J.P. Effect of magnesium and calciumphosphate coatings on osteoblastic responses to the titanium surface. J. Adv. Prosthodont. 2013, 5, 402–408. [CrossRef] [PubMed]Cho, L.R.; Kim, D.G.; Kim, J.H.; Byon, E.S.; Jeong, Y.S.; Park, C.J. Bone response of mg ion-implanted clinical implants with the plasma source ion implantation method. Clin. Oral Implants Res. 2010, 21, 848–856.
- Cho, L.R.; Kim, D.G.; Kim, J.H.; Byon, E.S.; Jeong, Y.S.; Park, C.J. Bone response of mg ion-implanted clinical implants with theplasma source ion implantation method. Clin. Oral Implants Res. 2010, 21, 848–856. [CrossRef] [PubMed]Li, X.; Li, Y.; Liao, Y.; Li, J.; Zhang, L.; Hu, J. The effect of magnesium incorporated hydroxyapatite coating on titanium implant fixation in ovariectomized rats. Int. J. Oral Maxillofac. Implants 2014, 29, 196–202.
- Li, X.; Li, Y.; Liao, Y.; Li, J.; Zhang, L.; Hu, J. The effect of magnesium incorporated hydroxyapatite coating on titanium implantfixation in ovariectomized rats. Int. J. Oral Maxillofac. Implants 2014, 29, 196–202. [CrossRef] [PubMed]Tao, Z.S.; Zhou, W.S.; He, X.W.; Liu, W.; Bai, B.L.; Zhou, Q.; Huang, Z.L.; Tu, K.K.; Li, H.; Sun, T.; et al. A comparative study of zinc, magnesium, strontium-incorporated hydroxyapatite-coated titanium implants for osseointegration of osteopenic rats. Mater. Sci. Eng. C 2016, 62, 226–232.
- Tao, Z.S.; Zhou, W.S.; He, X.W.; Liu, W.; Bai, B.L.; Zhou, Q.; Huang, Z.L.; Tu, K.K.; Li, H.; Sun, T.; et al. A comparative study ofzinc, magnesium, strontium-incorporated hydroxyapatite-coated titanium implants for osseointegration of osteopenic rats. Mater.Sci. Eng. C 2016, 62, 226–232. [CrossRef]Zhao, S.F.; Jiang, Q.H.; Peel, S.; Wang, X.X.; He, F.M. Effects of magnesium-substituted nanohydroxyapatite coating on implant osseointegration. Clin. Oral Implant. Res. 2013, 24, 34–41.
- Zhao, S.F.; Jiang, Q.H.; Peel, S.; Wang, X.X.; He, F.M. Effects of magnesium-substituted nanohydroxyapatite coating on implantosseointegration. Clin. Oral Implant. Res. 2013, 24, 34–41. [CrossRef]Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193.
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G.Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [CrossRef]Liu, Y.; Wang, D.L.; Huang, Y.C.; Wang, T.B.; Zeng, H. Hydrogen inhibits the osteoclastogenesis of mouse bone marrow mononuclear cells. Mater. Sci. Eng. C 2020, 110, 110640.
- Liu, Y.; Wang, D.L.; Huang, Y.C.; Wang, T.B.; Zeng, H. Hydrogen inhibits the osteoclastogenesis of mouse bone marrowmononuclear cells. Mater. Sci. Eng. C 2020, 110, 110640. [CrossRef]Mraied, H.; Wang, W.; Cai, W. Influence of chemical heterogeneity and microstructure on the corrosion resistance of biodegradable WE43 magnesium alloys. J. Mater. Chem. B 2019, 7, 6399–6411.
- Mraied, H.; Wang, W.; Cai, W. Influence of chemical heterogeneity and microstructure on the corrosion resistance of biodegradableWE43 magnesium alloys. J. Mater. Chem. B 2019, 7, 6399–6411. [CrossRef] [PubMed]Wang, Q.; Tan, L.; Xu, W.; Zhang, B.; Yang, K. Dynamic behaviors of a Ca–P coated AZ31B magnesium alloy during in vitro and in vivo degradations. Mater. Sci. Eng. B 2011, 176, 1718–1726.
