Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Shima Tavakoli and Version 3 by Catherine Yang.
Hydrogels represent a class of materials that are widely used in soft tissue engineering of skin, blood vessel, muscle, and fat. Hydrogels are three-dimensional (3D) networks consisting of physically or chemically crosslinked bonds of hydrophilic polymers. The insoluble hydrophilic structures demonstrate a remarkable potential to absorb wound exudates and allows oxygen diffusion to accelerate healing.
- skin substitutes
- sprayable “smart” hydrogels
1. Sprayable “In Situ” Forming Hydrogels for Wound Applications
In recent years, “in situ” forming, so called “smart” wound dressings have been proposed to overcome key limitations of conventional dressings used for clinical applications. “Smart” hydrogels can be easily crosslinked by using different chemical or physical strategies such as photo-, thermo-, or ionic-crosslinking [1][2][3][56,57,58]. Among the different types of “smart” hydrogels, sprayable dressings are the most suitable “in situ” forming dressings to cover wounds. Those hydrogels exhibit numerous advantages, such as simple application without any specialist’s aid, patient satisfaction, and low production costs. Moreover, spray delivery can increase the penetration of the nanocomposite hydrogel into the wound area, thereby contributing to the improved delivery of active ingredients or therapeutic formulation to the wound [2][4][5][13,57,59]. However, the formulation of a sprayable wound dressing needs to be carefully adjusted to have an optimal viscosity to be applied as a spray-on dressing and cover the wound site uniformly [6][60]. Currently, several “in situ” forming sprayable hydrogels are available as wound dressings. Here, thwe researchers describe recent advances, synthesis process, and biological applications of sprayable hydrogels used as wound dressings, with a particular focus on the use of natural hydrogels.
1.1. Methacrylated Gelatin
Gelatin is a type of natural hydrophilic polymer obtained after hydrolysis and denaturation of collagen under high temperature. Gelatin has some advantages as a wound dressing, including high biocompatibility, solubility, degradability, easy extraction, and synthesis [7][8][61,62]. Moreover, gelatin polymers mimic, to some extent, the natural dermal extracellular matrix and can be therefore easily employed for wound dressings. For example, in a study by Neumann et al. [9][63], a gelatin-based spray-on foam bandage for use on skin wounds was developed. The aqueous gel is first sprayed on the wound site from aerosol containers and effectively covers the uneven wound surface. Subsequently, the sprayed foam dries and adheres to the wound, providing a three-dimensional matrix, which reduces evaporative water loss. Moreover, this foam also possesses antimicrobial activity against Gram-positive, Gram-negative, and fungal contaminants [9][63].
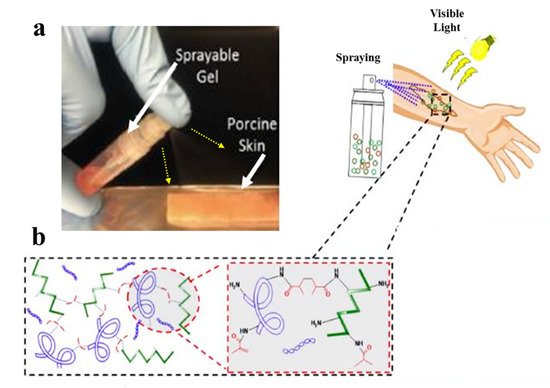
However, gelatin is characterized by insufficient and uncontrollable mechanical properties and high degradation rate for wound healing applications. To overcome these limitations, specific modifications of gelatin such as its methacrylation or blending with other hydrogels, may be applied [10][64]. Accordingly, Annabi et al. [11][65] employed methacrylated gelatin (GelMA) and methacryloyl-substituted recombinant human tropoelastin (MeTro) to synthesize a sprayable MeTro/GelMA composite hydrogel. The authors demonstrated that this sprayable hydrogel can be efficiently crosslinked under visible light exposure. Figure 13 demonstrates spraying of the MeTro/GelMA composite hydrogel over porcine skin and the interactions between monomers with methacrylated groups under light exposure.
