Alkaline phosphatase (ALP) is one of the vital phospho-ester bond cleaving biocatalysts that has inevitable significance in cellular systems, viz., early-stage osteoblast differentiation, cell integrity in tissues, bone mineralization, cancer biomarker, liver dysfunction, cellular osmotic pressure, protein folding and many more. Variation from optimal levels of ALP in intra and extracellular fluids can cause severe diseases, including death. Due to these reasons, ALP is considered as a vital biomarker for various preclinical and medical diagnosis. Fluorescence image-based diagnosis is the most widely used method, owing to its simplicity, robustness, non-invasive properties and excellent spatio-temporal resolution (up to the nM/pM level), as compared to conventional analytical techniques, such as the electroanalytical method, nuclear magnetic resonance (NMR) and high-performance liquid chromatography (HPLC). Most of the reviews reported for ALP’s recognition in the literature scarcely explain the structurally related, photophysical and biophysical parameters; and the sub-cellular localizations. Considering these facts, in order to enhance the opto-analytical parameters of fluorescence-based diagnostic materials at the cellular level, herein we have systematically documented recent developments in the opto-analytical capabilities of quencher-free probes for ALP, used in in vitro (biological buffers) to in cellulo conditions, along with in vivo models.
- Alkaline phosphatase (ALP)
- Fluorescent probes
- Intramolecular charge transfer
- Photoinduced charge transfer
- Two photon fluorescence
- Nuclear magnetic resonance
1. Introduction
2. Conceptual Strategies for the Design of ALP Fluorescent Probes in Cellulo Recognition
Most ALP recognition strategies rely on turn-on methods of optical responses in biological fluids. It is one of the essential criteria for ideal biomolecular probes, which trigger significant switch on responses upon interactions with specific analytes in physiological conditions. Selective recognition capabilities of rationally designed probes, either in chemo-dosimetric (non-reversible, chemical changes) or reversible (non-covalently held) types, substantially induce perturbation in the electronic/rotational/translational energy levels of