- Wang, Q.; Tan, L.; Xu, W.; Zhang, B.; Yang, K. Dynamic behaviors of a Ca–P coated AZ31B magnesium alloy during in vitro andin vivo degradations. Mater. Sci. Eng. B 2011, 176, 1718–1726. [CrossRef]Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-based biodegradable alloys: Degradation, application, and alloying elements. Interv. Med. Appl. Sci. 2017, 9, 27–38.
- Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-based biodegradable alloys: Degradation, application, andalloying elements. Interv. Med. Appl. Sci. 2017, 9, 27–38. [CrossRef] [PubMed]Han, P.; Cheng, P.; Zhang, S.; Zhao, C.; Ni, J.; Zhang, Y.; Zhong, W.; Hou, P.; Zhang, X.; Zheng, Y.; et al. In vitro and in vivo studies on the degradation of high-purity Mg (99.99 wt.%) screw with femoral intracondylar fractured rabbit model. Biomaterials 2015, 64, 57–69.
- Han, P.; Cheng, P.; Zhang, S.; Zhao, C.; Ni, J.; Zhang, Y.; Zhong, W.; Hou, P.; Zhang, X.; Zheng, Y.; et al. In vitro and in vivostudies on the degradation of high-purity Mg (99.99 wt.%) screw with femoral intracondylar fractured rabbit model. Biomaterials2015, 64, 57–69. [CrossRef]Bonithon, R.; Kao, A.P.; Fernández, M.P.; Dunlop, J.N.; Blunn, G.W.; Witte, F.; Tozzi, G. Multi-scale mechanical and morphological characterisation of sintered porous magnesium-based scaffolds for bone regeneration in critical-sized defects. Acta Biomater. 2021, 127, 338–352.
- Bonithon, R.; Kao, A.P.; Fernández, M.P.; Dunlop, J.N.; Blunn, G.W.; Witte, F.; Tozzi, G. Multi-scale mechanical and morphologicalcharacterisation of sintered porous magnesium-based scaffolds for bone regeneration in critical-sized defects. Acta Biomater. 2021,127, 338–352. [CrossRef]Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57.
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [CrossRef]Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563.
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of fourmagnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [CrossRef]Chen, K.; Lu, Y.; Tang, H.; Gao, Y.; Zhao, F.; Gu, X.; Fan, Y. Effect of strain on degradation behaviors of WE43, Fe and Zn wires. Acta Biomater. 2020, 113, 627–645.
- Chen, K.; Lu, Y.; Tang, H.; Gao, Y.; Zhao, F.; Gu, X.; Fan, Y. Effect of strain on degradation behaviors of WE43, Fe and Zn wires.Acta Biomater. 2020, 113, 627–645. [CrossRef]Mao, L.; Shen, L.; Chen, J.; Wu, Y.; Kwak, M.; Lu, Y.; Xue, Q.; Pei, J.; Zhang, L.; Yuan, G.; et al. Enhanced bioactivity of Mg-Nd-Zn-Zr alloy achieved with nanoscale MgF2 surface for vascular stent application. ACS Appl. Mater. Interfaces 2015, 7, 5320–5330.
- Mao, L.; Shen, L.; Chen, J.; Wu, Y.; Kwak, M.; Lu, Y.; Xue, Q.; Pei, J.; Zhang, L.; Yuan, G.; et al. Enhanced bioactivity ofMg-Nd-Zn-Zr alloy achieved with nanoscale MgF2 surface for vascular stent application. ACS Appl. Mater. Interfaces 2015, 7,5320–5330. [CrossRef]Holweg, P.; Herber, V.; Ornig, M.; Hohenberger, G.; Donohue, N.; Puchwein, P.; Leithner, A.; Seibert, F. A lean magnesium–zinc–calcium alloy ZX00 used for bone fracture stabilization in a large growing animal model. Acta Biomater. 2020, 113, 646–659.
- Holweg, P.; Herber, V.; Ornig, M.; Hohenberger, G.; Donohue, N.; Puchwein, P.; Leithner, A.; Seibert, F. A lean magnesium–zinc–calcium alloy ZX00 used for bone fracture stabilization in a large growing animal model. Acta Biomater. 2020, 113, 646–659.[CrossRef]Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W.; et al. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials 2016, 81, 84–92.