1.2. Methacrylated Kappa-Carrageenan
Methacrylated kappa-carrageenan (KaMA) is a hydrogel based on kappa-carrageenan (κCA), a natural linear water-soluble polysaccharide with one sulfated group per disaccharide (25 to 30% ester sulfate content) [12][52]. κCA resembles natural glycosaminoglycan structure and, hence, provides similar physical and mechanical properties to native human skin [13][66]. Importantly, the KaMA hydrogel can be crosslinked chemically under visible light exposure and physically by ion interactions. Recently, Mihaila et al. [2][57] introduced a dual-crosslinkable hydrogel based on KaMA for tissue engineering applications using potassium ions and UV light exposure. The authors used KaMA with different methacrylation degrees (low, medium, and high) and compared distinct mechanical properties of those hydrogels. n another study, Mokhtari et al. [14][67] reported the addition of dopamine functionalized graphene oxide to the dual-crosslinkable KaMA hydrogel network. This modification eventually resulted in increased shear-thinning behavior, which, in turn, improved the injection and spraying abilities of the hydrogel. However, Mokhtari et al. and Mihaila et al. used UV light in their studies, it can cause cell death, DNA damage, immunosuppression, cancer, and accelerating tissue aging [11][65].
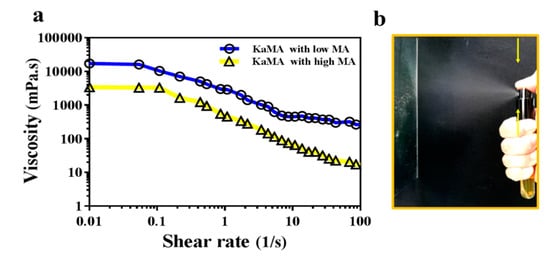
Therefore, in a recent study, thwe researchers optimized this method, using a dual-crosslinking KaMA hydrogel prepared under visible light exposure and ion interaction [6][60]. Furthermore, we demonsthrated that the methacrylation degree (MA) and polymer concentration improved the shear-thinning behavior and viscosity of the KaMA solution. This resulted in excellent spraying properties of KaMA, as confirmed by rheological tests (Figure 24) [6][60]. Generally, spray- or inject-ability of hydrogels could be facilitated with lower viscosity of primary solution due to the reduction in droplet size after pumping, which can vary based on MA degree and polymer concentration [3][6][58,60].
In another study, thwe researchers proposed a “smart in situ” forming nanocomposite hydrogel dressing based on polydopamine modified ZnO (ZnO/PD) in KaMA matrix for diabetic wounds [12][52]. In this study, a KaMA hydrogel containing ZnO/PD nanoparticles was crosslinked solely by exposure to visible light and evaluated thereafter. In addition, L-glutamic acid was added to the KaMA hydrogel matrix to accelerate wound contraction. TheOur results revealed that ZnO/PD and L-glutamic acid could be efficiently encapsulated in the hydrogel network with a controllable release profile. Eventually, in vitro and in vivo tests demonstrated that the nanocomposite KaMA hydrogel spray containing ZnO/PD and L-glutamic acid proved to be highly effective as hemostatic and antibacterial dressing, thus, facilitating a rapid wound closure (Figure 35).
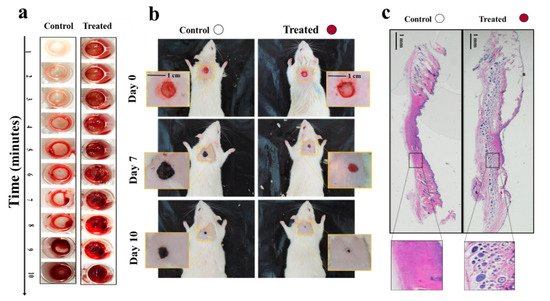
Figure 35. (a) Comparison of blood clot formation time in vitro between the control surface (surface without hydrogel) and the treated surface, containing the nanocomposite hydrogel with ZnO/PD and L-glutamic acid; (b) differences in wound size between control (untreated) wounds and wounds covered with the nanocomposite hydrogel containing ZnO/PD and L-glutamic acid in vivo; (c) histological analyses comparing the appearance of control wounds versus wounds treated with the nanocomposite hydrogel with ZnO/PD and L-glutamic acid in vivo [12][52].