fluorescent units. There are several photophysical properties that have been documented in the literature, such as photoinduced electron transfer (PET), excited state intramolecular proton transfer (ESIPT), intramolecular charge transfer (ICT), C=N isomerization inhibitions, twisted intramolecular charge transfer (TICT) and aggregated induced emission (AIE) mechanisms, which were exploited in the design of various types of ALP probes [5355][5456][5557][5658]. It is evident that most of the reported ALP probes specially designed for cell imaging studies are constructed based on conjugations of fluorescent reporters (fluorophore part), linkers and receptor units. Linkers usually help to hold the fluorophore moiety and receptor unit together without causing steric hindrance in the biocatalytic reaction site. Additionally, receptors (for phosphatase its phosphoester link) help to recognize the biocatalytic reaction sites within the biomolecules in question. Pertaining to the conjoining of these concepts, various ALP probes have been designed through the conjugation of fluorescent cores with phosphoester moieties either directly (without linker) or indirectly (with linker). Given the size of a fluorophore, in order to avoid steric hindrance at the dephosphorylation reaction site, most fluorescent reporters have been constructed with a structurally flexible linker. To achieve the best site-specific diagnostics, sometimes ALP probes are associated with targeting components/functional groups. Depending the requirements, subcellular localization (mitochondria, lysosome, endoplasmic reticulum, etc.) of probes will be regulated by incorporating conventional or non-classical targeting functional groups. The photophysical properties of the designed fluorescent materials depend on core unit, substituents and molecular geometry. Conventionally, symmetric and asymmetric molecular structures substantially regulate the photophysical properties of the fluorophoric units through the cumulative dipole moments of each bond, along with their transitional frequencies (energies) within the molecules. With careful consideration of these facts, various fluorescent probes with peculiar absorption and emission behavior have been designed to recognize biomolecules. Differently, based on