1. Introduction
Revolutionary developments in ultrasensitive sequencing approaches have led to the successful implementation of liquid biopsies to inform clinical decisions in patients with advanced
non-small cell lung cancer (NSCLC
) [1][2][3][4][1,2,3,4]. Liquid biopsies provide a minimally invasive method of cancer assessment via the analysis of circulating analytes, such as circulating nucleic acids (circulating tumor DNA (ctDNA) and cell-free RNA (cfRNA)), circulating tumor cells (CTCs), microRNAs, extracellular vesicles, tumor-educated platelets, and tumor-specific cell-free DNA methylation. These analytes can be found in several biological fluids, including plasma, serum, urine, pleural fluid, saliva, and cerebrospinal fluid (CSF)
[3][5][6][7][8][9][10][11][3,5,6,7,8,9,10,11]. The presence of cell-free nucleic acid fragments in the blood was first described by Mandel and Metais in 1948
[12]. Cell-free DNA (cfDNA) consists of fragments of nonencapsulated extracellular DNA, which usually measure between 150 and 200 bp in length and are passively released into the blood by apoptotic and necrotic cells
[13][14][15][13,14,15]. Plasma cfDNA is mostly released by endothelial and peripheral blood mononuclear cells (PBMCs)
[14][15][16][17][14,15,16,17]; in patients with cancer, a small fraction of cfDNA consists of DNA fragments released into the blood by tumor cells, termed ctDNA
[18]. Although several studies have demonstrated that serum has higher concentrations of cfDNA compared to plasma, most of the cfDNA in serum is originated from lysed PBMCs, which makes it more challenging to isolate the ctDNA
[17][19][20][21][17,19,20,21]. Therefore, in an effort to reduce PBMC contamination, plasma samples are preferred for ctDNA analysis
[17]. While plasma ctDNA is relatively prevalent in most patients with advanced solid malignancies (except CNS tumors), the sensitive detection of ctDNA has been historically challenging, largely due to the short half-life of ctDNA (15 min to 2.5 h), and assay specifications are required to mitigate the rate of false-positive variant-calling from clonal hematopoiesis (CH) and background sequencing error
[7][22][23][7,22,23]. With the development of next-generation sequencing (NGS), minimally invasive genotyping via ctDNA now plays an increasingly essential role in clinical practice, particularly via molecular profiling, to inform treatment decisions and serves as a powerful molecular response metric for translational research and precision medicine
[3][24][3,24]. In fact, two commercially available NGS-based assays (Guardant360
®, Guardant Health, Redwood City, CA, USA and FoundationOne
® Liquid CDx, Foundation Medicine INC., Cambridge, MA, USA) have received approval in 2020 by the US Food & Drug Administration (FDA) for identifying genomic alterations in the plasma of patients with advanced-stage solid malignancies, including NSCLC
[25][26][25,26]. Beyond molecular profiling, minimally invasive genotyping via ctDNA for NSCLC is under active investigation for treatment-response monitoring, minimal residual disease (MRD) detection, and early cancer detection
[13][15][13,15].
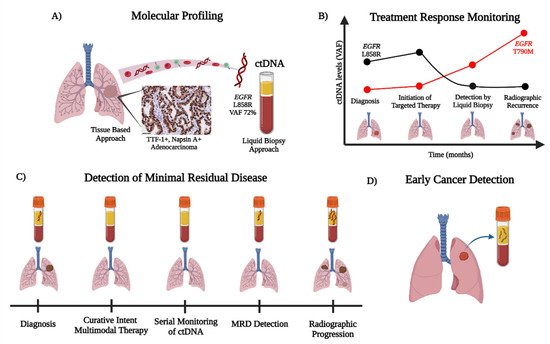
The current applications of ctDNA in the management of NSCLC and future directions for improving personalized care are summarized in In this review, we outline the current applications of ctDNA in the management of NSCLC and discuss future directions for improving personalized care (Figure 1).
Figure 1. Overview of ctDNA detection and the workflow for NSCLC. The detection of ctDNA through liquid biopsies can be utilized for (A) molecular profiling, (B) treatment response monitoring, (C) detection of minimal residual disease, and (D) early cancer detection. (A) ctDNA can be readily used to identify cancer-related aberrations (e.g., detection of the EGFR L858R) with ultrasensitive detection, for ease of use when tissue is limited or exhausted, or as a complementary diagnostic tool with rapid turn-around times. (B) The ease of ctDNA detection by serial blood draws permits treatment response monitoring in the evolution of tumor biology, with the detection of potential mechanisms of acquired resistance (e.g., EGFR T790M on first-generation EGFR TKIs). (C) ctDNA is a powerful tool for minimal residual disease (MRD) detection after curative intent multimodal therapy (i.e., chemotherapy, radiation, and immunotherapy) in locally advanced NSCLC. ctDNA MRD positivity can be detected generally prior to radiographic progression to aid in decision making for patient care. (D) In an effort to improve early cancer detection in high-risk individuals, minimally invasive ctDNA analysis via mutational, methylation, and/or fragmentation profiles offers promising potential to complement radiographic screening with low dose CT chest (LDCT chest).