1.3. Chitosan
Chitosan is a natural cationic copolymer with hydrophilic properties, that can be degraded by human enzymes, thus, showing high biocompatibility and biodegradability [15][16][68,69]. Moreover, hitosan-based hydrogels demonstrate excellent bioadhesive, bacteriostatic, and hemostatic properties, with the ability to bind with red blood cells, causing blood to clot [17][18][70,71]. These unique properties make chitosan a promising biopolymer for tissue engineering purposes. In the study of Gholizadeh et al. [19][72], a sprayable thermo-crosslinkable hydrogel based on chitosan biopolymer was tested for a local treatment of nasal wounds. The authors demonstrated that the developed formulation can undergo a rapid liquid-to-gel phase change within approximately 5 min at 32 °C, which is proportional to the human nasal cavity temperature range [19][72]. Another study performed by Mattioli-Belmonte et al. [20][73] revealed the effectiveness of chitin nanofibril/chitosan glycolate-based hydrogels for healing of skin lesions [20][73]. This sprayable hydrogel was compared with two other forms of dressings with similar components, including a gel and a gauze form. Interestingly, the results of this study indicated that the sprayable hydrogel form seems to be a more effective treatment option in healing of superficial skin lesions.
LQD (Brancaster Pharma) wound spray is a new spray-on dressing that contains chitosan FH02™, a unique form of chitosan in aqueous solution. This sprayable wound dressing is primarily indicated for the local treatment of chronic wounds, such as leg ulcers and diabetic foot ulcers, but also for acute wounds and epidermal and superficial partial-thickness burns [21][74]. Several clinicians have evaluated this product in various clinical settings and confirmed its easy and safe application [21][22][74,75]. Moreover, LQD wound spray shows some antibacterial and hemostatic properties due to the presence of chitosan [23][76].
2. Acellular Hydrogel-Based Wound Dressings
There are also conventional wound dressings based on hydrogels which can be produced as films or sheets. Hydrogel wound dressing films can be synthesized from natural or synthetic crosslinked polymers. There are various types of hydrogel dressings, based either on synthetic (poly (methacrylates), poly-vinylpyrrolidine, polyvinyl alcohol, and polyurethane) or natural components [24][77]. Recently, a comprehensive review article by Cascone et al. [25][78] summarized acellular hydrogel-based commercial wound dressings for biomedical applications. Table 12 summarizes the recent and most frequently used commercial acellular hydrogel-based wound dressings, along with their main components and applications [25][78].
| Product | Company | Main Component | Application |
|---|---|---|---|
| Algisite | Smith and Nephew | Alginate | Lacerations, abrasions, skin tears and minor burn wounds |
| Medihoney | Derma Sciences | Alginate | Partial to full-thickness wounds and burns |
| Kaltostat | Convatec | Alginate | Moderate to heavily exuding wounds chronic and acute wounds |
| NU-GEL | Systagenix | Alginate | Management of chronic wounds throughout all stages of healing |
| Condress | Smith and Nephew | Collagen | Chronic and acute wounds |
| Helix3-cm | Amerx Health Care | Collagen | Chronic and acute wounds |
| DermaFilm | DermaRite | Hydrocolloids | Abrasions, closed surgical wounds, superficial ulcers, and skin grafts |
| Comfeel | Coloplast | Hydrocolloids | Designed for difficult-to-dress areas |
| CovaWound | Covalon | Hydrocolloids | Pressure, leg and foot ulcers, superficial partial-thickness burns |
| Inadine | Systagenix | Polyethylene glycol | Open wounds that may become infected |
| Sofargen | Sofar | Colloidal silica | For abrasions, grazes, first- and second-degree burns and injuries |
| Cutimed | Bsn Medical | Dialkylcarbamoyl-chloride | Treatment of necrotic and sloughy tissues in chronic wounds |
| Kendall | Cardinal Health | Glycerin formulation | First- and second-degree burns and partial- and full-thickness wounds |
2.1. Commercial Dermal Substitutes Based on Natural Hydrogels
Natural hydrogels are frequently used as dermal skin substitutes due to their unique properties such as high water content, softness, flexibility, and biocompatibility [24][26][77,79]. Therefore, there are several dermal substitutes on the market that are based on natural hydrogel components.