a complexation and decomplexation strategy, metal complexes have been used to detect ALP activity [5759][5860][5961][6062]. In this strategy, ALP is indirectly monitored based on the PPi concentration in in vitro digestion assay protocols. Initially, PPi selective fluorescent ligand-metal complexes are prepared; then, metal ions are extruded from the fluorescent ligands by the sequential addition of PPi ions. Eventually, the optical properties of the fluorescent ligand are regulated based on complexation and decomplexation processes in a sequential manner. Upon performing the PPi digestion in the presence of ALP, the residual amount of PPi in the reaction mixture is monitored. Residual PPi in the reaction mixture for ALP activity has been monitored through opto-analytical methods (UV–Vis and fluorescence methods). It is worth mentioning that most efficient ALP probes are associated with the systematic conjugation of the phosphorylated terminus through the self-immobilized moiety (linkers) to the fluorescent reporter, in association with subcellular organelles targeting functional groups, such as mitochondria, lysosomes and the Golgi apparatus, in a sterically free environment (Figure 1). In contrast, indirect methods, such as the use of PPi-regulated organometallic complexes and nano-material-based techniques, could be more effective in in vitro diagnostics rather than in vivo ALP model-based recognition. If these materials are designed rationally, considering site-specific qualitative and quantitative tracking of phosphatase substrates (PPi, nucleotides, etc.), they may provide novel platforms for a future generation of fluorescent materials for ALP activity monitoring based on the indirect approach. Furthermore, this strategy using both the phosphatase and its substrates could be helpful to elucidate the disease states in a biological system at the molecular level.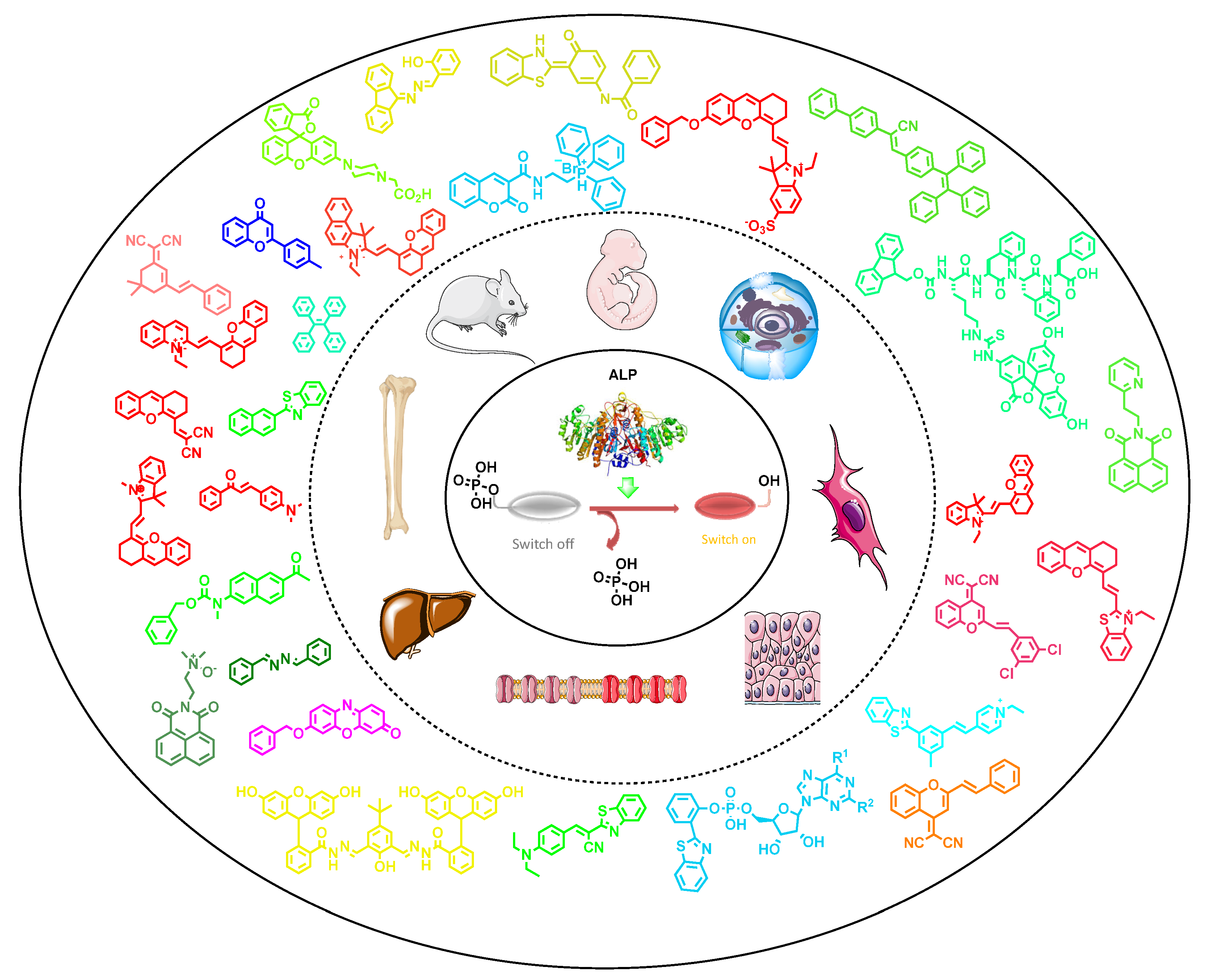
3. Small-Molecule-Based Fluorescent Probes
Small molecular probes are always advantageous compared to labelled methods, where biomolecules such as peptides and nucleic acids are generally covalently attached to the fluorophores [61][62][63][64][65][66][67]. In this labelling approach, where biomacromolecules are site specifically modified with fluorescent