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W.; et al. Vascularized bone grafting fixed bybiodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials 2016, 81, 84–92. [CrossRef] [PubMed]Plaass, C.; von Falck, C.; Ettinger, S.; Sonnow, L.; Calderone, F.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Waizy, H.; Daniilidis, K.; et al. Bioabsorbable magnesium versus standard titanium compression screws for fixation of distal metatarsal osteotomies—3 year results of a randomized clinical trial. J. Orthop. Sci. 2018, 23, 321–327.
- Plaass, C.; von Falck, C.; Ettinger, S.; Sonnow, L.; Calderone, F.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Waizy, H.;Daniilidis, K.; et al. Bioabsorbable magnesium versus standard titanium compression screws for fixation of distal metatarsalosteotomies—3 year results of a randomized clinical trial. J. Orthop. Sci. 2018, 23, 321–327. [CrossRef] [PubMed]Acar, B.; Kose, O.; Turan, A.; Unal, M.; Kati, Y.A.; Guler, F. Comparison of bioabsorbable magnesium versus titanium screw fixation for modified distal chevron osteotomy in hallux valgus. Biomed. Res. Int. 2018, 2018, 5242808.
- Acar, B.; Kose, O.; Turan, A.; Unal, M.; Kati, Y.A.; Guler, F. Comparison of bioabsorbable magnesium versus titanium screwfixation for modified distal chevron osteotomy in hallux valgus. Biomed. Res. Int. 2018, 2018, 5242808. [CrossRef] [PubMed]Klauser, H. Internal fixation of three-dimensional distal metatarsal i osteotomies in the treatment of hallux valgus deformities using biodegradable magnesium screws in comparison to titanium screws. Foot Ankle Surg. 2019, 25, 398–405.
- Klauser, H. Internal fixation of three-dimensional distal metatarsal i osteotomies in the treatment of hallux valgus deformitiesusing biodegradable magnesium screws in comparison to titanium screws. Foot Ankle Surg. 2019, 25, 398–405. [CrossRef][PubMed]Atkinson, H.D.; Khan, S.; Lashgari, Y.; Ziegler, A. Hallux valgus correction utilising a modified short scarf osteotomy with a magnesium biodegradable or titanium compression screws—A comparative study of clinical outcomes. BMC Musculoskelet. Disord. 2019, 20, 334.
- Atkinson, H.D.; Khan, S.; Lashgari, Y.; Ziegler, A. Hallux valgus correction utilising a modified short scarf osteotomy with amagnesium biodegradable or titanium compression screws—A comparative study of clinical outcomes. BMC Musculoskelet.Disord. 2019, 20, 334. [CrossRef] [PubMed]Sukotjo, C.; Lima-Neto, T.J.; Júnior, J.F.S.; Faverani, L.P.; Miloro, M. Is there a role for absorbable metals in surgery? A systematic review and meta-analysis of Mg/Mg alloy based implants. Materials 2020, 13, 3914.
- Sukotjo, C.; Lima-Neto, T.J.; Júnior, J.F.S.; Faverani, L.P.; Miloro, M. Is there a role for absorbable metals in surgery? A systematicreview and meta-analysis of Mg/Mg alloy based implants. Materials 2020, 13, 3914. [CrossRef]Zhang, X.; Yuan, G.; Mao, L.; Niu, J.; Fu, P.; Ding, W. Effects of extrusion and heat treatment on the mechanical properties and biocorrosion behaviors of a Mg-Nd-Zn-Zr alloy. J. Mech. Behav. Biomed. Mater. 2012, 7, 77–86.
- Zhang, X.; Yuan, G.; Mao, L.; Niu, J.; Fu, P.; Ding, W. Effects of extrusion and heat treatment on the mechanical properties andbiocorrosion behaviors of a Mg-Nd-Zn-Zr alloy. J. Mech. Behav. Biomed. Mater. 2012, 7, 77–86. [CrossRef]Gareb, B.; van Bakelen, N.B.; Buijs, G.J.; Jansma, J.; de Visscher, J.; Hoppenreijs, T.J.M.; Bergsma, J.E.; van Minnen, B.; Stegenga, B.; Bos, R.R.M. Comparison of the long-term clinical performance of a biodegradable and a titanium fixation system in maxillofacial surgery: A multicenter randomized controlled trial. PLoS ONE 2017, 12, e0177152.