The Integra dermal regeneration template is one of many off-the-shelf acellular products for the regeneration of dermal tissue [27][80]. Integra artificial dermis is manufactured as a biosynthetic template composed of a porous matrix of crosslinked bovine tendon collagen and glycosaminoglycan, and is covered with a semi-permeable polysiloxane (silicone) layer. This silicone membrane controls water vapor loss, provides a flexible adherent covering for the wound surface, and adds increased tear strength to the template [28][81]. Importantly, the collagen–glycosaminoglycan biodegradable matrix of the Integra enables sufficient ingrowth of host blood vessels and fibroblasts, and provides immediate wound closure and permanent regeneration of the dermis. The Integra is applied on a debrided wound bed, especially in burn injuries [29][30][82,83]. In particular, it is indicated for use in partial- and full-thickness wounds, pressure ulcers, venous ulcers, diabetic ulcers, chronic vascular ulcers, as well as surgical, traumatic, and draining wounds [31][32][84,85]. First, the membrane-covered Integra is placed on the wound usually for 3–6 weeks and allowed to be invaded by fibroblasts and vascularize, before adding keratinocytes. Once the neodermis is formed, the silicone membrane is removed, and autologous split-thickness skin can be applied over the neodermis [33][34][3,86].
Another dermal substitute, Matriderm, is based on a highly porous membrane consisting of bovine type I collagen and elastin [35][87]. The collagen used for the matrix is extracted from bovine dermis, and elastin is derived from bovine nuchal ligament by hydrolysis. Matriderm is employed for dermal regeneration, especially in burn and chronic wounds [36][37][88,89]. Due to its hemostatic properties, Matriderm was also reported to reduce the risk of hematoma formation, which often occurs after skin grafting [38][90]. There are several reports of effective skin engraftment using Matriderm in a one-step procedure in the clinics [39][40][91,92]. Furthermore, one study compared the effectiveness of using Matriderm versus Integra in full-thickness skin defects of immune-incompetent rats [41][93]. Interestingly, although the structure, composition, and crosslinking of these two dermal substitutes are different, the results of this study revealed no significant differences between Matriderm and Integra regarding neodermis formation or vascularization potential, and acceptance of grafts. Moreover, the crosslinking of Integra with glutaraldehyde results in higher resistance of Integra to degradation in comparison with Matriderm, which lacks any crosslinking in its preparation [41][93].
Overall, naturally derived skin substitutes frequently have superior biocompatibility over synthetic polymers. However, it must be noted that they are prone to some batch-to-batch variations, leading to unstable physical and chemical features in some cases [42][94]. Moreover, mechanical properties of skin substitutes derived from natural scaffolds are often poor and need to be strengthened by biodegradable meshes [43][95].
2.2. Commercial Dermal Substitutes Based on Synthetic Hydrogels
Synthetic hydrogels used as skin templates represent some advantages as compared to naturally derived polymers [44][96]. First, they demonstrate predictable and controllable characteristics, such as easy shape control, low production costs, and stable mechanical properties. Second, synthetic scaffolds are convenient to produce in terms of their stable formulation. However, synthetic materials should be selected precisely to exhibit low risk of transplant rejection and disease transmission for bioapplications [45][97].
Polyvinyl alcohol (PVA) is one of the most frequently used synthetic polymers employed as a dermal wound dressing [46][98]. PVA hydrogels are often used in combination with other polysaccharide-based hydrogels, such as starch, alginate, and carrageenan, due to its insufficient elasticity, membrane stiffness, and inadequate hydrophilic properties [26][47][48][79,99,100].
Polyurethane (PU) is another synthetic polymer frequently used in various commercial wound dressings. One example is the NovoSorb biodegradable temporizing matrix (BTM). This is a fully synthetic temporary dermal replacement template based on a biodegradable PU foam, allowing for the infiltration of cells and serving as a matrix for the reconstruction of deeper layers (dermis) of the skin. The PU foam is covered by a non-biodegradable PU seal designed to physiologically close the wound and limit evaporative moisture loss [49][101]. The BTM is applied particularly in deep burns, when split thickness skin grafts alone are insufficient for operative tissue reconstruction [50][102]. Moreover, the BTM was already successfully used for the reconstruction of defects following serial debridement of necrotizing fasciitis, which are associated with a significant mortality rate [50][102]. Moreover, the application of the BTM substitute has been also reported for the reconstruction, following radical debridement and necrotizing fasciitis wounds, which are often deep and poorly vascularized [51][52][103,104]. Interestingly, these studies revealed that the PU structure of the BTM was not affected by the underlying wound infection. Moreover, the authors demonstrated that the BTM continued to integrate into the wound bed following drainage of infected collections through perforations in the seal. This, in turn, resulted in improved healing of those deep and extensive wounds [53][54][105,106]. In addition, the BTM has been also used for the reconstruction of soft tissue defects, such as on the anterior neck, lateral chest and flank, and knee from mid-thigh to distal leg [54][106].