2. ctDNA for Early Cancer Detection
A low-dose CT scan of the chest (LDCT chest) has been the current standard for lung cancer screening in the US since the National Lung Screening Trial (NLST)
[27][128]. Within this randomized trial, which compared annual LDCT chest for 3 years versus a single chest radiography among asymptomatic participants at high-risk for lung cancer, the LDCT chest was associated with a 20% relative reduction in mortality from lung cancer
[27][128]. However, not all patients will benefit from an LDCT chest as current guidelines do not recommend this screening test for patients without a smoking history or those who have not smoked for many years
[27][128]. Furthermore, LDCT chest has a 96% false-positive rate, thus leading to an excessive use of diagnostic testing for asymptomatic individuals
[27][128]. Incorporating ctDNA analysis into screening may help to address these issues by improving the detection of lung cancer in a broader patient demographic and reducing the number of diagnostic procedures.
The majority of LDCT chest findings are small pulmonary nodules and ground-glass opacities, which may not be malignant or may shed very low concentrations of ctDNA
[28][129]. Akin to the challenges of ctDNA detection in the MRD setting, two major challenges for ctDNA detection during screening are a low VAFs, due to a small tumor volume, and distinguishing tumor-derived mutations from CH
[28][129]. However, as opposed to MRD detection, screening does not provide the option of tumor-informed variant calling, thus making it difficult to detect true biological mutations with a low VAF
[28][129]. As shown by data from the TRACERx study
[29][38], ctDNA for a stage T1b NSCLC tumor of 1 cm is present at an estimated VAF of 0.008% (95% CI: 0.002–0.03%), which is just beyond the detection limit of most liquid biopsy technologies. Therefore, the early detection of stage ≤ T1b tumors via a tumor-naïve approach presents a significant challenge regarding the effective implementation of ctDNA assays in lung cancer screening protocols
[30][130].
Based on ctDNA detection via CAPP-Seq, Chabon et al. developed a machine-learning method, termed “Lung Cancer Likelihood in Plasma” (Lung-CLiP), to estimate the likelihood that a given blood sample contains cfDNA derived from lung cancer, discriminated from risk-matched controls
[28][129]. In addition to tumor-naïve sequencing with a panel of 255 genes that are recurrently mutated in lung cancer, the authors leveraged the unique properties of NSCLC-derived mutations relative to CH mutations (e.g., shorter cfDNA fragment size and tobacco-smoking-associated mutational signatures) to reduce the rate of false-positive variant calling
[28][129]. Lung-CLiP was trained on a discovery cohort of 104 patients with early-stage NSCLC and 56 risk-matched controls who underwent annual LDCT chest screening
[28][129]. At a specificity of 98%, the algorithm yielded a sensitivity of 41% in patients with stage I disease, 54% in patients with stage II disease, and 67% in patients with stage III disease
[28][129]. Compared to tumor-informed ctDNA analysis, Lung-CLiP achieves a similar level of performance without requiring tumor tissue genotyping
[28][129]. Next, the authors prospectively validated Lung-CLiP in a cohort of 46 patients with early-stage NSCLC and 48 risk-matched controls, all of whom were enrolled at a different institution
[28][129]. This independent validation demonstrated a statistically similar level of performance within each stage of disease, thus supporting the external validity of Lung-CLiP to predict the likelihood of lung cancer among similar types of patients
[28][129]. Since most of the patients enrolled in this
res
earchtudy were smokers and displayed incidentally diagnosed lung cancer, further work will be important to study the applicability of Lung-CLiP to never-smokers and patients who otherwise undergo LDCT chest screening.
Methylation-based ctDNA analysis presents an alternative strategy for lung cancer screening that largely avoids the issue of false-positives from CH mutations
[31][32][33][34][48,49,131,132]. As part of the Circulating Cell-free Genome Atlas (CCGA) study, Liu et al. demonstrated a method of targeted cfDNA methylation analysis for multi-cancer early detection
[35][133]. The authors applied bisulfite sequencing targeting a panel of >100,000 informative methylation regions, identified from the first sub-study of CCGA, to plasma cfDNA samples from 6689 participants (2482 with cancer across >50 different cancer types and 4207 without cancer)
[35][133]. Using a methylation-based classifier developed from a training set of the cohort, independent validation yielded a specificity of 99.3%, with a <1% rate of false-positives across all cancer types
[35][133]. Sensitivity of stage I-III disease detection was 43.9% in all cancer types and increased to 67.3% in a pre-specified set of 12 high-signal cancer types, including lung cancer
[35][133]. As expected, the sensitivity of detection improved with increasing disease stage
[35][133]. For lung cancer, sensitivity ranged from ~25% for stage I disease to ~90% for stage IV disease
[35][133]. Of note, localizing the tissue of origin (TOO) was predicted with 93% accuracy
[35][133].