- Gareb, B.; van Bakelen, N.B.; Buijs, G.J.; Jansma, J.; de Visscher, J.; Hoppenreijs, T.J.M.; Bergsma, J.E.; van Minnen, B.; Stegenga, B.;Bos, R.R.M. Comparison of the long-term clinical performance of a biodegradable and a titanium fixation system in maxillofacialsurgery: A multicenter randomized controlled trial. PLoS ONE 2017, 12, e0177152. [CrossRef]Henderson, S.E.; Verdelis, K.; Maiti, S.; Pal, S.; Chung, W.L.; Chou, D.T.; Kumta, P.N.; Almarza, A.J. Magnesium alloys as a biomaterial for degradable craniofacial screws. Acta Biomater. 2014, 10, 2323–23320.
- Henderson, S.E.; Verdelis, K.; Maiti, S.; Pal, S.; Chung, W.L.; Chou, D.T.; Kumta, P.N.; Almarza, A.J. Magnesium alloys as abiomaterial for degradable craniofacial screws. Acta Biomater. 2014, 10, 2323–23320. [CrossRef]Lee, J.Y.; Lee, J.W.; Pang, K.M.; Kim, H.E.; Kim, S.M.; Lee, J.H. Biomechanical evaluation of magnesium-based resorbable metallic screw system in a bilateral sagittal split ramus osteotomy model using three-dimensional finite element analysis. J. Oral Maxillofac. Surg. 2014, 72, 402.
- Lee, J.Y.; Lee, J.W.; Pang, K.M.; Kim, H.E.; Kim, S.M.; Lee, J.H. Biomechanical evaluation of magnesium-based resorbable metallicscrew system in a bilateral sagittal split ramus osteotomy model using three-dimensional finite element analysis. J. Oral Maxillofac.Surg. 2014, 72, 402. [CrossRef]Lee, J.H.; Han, H.S.; Kim, Y.C.; Lee, J.Y.; Lee, B.K. Stability of biodegradable metal (Mg-Ca-Zn alloy) screws compared with absorbable polymer and titanium screws for sagittal split ramus osteotomy of the mandible using the finite element analysis model. J. Cranio-Maxillofac. Surg. 2017, 45, 1639–1646.
- Lee, J.H.; Han, H.S.; Kim, Y.C.; Lee, J.Y.; Lee, B.K. Stability of biodegradable metal (Mg-Ca-Zn alloy) screws compared withabsorbable polymer and titanium screws for sagittal split ramus osteotomy of the mandible using the finite element analysismodel. J. Cranio-Maxillofac. Surg. 2017, 45, 1639–1646. [CrossRef] [PubMed]Schaller, B.; Saulacic, N.; Beck, S.; Imwinkelried, T.; Goh, B.T.; Nakahara, K.; Hofstetter, W.; Iizuka, T. In vivo degradation of a new concept of magnesium-based rivet-screws in the minipig mandibular bone. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 247–254.
- Schaller, B.; Saulacic, N.; Beck, S.; Imwinkelried, T.; Goh, B.T.; Nakahara, K.; Hofstetter, W.; Iizuka, T. In vivo degradation of anew concept of magnesium-based rivet-screws in the minipig mandibular bone. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69,247–254. [CrossRef] [PubMed]Naujokat, H.; Ruff, C.B.; Kluter, T.; Seitz, J.M.; Acil, Y.; Wiltfang, J. Influence of surface modifications on the degradation of standard-sized magnesium plates and healing of mandibular osteotomies in miniature pigs. Int. J. Oral Maxillofac. Surg. 2020, 49, 272–283.
- Naujokat, H.; Ruff, C.B.; Kluter, T.; Seitz, J.M.; Acil, Y.; Wiltfang, J. Influence of surface modifications on the degradation ofstandard-sized magnesium plates and healing of mandibular osteotomies in miniature pigs. Int. J. Oral Maxillofac. Surg. 2020, 49,272–283. [CrossRef] [PubMed]Byun, S.H.; Lim, H.K.; Cheon, K.H.; Lee, S.M.; Kim, H.E.; Lee, J.H. Biodegradable magnesium alloy (WE43) in bone-fixation plate and screw. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2505–2512.