3. Hydrogel Dressings with Integrated Sensors
Nowadays, hydrogel wound dressings are employed to protect and seal injury site. In addition, many of them have been designed to release drugs or compounds in a controllable manner to prevent infection and accelerate the wound healing process [55][56][107,108]. However, they are incapable of providing reliable information regarding general healing status, like its bacterial density, oxygenation amount, inflammation level, temperature, and pH [55][107]. These limitations inspired the development of sensor-based wound dressings, which can provide important information about conditions of the wound. These kinds of wound dressings offer several advantages, including improved wound care treatment, reduced hospitalization time and healthcare costs, and reduction in wound dressing exchanges [57][109].
Recently, various sensors have been developed to measure different biomarkers such as pH, temperature, moisture, oxygen of wound, mechanical and electrical properties of skin or wound, and upregulation or downregulation of enzyme levels [55][107]. Importantly, all of the sensors should have some essential characteristics, including proportional flexibility to the hydrogel film and to body contours, biocompatibility, and non-toxicity to the immune system. Moreover, it is critical that such sensors are resistant to wound exudate. Additionally, for degradable wound dressings, the integrated sensors should be degraded with a proportional degradation rate to the hydrogel matrix rate and with non-toxic degradation debris to the immune system [55][58][59][107,110,111]. Moreover, it is vitally important to design novel dressings with integrated sensors, which are able to monitor the early status of the wound [60][112]. Among different biomarkers, temperature is considered as one of the most promising indicators for an early detection of the inflammation and infection in the wound bed; indeed, abnormal wound temperature variations can be selected as an early predictor of infection before any other symptom emergence [61][113]. On the other hand, it is also important to select appropriate sensors to measure a particular biomarker based on the wound type [62][114]. For this, however, the integration of more than one sensor into a wound dressing is needed. This approach can provide even more accurate information about wound status. However, such multitude sensors-based dressings need a precise design and are, therefore, more expensive.
In a study performed by Tamayol et al. [63][115], pH-responsive alginate-based hydrogel microfibers were used for long-term monitoring of the epidermal wound environment. First, mesoporous microparticles of polyester beads were loaded with a pH sensitive dye, and then, embedded in the alginate hydrogel microfibers by using a microfluidic spinning method for fabricating fibers with diverse shapes and sizes. The pH of wounds is a critical parameter related to angiogenesis, protease activity, and bacterial infection [64][116]. For example, chronic non-healing wounds are known to have a high alkaline environment, while the healing process occurs more efficiently in an acidic condition [65][117]. Thus, fabricated dermal patches capable of continuous pH measurement of the wound are crucial to monitor the healing process and guide the point-of-care treatment to finally improve chronic wound therapy outcome. Figure 46 demonstrates colorimetric pH sensor-based hydrogels carrying beads loaded with pH-sensitive dye and its application on a wound. Importantly, the color of this wound dressing changes rapidly under acidic or basic environments, slowing for long-term monitoring of wound.
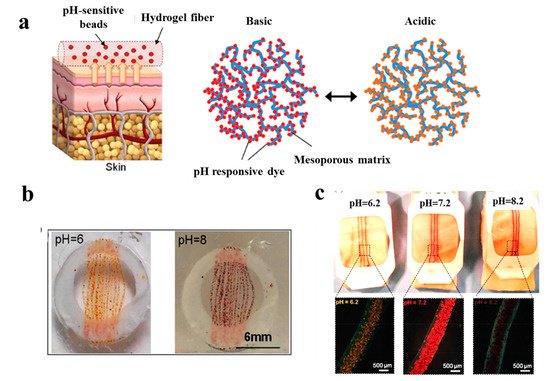
Figure 46. (a) Schematic illustrating the application of a pH sensor-based hydrogel containing pH-sensitive beads on skin and the color changes in basic and acidic environment; (b) macroscopic pictures of hydrogel fiber-based patches with pH-sensitive beads under acidic and basic conditions; (c) images representing color changes of pH-sensitive dressings placed on pig skin when sprayed with solutions of different pH values [63][115].