Recently, Klein et al. published their findings from the third and final sub-study of CCGA, a large clinical validation of the methylation-based classifier among 4077 independently enrolled participants (2823 with cancer and 1254 without cancer)
[36][50]. Similar to results reported from the second sub-study by Liu et al., the specificity for detecting cancer was 99.5%
[36][50]. For lung cancer, the sensitivity of detection across all stages was 74.8%, ranging from 21.9% for stage I disease to 95.2% for stage IV disease.
[36][50]. TOO was predicted with 88.7% accuracy
[36][50]. Altogether, these results suggest that methylation-based analysis of ctDNA for multi-cancer early detection may complement existing screening tests for individual cancers. However, given that CCGA is a case-control study, further work will be important for studying the utility of this methylation-based test for screening populations. The ongoing SUMMIT study (NCT03934866) is specifically for UK NHS patients who are at high risk of lung cancer, all of whom will have ctDNA analysis and screening LDCT, with follow-up over several years
[37][134].
In an effort to increase the sensitivity of early lung cancer detection, compared to targeted sequencing of either mutations or methylation in ctDNA, Mathios et al. performed a genome-wide analysis of cfDNA fragmentation profiles
[38][51]. Their method, termed the DNA evaluation of fragments for early interception (DELFI)
[39][135], combines an analysis of genome-wide cfDNA fragmentomes (i.e., evaluating the size distribution and frequency of millions of cfDNA fragments) with clinical risk factors and CEA levels within a machine learning model to detect lung cancer
[38][51]. The authors assessed blood samples from 365 participants who were enrolled in a prospective observational trial (LUCAS cohort), most of whom were symptomatic individuals at high risk of lung cancer
[38][51]. Among these individuals, 129 had lung cancer diagnosed by tissue biopsy after blood collection, 87 had benign nodules, and 149 were not biopsied due to the low clinical and radiographic suspicion of cancer
[38][51].
Median DELFI scores were similar among individuals with benign lesions (0.21) versus those without a biopsy (0.16) and were significantly higher among patients with lung cancer (ranging from 0.35 to 0.99 for stage I to stage IV, respectively)
[38][51]. Among all patients in the LUCAS cohort, the DELFI approach detected lung cancer with an area under the curve (AUC) of 0.90
[38][51]. The AUC increased to 94% when considering only the majority of patients at higher risk for lung cancer (i.e., age 50–80 with a >20 pack per year smoking history)
[38][51]. The predictive performance of DELFI was externally validated in an independent cohort of 385 participants without cancer and 46 participants with predominantly early-stage cancer, and yielded similar levels of sensitivity and specificity as in those observed by the model applied to the LUCAS cohort
[38][51]. Furthermore, the incorporation of genome-wide cfDNA fragmentation data from the binding sites of ASCL1, a transcription factor differentially overexpressed in small cell lung cancer (SCLC), may even enable SCLC patients to be distinguished from NSCLC patients with an AUC of 0.98
[38][51]. Altogether, these findings suggest that ctDNA analysis via DELFI could potentially enhance lung cancer screening
[38][51]. The authors propose a schema in which patients with positive DELFI pre-screening may proceed to LDCT chest analysis for further diagnostic workup
[38][51]. DELFI-L101 (NCT04825834) is an ongoing prospective trial aiming to evaluate a DELFI-based assay among individuals eligible for lung cancer screening
[40][136]. Further work will be important for assessing the utility of DELFI in combination with LDCT chest and other markers of early lung cancer detection.
3. Conclusion
In conclusion, ctDNA analysis via mutational, methylation, and/or fragmentation profiles offers promising potential for improving early lung cancer detection [41][42]. While positive ctDNA screening alone may not be adequate, its minimally invasive nature can provide a valuable pre-screening tool for a broader patient demographic and increase the uptake and adherence of screening with LDCT chest. Perhaps, future studies for cancer interception may utilize ctDNA technologies, such as DELFI and Grail Galleri™, for the emergence of pre-cancerous lesions, to lessen cancer burdens in high-risk patients with a smoking history [36][38]. Therefore, future clinical studies centered on validating multi-omic prediction models that integrate liquid biopsy markers and LDCT will be important for optimizing lung cancer screening moving forward.In conclusion, ctDNA analysis via mutational, methylation, and/or fragmentation profiles offers promising potential for improving early lung cancer detection [52,53]. While positive ctDNA screening alone may not be adequate, its minimally invasive nature can provide a valuable pre-screening tool for a broader patient demographic and increase the uptake and adherence of screening with LDCT chest. Perhaps, future studies for cancer interception may utilize ctDNA technologies, such as DELFI and Grail Galleri™, for the emergence of pre-cancerous lesions, to lessen cancer burdens in high-risk patients with a smoking history [50,51]. Therefore, future clinical studies centered on validating multi-omic prediction models that integrate liquid biopsy markers and LDCT will be important for optimizing lung cancer screening moving forward.