- Byun, S.H.; Lim, H.K.; Cheon, K.H.; Lee, S.M.; Kim, H.E.; Lee, J.H. Biodegradable magnesium alloy (WE43) in bone-fixation plateand screw. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2505–2512. [CrossRef]Byun, S.H.; Lim, H.K.; Lee, S.M.; Kim, H.J.; Kim, S.M.; Lee, J. Biodegradable magnesium alloy (ZK60) with a poly(l-lactic)-acid polymer coating for maxillofacial surgery. Metals 2020, 10, 724.
- Byun, S.H.; Lim, H.K.; Lee, S.M.; Kim, H.J.; Kim, S.M.; Lee, J. Biodegradable magnesium alloy (ZK60) with a poly(l-lactic)-acidpolymer coating for maxillofacial surgery. Metals 2020, 10, 724. [CrossRef]Schaller, B.; Burkhard, J.P.M.; Chagnon, M.; Beck, S.; Imwinkelried, T.; Assad, M. Fracture healing and bone remodeling with human standard-sized magnesium versus polylactide-Co-glycolide plate and screw systems using a mini-swine craniomaxillofacial osteotomy fixation model. J. Oral Maxillofac. Surg. 2018, 76, 2138–2150.
- Schaller, B.; Burkhard, J.P.M.; Chagnon, M.; Beck, S.; Imwinkelried, T.; Assad, M. Fracture healing and bone remodeling with hu-man standard-sized magnesium versus polylactide-Co-glycolide plate and screw systems using a mini-swine craniomaxillofacialosteotomy fixation model. J. Oral Maxillofac. Surg. 2018, 76, 2138–2150. [CrossRef]Kim, B.J.; Piao, Y.; Wufuer, M.; Son, W.C.; Choi, T.H. Biocompatibility and efficiency of biodegradable magnesium-based plates and screws in the facial fracture model of beagles. J. Oral Maxillofac. Surg. 2018, 76, 1055.
- Kim, B.J.; Piao, Y.; Wufuer, M.; Son, W.C.; Choi, T.H. Biocompatibility and efficiency of biodegradable magnesium-based platesand screws in the facial fracture model of beagles. J. Oral Maxillofac. Surg. 2018, 76, 1055. [CrossRef]Naujokat, H.; Seitz, J.M.; Acil, Y.; Damm, T.; Moller, I.; Gulses, A.; Wiltfang, J. Osteosynthesis of a cranio-osteoplasty with a biodegradable magnesium plate system in miniature pigs. Acta Biomater. 2017, 62, 434–445.
- Naujokat, H.; Seitz, J.M.; Acil, Y.; Damm, T.; Moller, I.; Gulses, A.; Wiltfang, J. Osteosynthesis of a cranio-osteoplasty with abiodegradable magnesium plate system in miniature pigs. Acta Biomater. 2017, 62, 434–445. [CrossRef]Schaller, B.; Saulacic, N.; Imwinkelried, T.; Beck, S.; Liu, E.W.; Gralla, J.; Nakahara, K.; Hofstetter, W.; Iizuka, T. In vivo degradation of magnesium plate/screw osteosynthesis implant systems: Soft and hard tissue response in a calvarial model in miniature pigs. J. Cranio-Maxillofac. Surg. 2016, 44, 309–317.
- Schaller, B.; Saulacic, N.; Imwinkelried, T.; Beck, S.; Liu, E.W.; Gralla, J.; Nakahara, K.; Hofstetter, W.; Iizuka, T. In vivo degradationof magnesium plate/screw osteosynthesis implant systems: Soft and hard tissue response in a calvarial model in miniature pigs.J. Cranio-Maxillofac. Surg. 2016, 44, 309–317. [CrossRef]Zhang, D.; Ni, N.; Su, Y.; Miao, H.; Tang, Z.; Ji, Y.; Wang, Y.; Gao, H.; Ju, Y.; Sun, N.; et al. Targeting local osteogenic and ancillary cells by mechanobiologically optimized magnesium scaffolds for orbital bone reconstruction in canines. ACS Appl. Mater. Interfaces 2020, 12, 27889–27904.