In another study, a hydrogel-based dressing for the detection of enzymes was applied and studied in infection-sensing wound dressings [66][118]. In this regard, a modified chitosan hydrogel was functionalized with a fluorogenic substrate, which is released by enzymatic degradation (Figure 57). This model is capable of detecting the presence of different types of enzymes by using appropriate fluorogenic or chromogenic substrates. Moreover, it is highly useful for the detection of specific pathogenic bacteria in wound dressings.
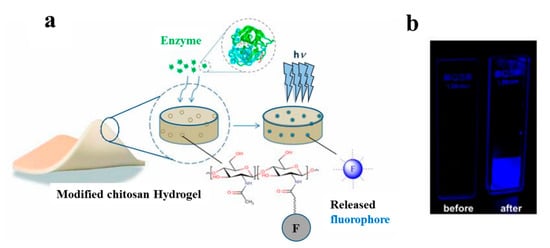
Occhiuzzi et al. [67][119] worked on a hydrogel membrane including a radio-frequency identification (RFID) epidermal sensor for monitoring wound conditions. This hydrogel membrane was synthesized based on a polyvinyl alcohol/xyloglucan (PVA/XG) hydrogel, which is able to absorb wound exudates and release water and drugs or biomolecules (e.g., antibacterial agents or growth factors), thus, providing appropriate conditions for the wound healing process. In addition, the epidermal sensor was capable of measuring the local temperature and could, thus, provide meaningful biological information about the status of the wound.
Moreover, an automated flexible wound dressing for the monitoring and treatment of chronic wounds was developed by Mostafalu et al. [68][120]. This dressing contains numerous components, including a patch with flexible temperature and pH sensors, a hydrogel release sheet, thermo-responsive drug-loaded carriers with an integrated microheater, and an electronic patch (Figure 68). First, potentiometric pH and temperature sensors provide information about bacterial infection and inflammation level, respectively. Afterwards, thermo-responsive drug carriers provide a controllable drug release system in response to temperature changes. The drug-carriers embedded within an alginate hydrogel patch are placed on top of a flexible heater, and then, the entire system is attached to a transparent medical tape to form a wearable matrix. Generally, this sophisticated approach allows for monitoring of pH and temperature in real-time with on-demand drug release. This flexible smart wound dressing has the potential to significantly impact the treatment of chronic wounds in the future.
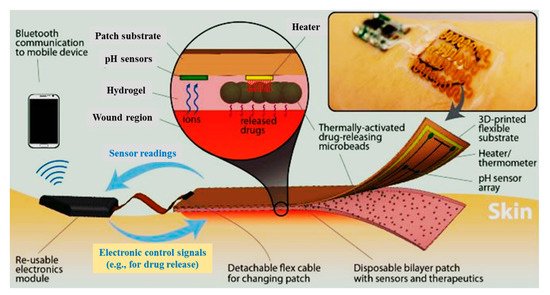
Figure 68. Schematic of a flexible “smart” wound dressing. The dressing is comprised of a pH sensor and a flexible heater to trigger thermo-responsive carriers containing drugs. Drug carriers are embedded in a sheet of alginate hydrogel, casted around the pH sensors and on the heater. Finally, the sensors and the heater are connected to an electronic module that is able to record the data from sensors and power the heater. The electronic module can also communicate with computers and smartphones wirelessly [68][120].
In general, smart wound dressing could revolutionize wound care quality and can have a major effect on therapeutic outcomes. They can solve many of the challenges associated with wound healing, in particular with chronic wounds, by allowing sensing, responding, and reporting information of the wound environment in real-time. Thus, smart wound dressing can improve wound management and long-term clinical outcomes. Moreover, sensors with integrated active drug delivery systems can rapidly respond to potential infections or hyper-inflammation in chronic wounds [55][107].