- Zhang, D.; Ni, N.; Su, Y.; Miao, H.; Tang, Z.; Ji, Y.; Wang, Y.; Gao, H.; Ju, Y.; Sun, N.; et al. Targeting local osteogenic andancillary cells by mechanobiologically optimized magnesium scaffolds for orbital bone reconstruction in canines. ACS Appl.Mater. Interfaces 2020, 12, 27889–27904. [CrossRef]Leonhardt, H.; Franke, A.; McLeod, N.M.H.; Lauer, G.; Nowak, A. Fixation of fractures of the condylar head of the mandible with a new magnesium-alloy biodegradable cannulated headless bone screw. Br. J. Oral Maxillofac. Surg. 2017, 55, 623–625.
- Leonhardt, H.; Franke, A.; McLeod, N.M.H.; Lauer, G.; Nowak, A. Fixation of fractures of the condylar head of the mandible witha new magnesium-alloy biodegradable cannulated headless bone screw. Br. J. Oral Maxillofac. Surg. 2017, 55, 623–625. [CrossRef][PubMed]Leonhardt, H.; Ziegler, A.; Lauer, G.; Franke, A. Osteosynthesis of the mandibular condyle with magnesium-based biodegradable. Headless compression screws show good clinical results during a 1-year follow-up period. J. Oral Maxillofac. Surg. 2021, 79, 637–643.
- Leonhardt, H.; Ziegler, A.; Lauer, G.; Franke, A. Osteosynthesis of the mandibular condyle with magnesium-based biodegradable.Headless compression screws show good clinical results during a 1-year follow-up period. J. Oral Maxillofac. Surg. 2021, 79,637–643. [CrossRef] [PubMed]Laiteerapong, A.; Lochaiwatana, Y.; Hirata, I.; Okazaki, M.; Mori, K.; Murakami, S.; Poolthong, S. A novel glass ionomer cement containing MgCO3 apatite induced the increased proliferation and differentiation of human pulp cells in vitro. Dent. Mater. J. 2012, 31, 772–778.
- Laiteerapong, A.; Lochaiwatana, Y.; Hirata, I.; Okazaki, M.; Mori, K.; Murakami, S.; Poolthong, S. A novel glass ionomer cementcontaining MgCO3 apatite induced the increased proliferation and differentiation of human pulp cells in vitro. Dent. Mater. J.2012, 31, 772–778. [CrossRef] [PubMed]Kong, Y.; Hu, X.; Zhong, Y.; Xu, K.; Wu, B.; Zheng, J. Magnesium-enriched microenvironment promotes odontogenic differentiation in human dental pulp stem cells by activating ERK/BMP2/Smads signaling. Stem Cell Res. Ther. 2019, 10, 378.
- Kong, Y.; Hu, X.; Zhong, Y.; Xu, K.; Wu, B.; Zheng, J. Magnesium-enriched microenvironment promotes odontogenic dif-ferentiation in human dental pulp stem cells by activating ERK/BMP2/Smads signaling. Stem Cell Res. Ther. 2019, 10, 378.[CrossRef]Zheng, J.M.; Kong, Y.Y.; Li, Y.Y.; Zhang, W. MagT1 regulated the odontogenic differentiation of BMMSCs induced by TGC-CM via ERK signaling pathway. Stem Cell Res. Ther. 2019, 10, 48.
- Zheng, J.M.; Kong, Y.Y.; Li, Y.Y.; Zhang, W. MagT1 regulated the odontogenic differentiation of BMMSCs induced by TGC-CMvia ERK signaling pathway. Stem Cell Res. Ther. 2019, 10, 48. [CrossRef]Lange, T.S.; Kirchberg, J.; Bielinsky, A.K.; Leuker, A.; Bank, L.; Ruzicka, T.; Scharffetter-Kochanek, K. Divalent cations (Mg2+, Ca2+) differentially influence the β1 integrin-mediated migration of human fibroblasts and keratinocytes to different extracellular matrix proteins. Exp. Dermatol. 1995, 4, 130–137.
- Lange, T.S.; Kirchberg, J.; Bielinsky, A.K.; Leuker, A.; Bank, L.; Ruzicka, T.; Scharffetter-Kochanek, K. Divalent cations (Mg2+,Ca2+) differentially influence the β1 integrin-mediated migration of human fibroblasts and keratinocytes to different extracellularmatrix proteins. Exp. Dermatol. 1995, 4, 130–137. [CrossRef]Lange, T.S.; Bielinsky, A.K.; Kirchberg, K.; Bank, I.; Herrmann, K.; Krieg, T.; Scharffetter-Kochanek, K. Mg2+ and Ca2+ differentially regulate β1 integrinmediated adhesion of dermal fibroblasts and keratinocytes to various extracellular matrix proteins. Exp. Cell Res. 1994, 214, 381–388.
- Lange, T.S.; Bielinsky, A.K.; Kirchberg, K.; Bank, I.; Herrmann, K.; Krieg, T.; Scharffetter-Kochanek, K. Mg2+ and Ca2+ differentiallyregulate β1 integrinmediated adhesion of dermal fibroblasts and keratinocytes to various extracellular matrix proteins. Exp. CellRes. 1994, 214, 381–388. [CrossRef]Heitz-Mayfield, L.J.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol. 2000 2010, 53, 167–181.
- Heitz-Mayfield, L.J.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol.2000 2010, 53, 167–181. [CrossRef]Takamori, Y.; Atsuta, I.; Nakamura, H.; Sawase, T.; Koyano, K.; Hara, Y. Histopathological comparison of the onset of peri-implantitis and periodontitis in rats. Clin. Oral Implants Res. 2017, 28, 163–170.
- Takamori, Y.; Atsuta, I.; Nakamura, H.; Sawase, T.; Koyano, K.; Hara, Y. Histopathological comparison of the onset of peri-implantitis and periodontitis in rats. Clin. Oral Implants Res. 2017, 28, 163–170. [CrossRef]Okawachi, H.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Sakaguchi, M.; Yamane, K.; Koyano, K. Effect of titanium surface calcium and magnesium on adhesive activity of epithelial-like cells and fibroblasts. Biointerphases 2012, 7, 27.
- Okawachi, H.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Sakaguchi, M.; Yamane, K.; Koyano, K. Effect of titanium surface calciumand magnesium on adhesive activity of epithelial-like cells and fibroblasts. Biointerphases 2012, 7, 27. [CrossRef]Diekmann, J.; Bauer, S.; Weizbauer, A.; Willbold, E.; Windhagen, H.; Helmecke, P.; Lucas, A.; Reifenrath, J.; Nolte, L.; Ezechieli, M. Examination of a biodegradable magnesium screw for the reconstruction of the anterior cruciate ligament: A pilot in vivo study in rabbits. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 1100–1109.
- Diekmann, J.; Bauer, S.; Weizbauer, A.; Willbold, E.; Windhagen, H.; Helmecke, P.; Lucas, A.; Reifenrath, J.; Nolte, L.; Ezechieli, M.Examination of a biodegradable magnesium screw for the reconstruction of the anterior cruciate ligament: A pilot in vivo studyin rabbits. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 1100–1109. [CrossRef]Fu, Y.M.; Yin, Y.; Guan, J.W.; Ji, Q.B.; Wang, Y.; Zhang, Q. The evaluation of a degradable magnesium alloy bio-transfix nail system compounded with bone morphogenetic protein-2 in a beagle anterior cruciate ligament reconstruction model. J. Biomater. Appl. 2019, 34, 687–698.
- Fu, Y.M.; Yin, Y.; Guan, J.W.; Ji, Q.B.; Wang, Y.; Zhang, Q. The evaluation of a degradable magnesium alloy bio-transfix nailsystem compounded with bone morphogenetic protein-2 in a beagle anterior cruciate ligament reconstruction model. J. Biomater.Appl. 2019, 34, 687–698. [CrossRef] [PubMed]Wang, J.; Xu, J.; Fu, W.; Cheng, W.; Chan, K.; Yung, P.S.; Qin, L. Biodegradable magnesium screws accelerate fibrous tissue mineralization at the tendon-bone insertion in anterior cruciate ligament reconstruction model of rabbit. Sci. Rep. 2017, 7, 40369.
- Wang, J.; Xu, J.; Fu, W.; Cheng, W.; Chan, K.; Yung, P.S.; Qin, L. Biodegradable magnesium screws accelerate fibrous tissuemineralization at the tendon-bone insertion in anterior cruciate ligament reconstruction model of rabbit. Sci. Rep. 2017, 7, 40369.[CrossRef] [PubMed]Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The role of magnesium in neurological disorders. Nutrients 2018, 10, 730.
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The role of magnesium in neurological disorders. Nutrients 2018, 10, 730. [CrossRef]Fei, J.; Wen, X.; Lin, X.; Saijilafu; Wang, W.; Ren, O.; Chen, X.; Tan, L.; Yang, K.; Yang, H.; et al. Biocompatibility and neurotoxicity of magnesium alloys potentially used for neural repairs. Mater. Sci. Eng. C 2017, 78, 1155–1163.
- Fei, J.; Wen, X.; Lin, X.; Saijilafu; Wang, W.; Ren, O.; Chen, X.; Tan, L.; Yang, K.; Yang, H.; et al. Biocompatibility and neurotoxicityof magnesium alloys potentially used for neural repairs. Mater. Sci. Eng. C 2017, 78, 1155–1163. [CrossRef]Chen, Y.J.; Cheng, F.C.; Chen, C.J.; Su, H.L.; Sheu, M.L.; Sheehan, J.; Pan, H.C. Downregulated expression of magnesium transporter genes following a high magnesium diet attenuates sciatic nerve crush injury. Neurosurgery 2019, 84, 965–976.
- Chen, Y.J.; Cheng, F.C.; Chen, C.J.; Su, H.L.; Sheu, M.L.; Sheehan, J.; Pan, H.C. Downregulated expression of magnesiumtransporter genes following a high magnesium diet attenuates sciatic nerve crush injury. Neurosurgery 2019, 84, 965–976.[CrossRef]Sheldon, A.; Aleman, M.; Costa, L.R.R.; Weich, K.; Howey, Q.; Madigan, J.E. Effects of magnesium with or without boron on headshaking behavior in horses with trigeminal-mediated headshaking. J. Vet. Intern. Med. 2019, 33, 1464–1472.
- Sheldon, A.; Aleman, M.; Costa, L.R.R.; Weich, K.; Howey, Q.; Madigan, J.E. Effects of magnesium with or without boron onheadshaking behavior in horses with trigeminal-mediated headshaking. J. Vet. Intern. Med. 2019, 33, 1464–1472. [CrossRef]Jamshidi, A.; Hasanzadeh, A.; Zonnour, A.; Dabiri, S.; Yazdani, N. Iatrogenic facial nerve injury in mastoidectomy: The impact of variables on the outcome. Am. J. Otolaryngol. 2022, 43, 103472.
- Jamshidi, A.; Hasanzadeh, A.; Zonnour, A.; Dabiri, S.; Yazdani, N. Iatrogenic facial nerve injury in mastoidectomy: The impact ofvariables on the outcome. Am. J. Otolaryngol. 2022, 43, 103472. [CrossRef]Gougoulias, N.; Hatzisotiriou, A.; Kapoukranidou, D.; Albani, M. Magnesium administration provokes motor unit survival, after sciatic nerve injury in neonatal rats. BMC Musculoskelet. Disord. 2004, 5, 33.
- Gougoulias, N.; Hatzisotiriou, A.; Kapoukranidou, D.; Albani, M. Magnesium administration provokes motor unit survival, aftersciatic nerve injury in neonatal rats. BMC Musculoskelet. Disord. 2004, 5, 33. [CrossRef]Vennemeyer, J.J.; Hopkins, T.; Kuhlmann, J.; Heineman, W.R.; Pixley, S.K. Effects of elevated magnesium and substrate on neuronal numbers and neurite outgrowth of neural stem/progenitor cells in vitro. Neurosci. Res. 2014, 84, 72–78.
- Vennemeyer, J.J.; Hopkins, T.; Kuhlmann, J.; Heineman, W.R.; Pixley, S.K. Effects of elevated magnesium and substrate onneuronal numbers and neurite outgrowth of neural stem/progenitor cells in vitro. Neurosci. Res. 2014, 84, 72–78. [CrossRef]