labels, usually suffers from various drawbacks—viz., susceptibility to cellular ions, proteases and nucleases; and always demanding transfecting agents to enter the cells. In contrast, small molecules can enter the cells through passive modes without getting degraded by proteases or nucleases [6668][6769][6870]. Furthermore, small molecules are usually not accompanied by quenching units (such as BHQ) and can have a ability to trigger dual emission (AIE-based materials monomer ↔ excimer) depending on the molecular confinements. Considering these facts, various small molecule based blue/green/red/NIR emissive fluorescent materials were designed for the recognition of ALP. Zhang et al. reported phosphorylated tetraphenylethylene-based, AIE-based fluorescent probes ALP-1, ALP-2 and ALP-3 [6971] for monitoring of ALPs during osteogenic differentiation of stem cells (Figure 2). In these probes, hydrophilicities, were tuned by incorporating mono-, di-, and tetra-phosphate units at the periphery, resulting in a very low quantum yield in aqueous conditions. Probes showed characteristics λmax at ~338 (±4) nm and λem at 460 (±10) nm in UV–Vis and fluorescence spectra, respectively. In contrast, upon incubation with ALP, the probes showed a turn response within 5 min, at the bluish green region centered at λem = ~470 nm, which was blue shifted to λem = ~450 nm after 60 min.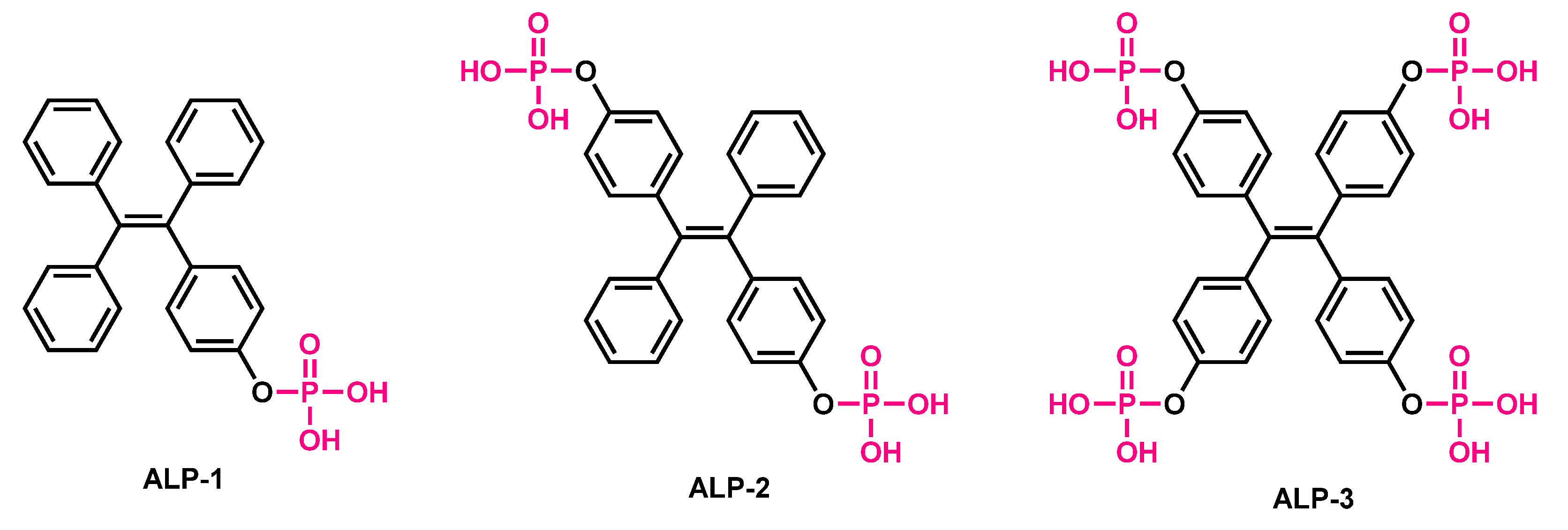
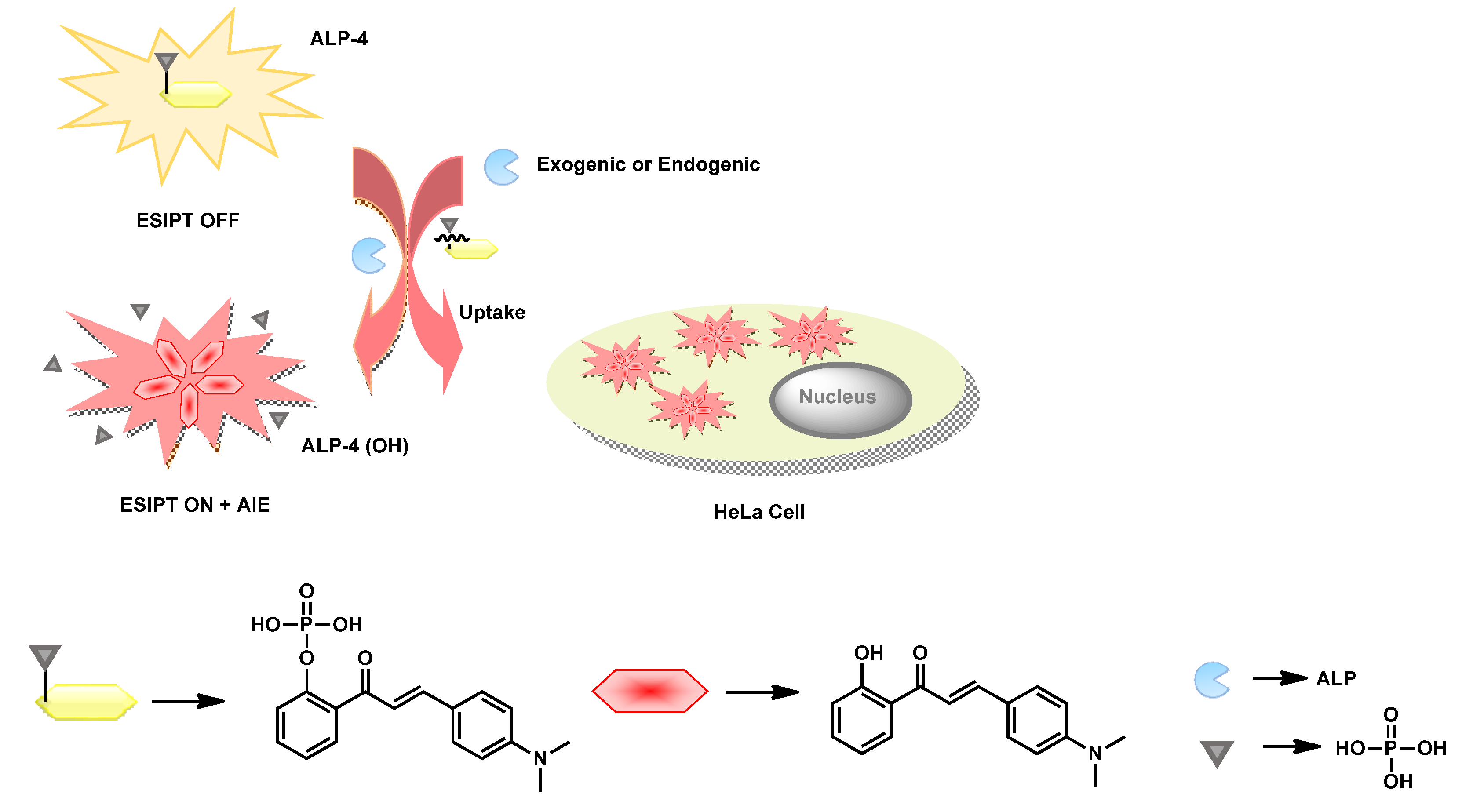
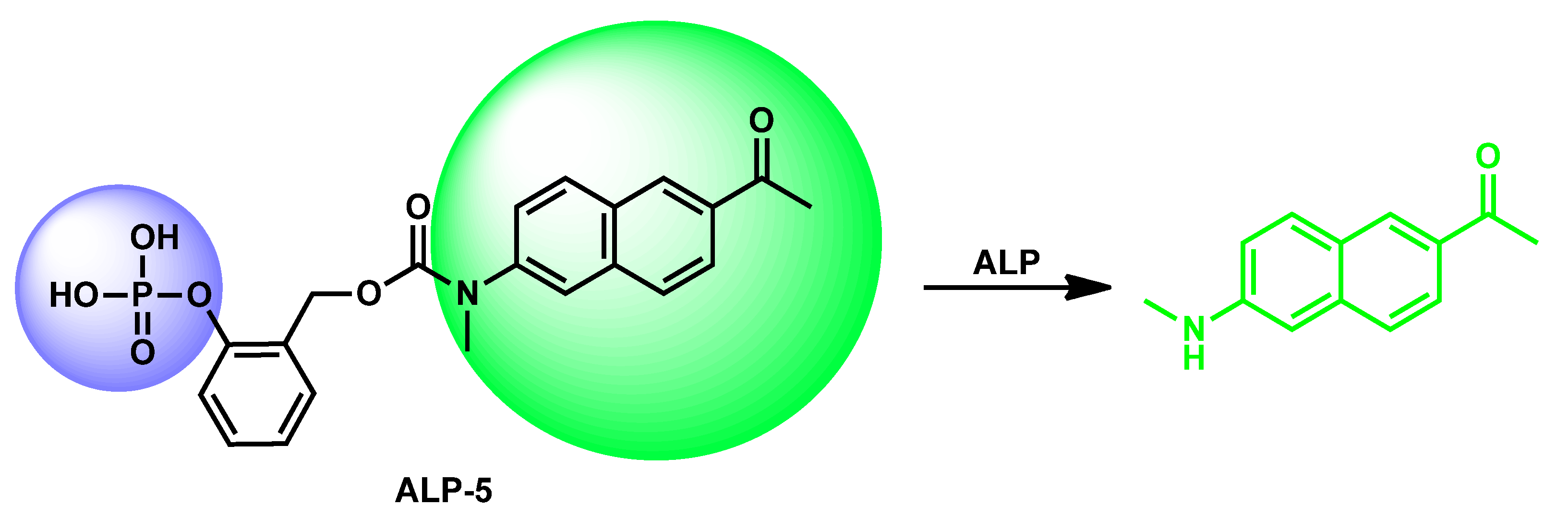
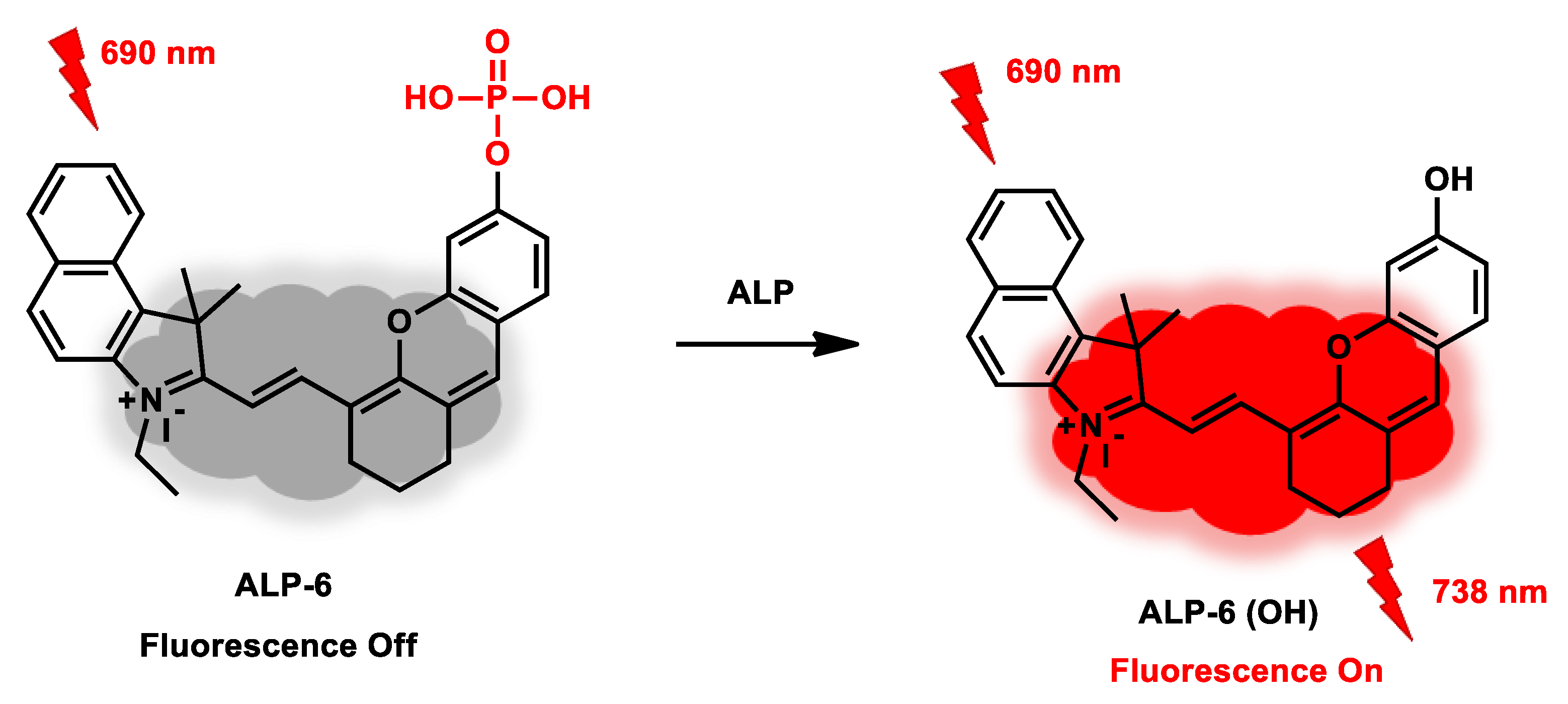
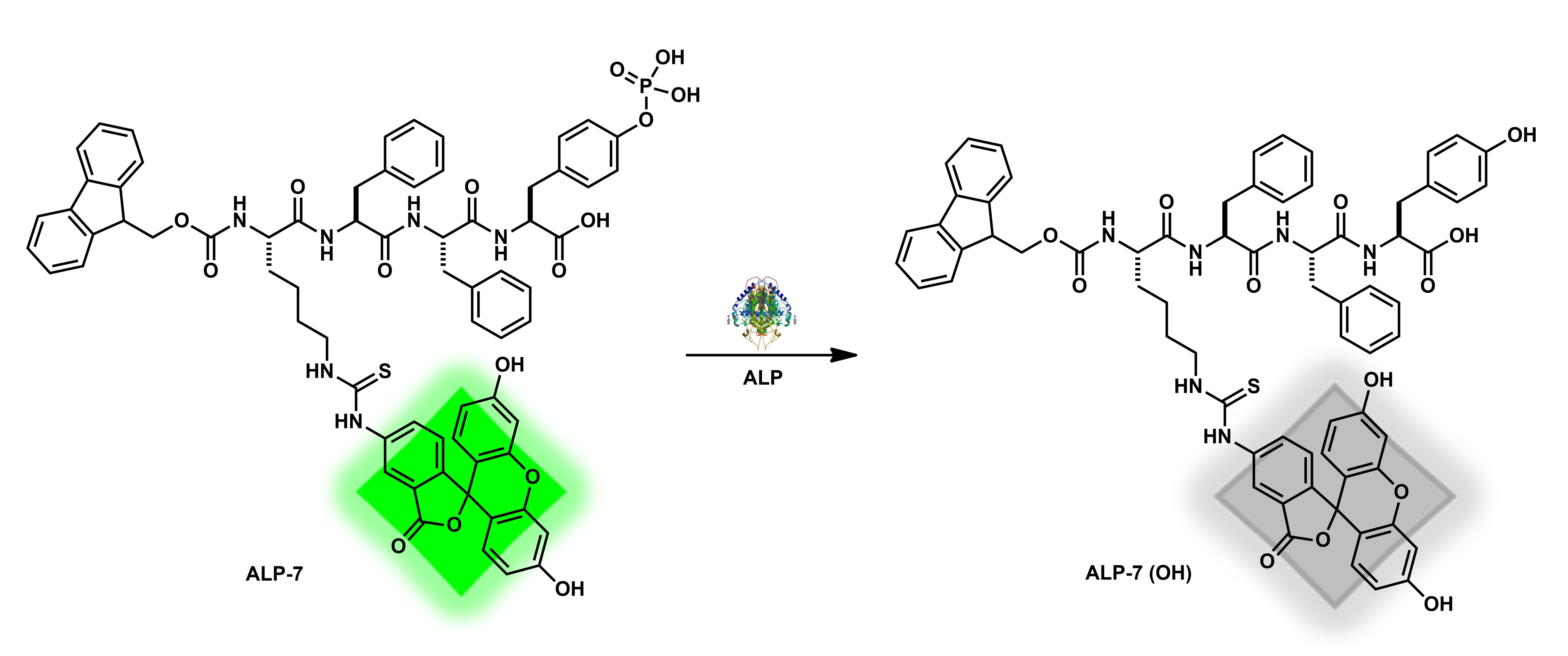
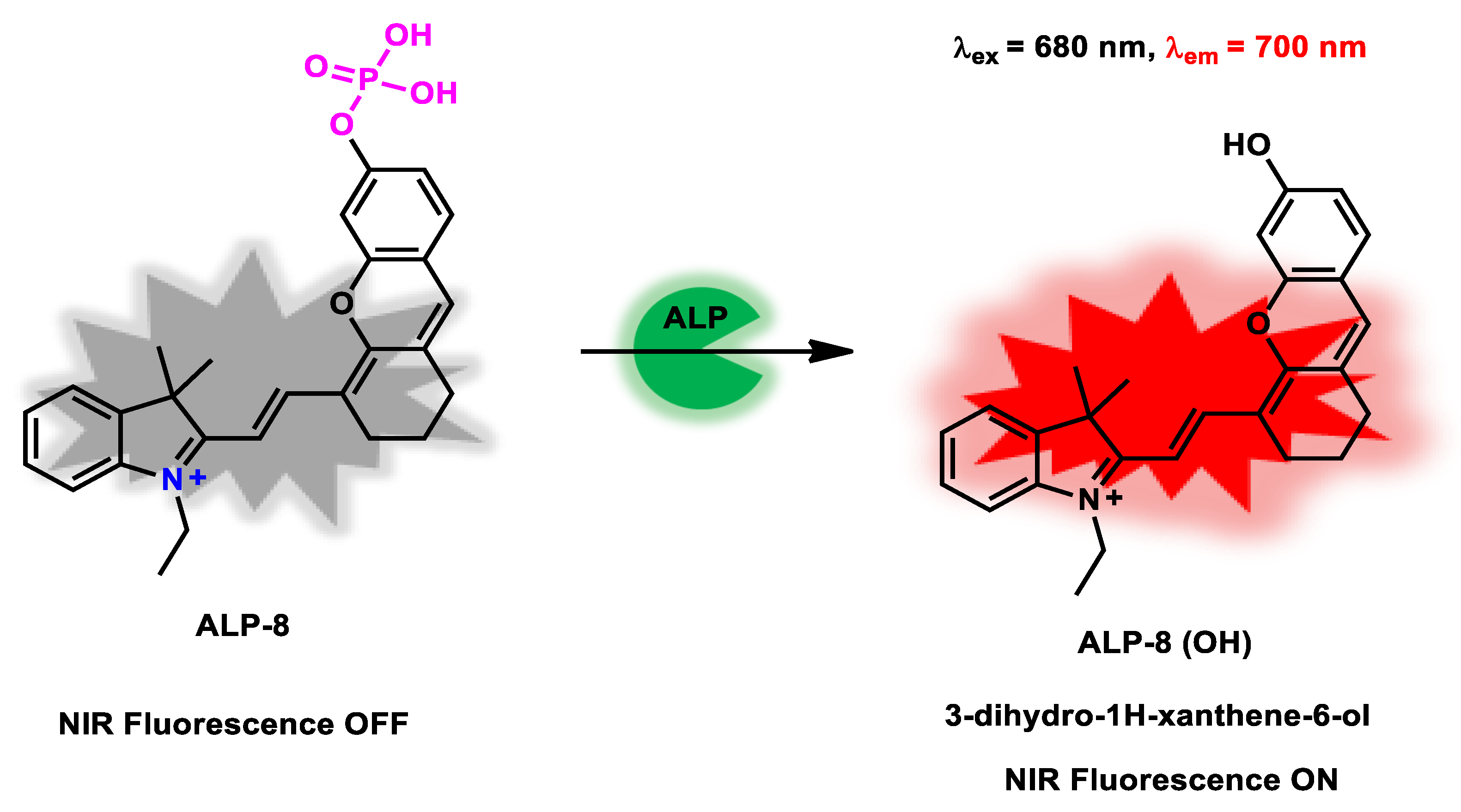
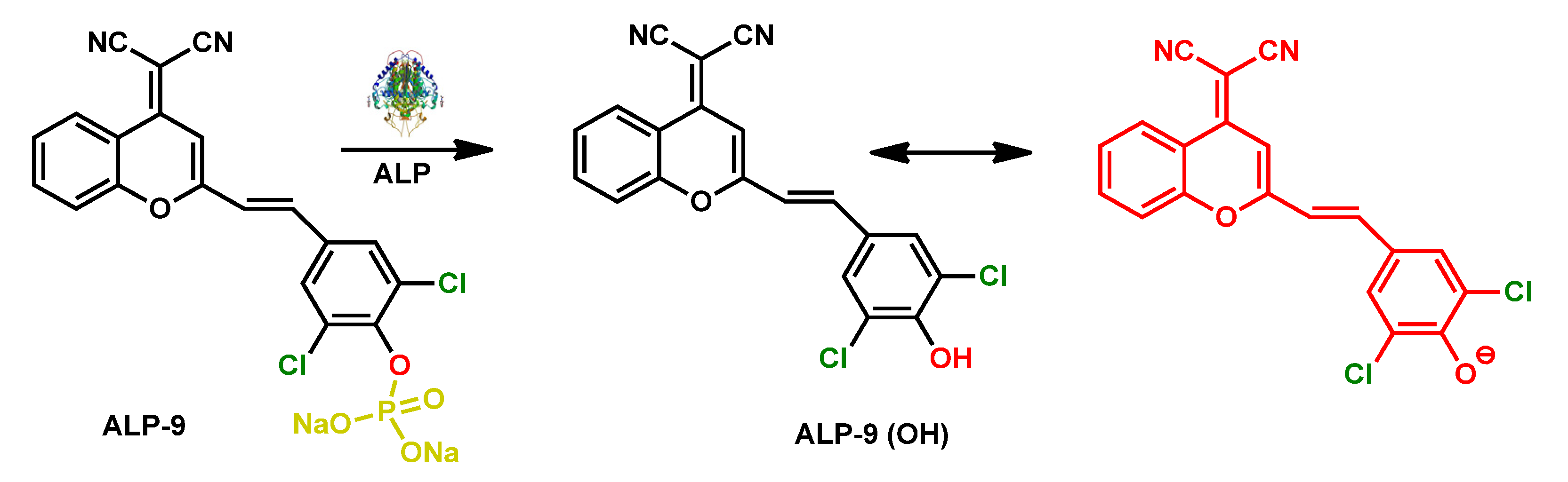
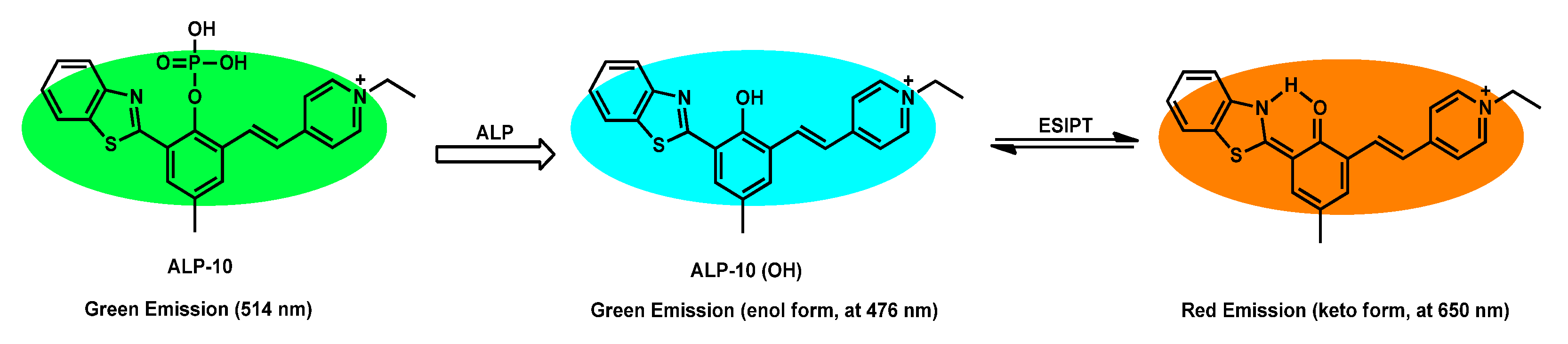
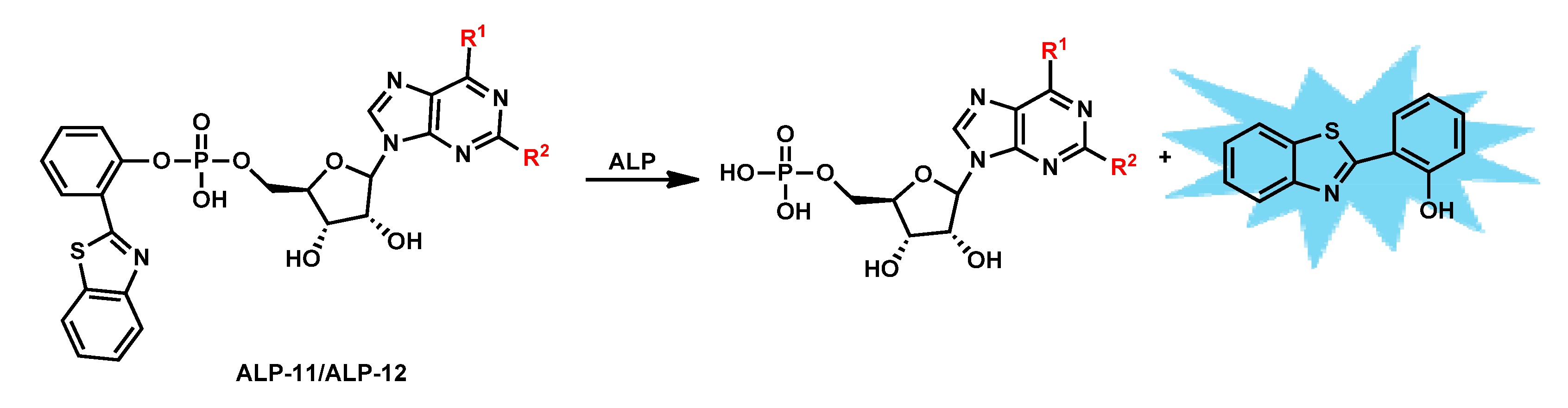
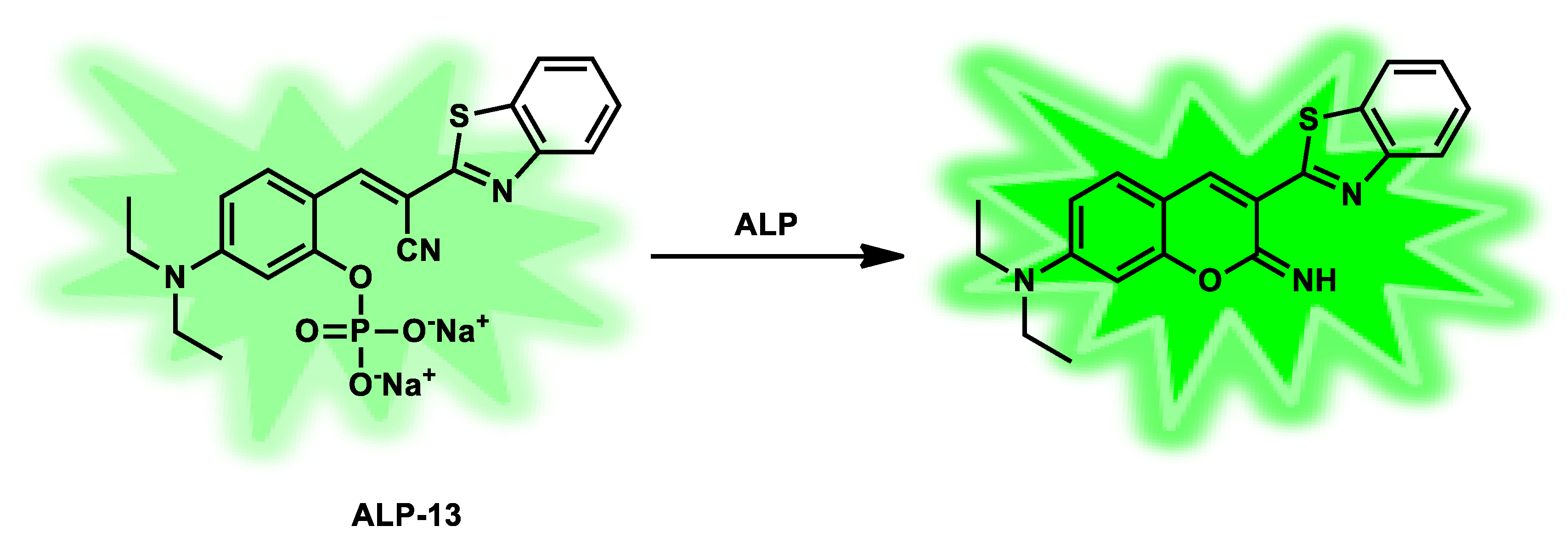
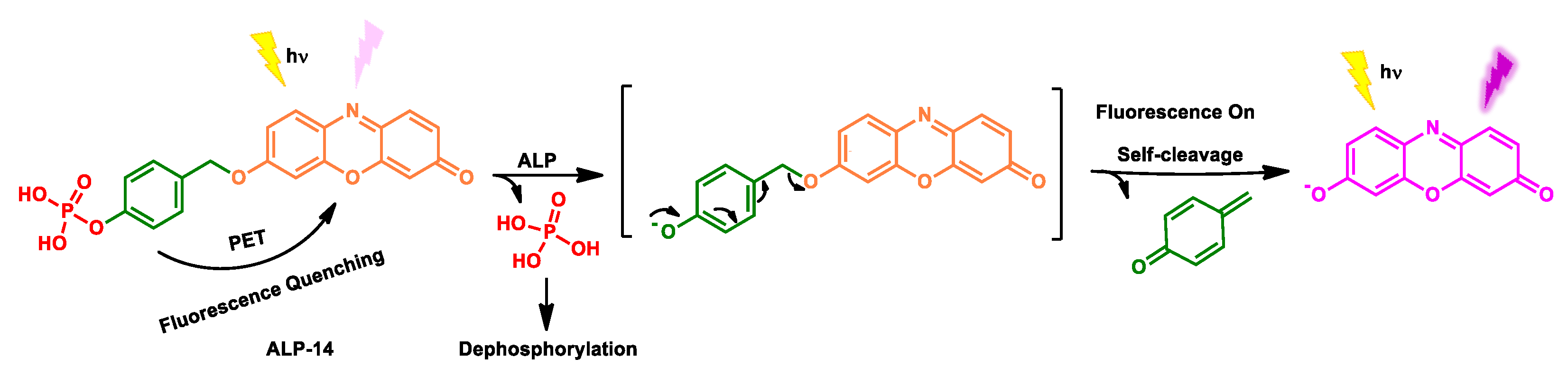
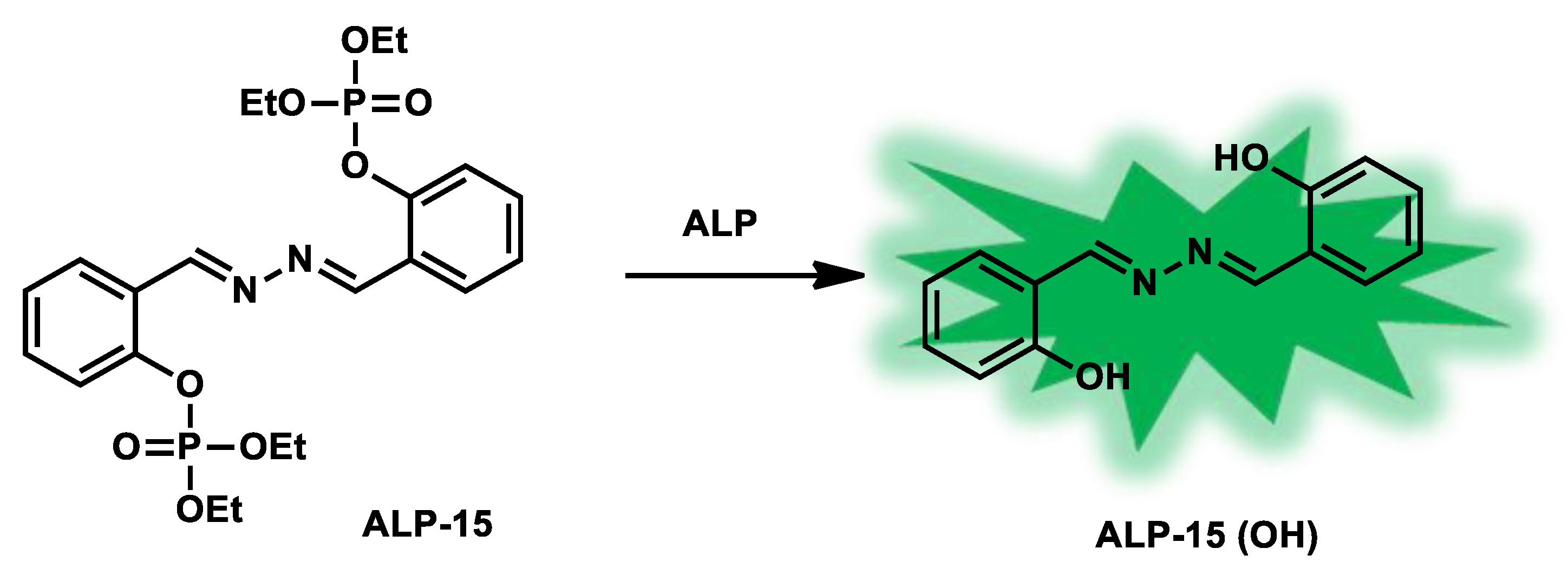
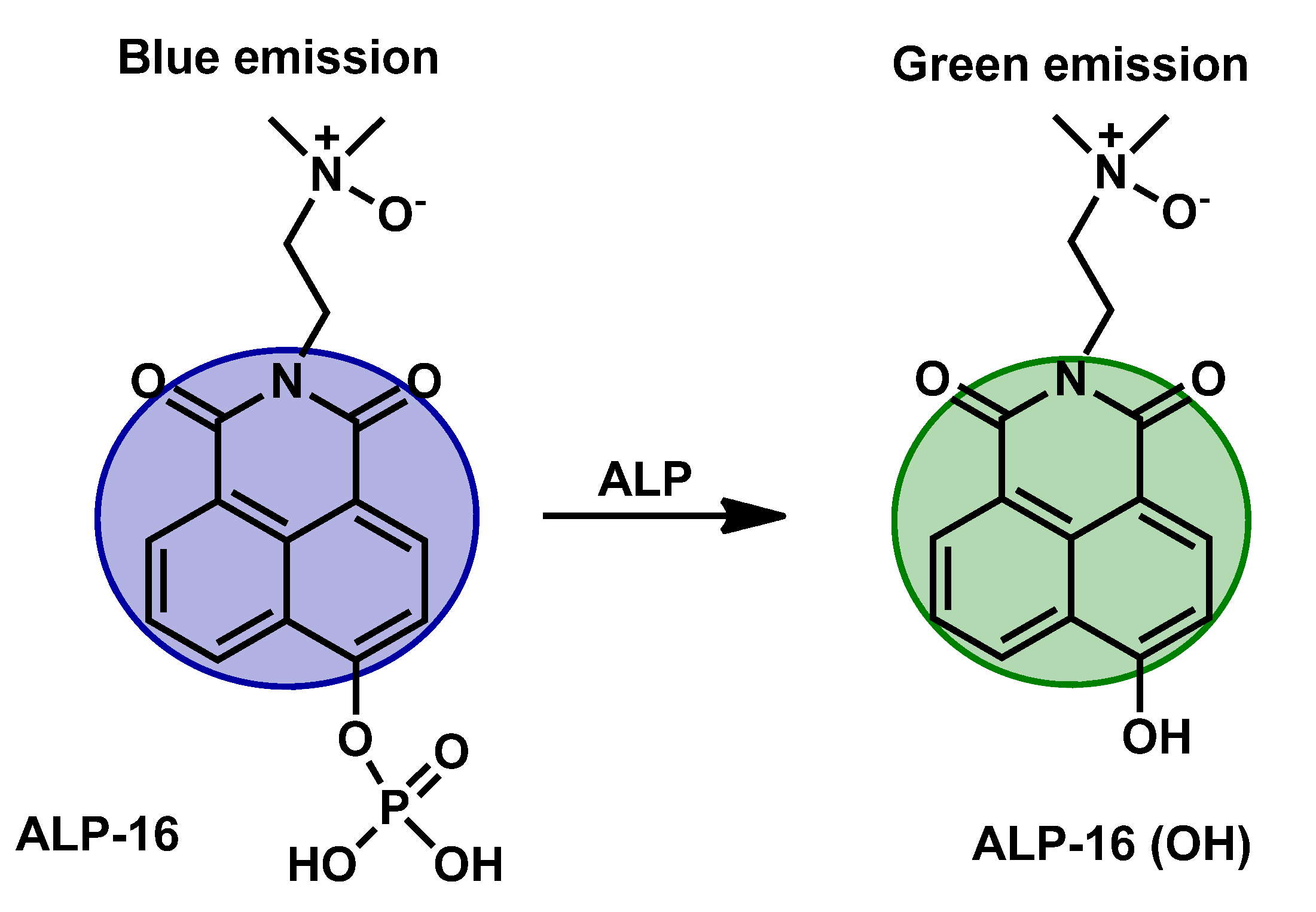
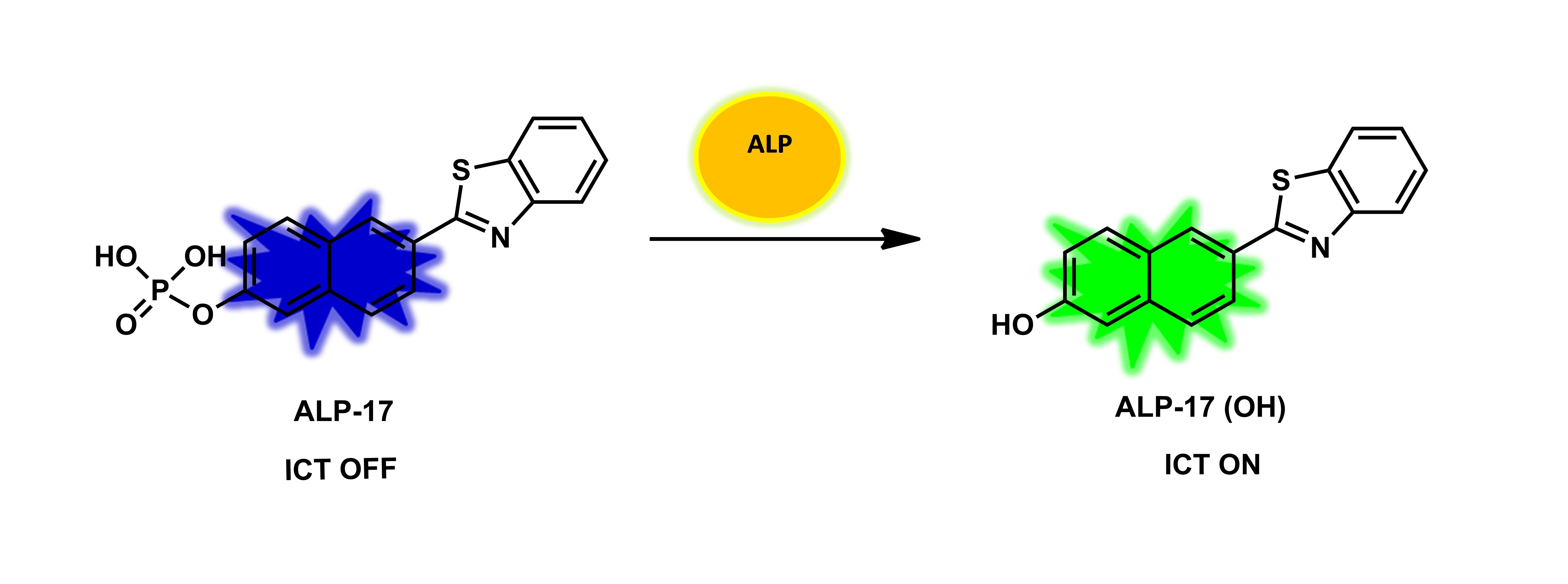 Figure 15. The structure of ALP-17 and a plausible phosphatase recognition mechanism.
Figure 15. The structure of ALP-17 and a plausible phosphatase recognition mechanism.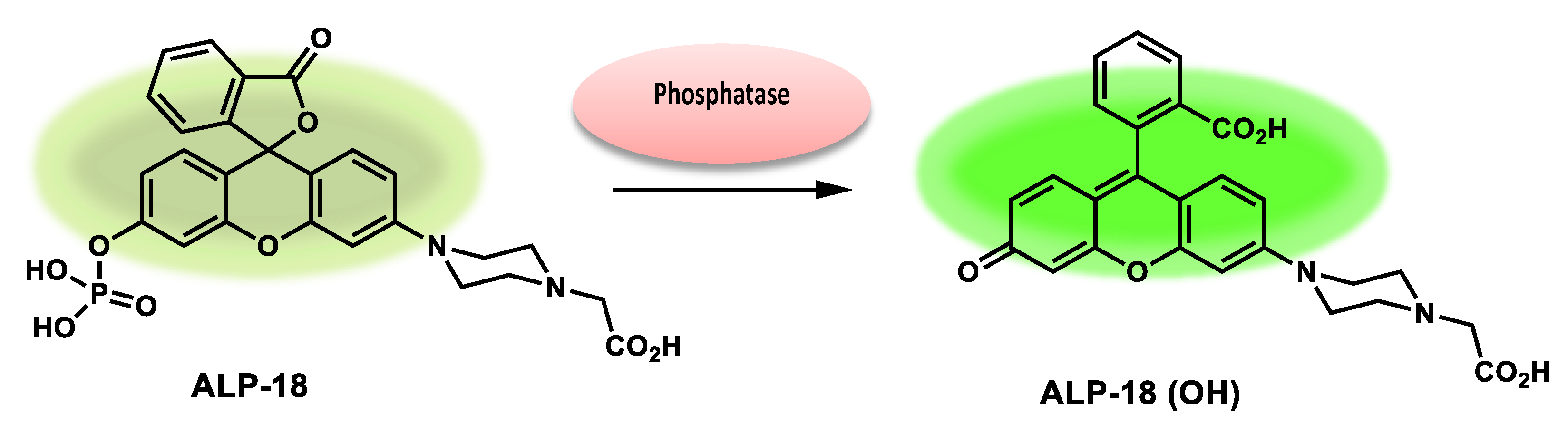
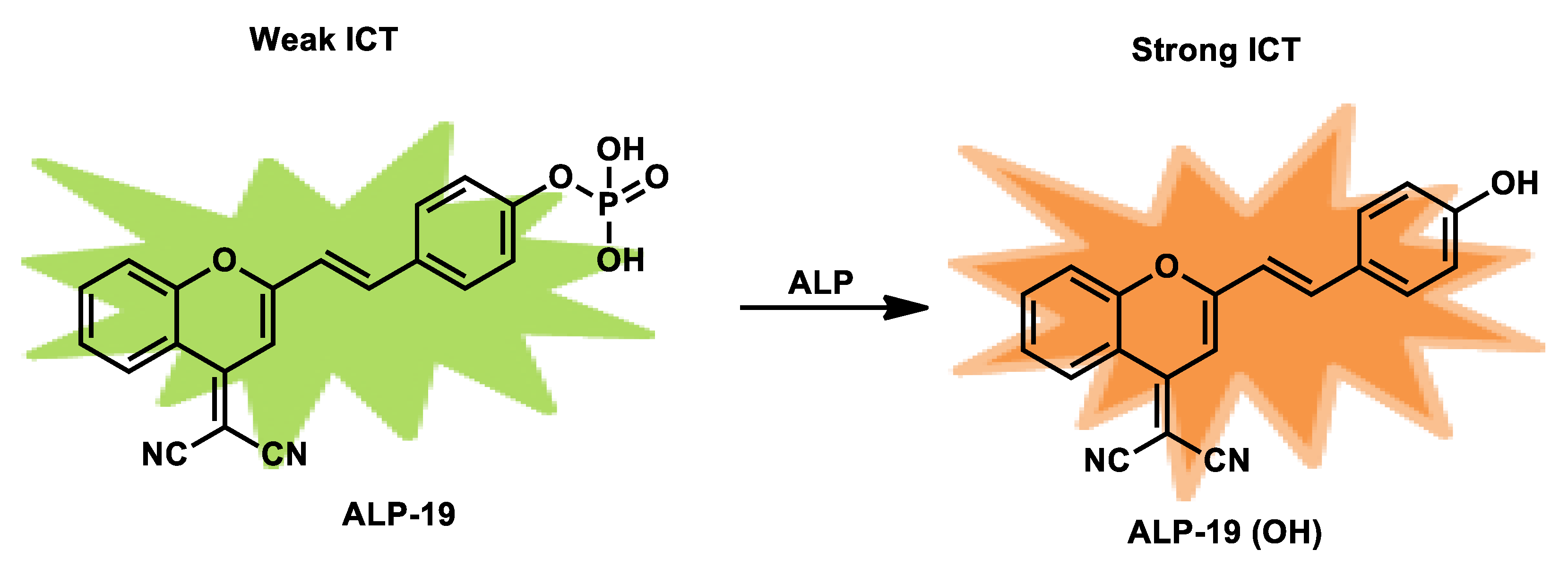
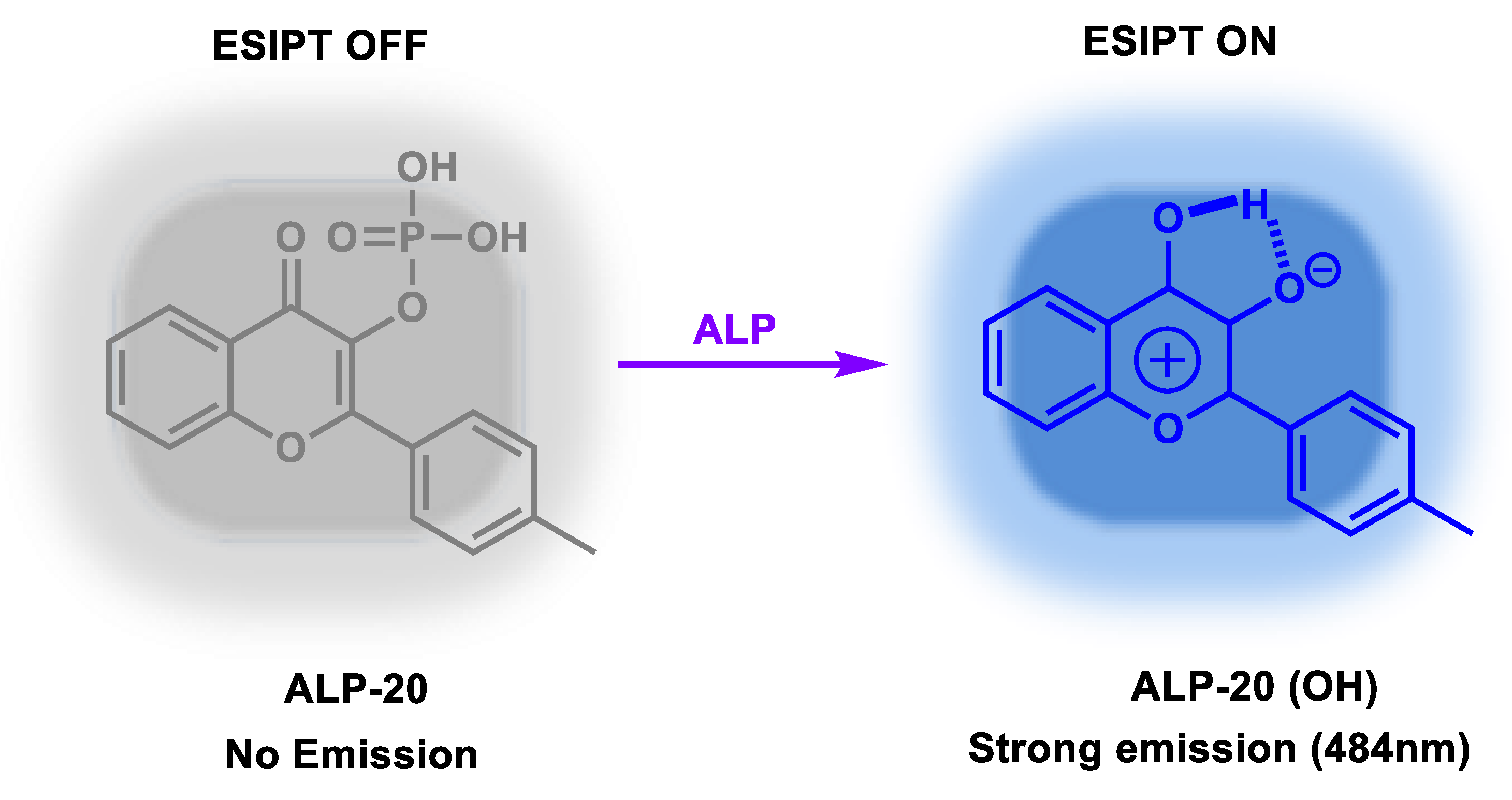
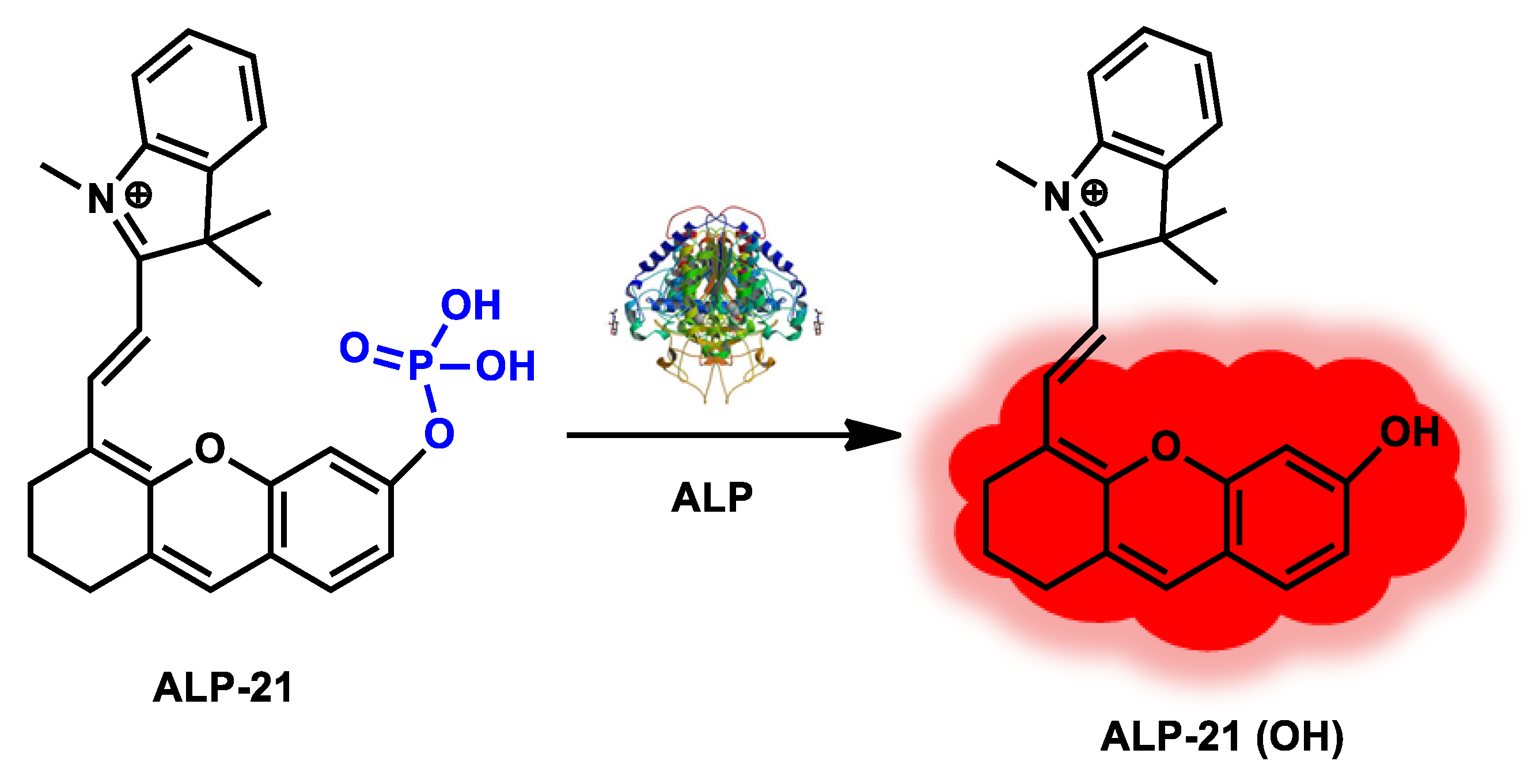
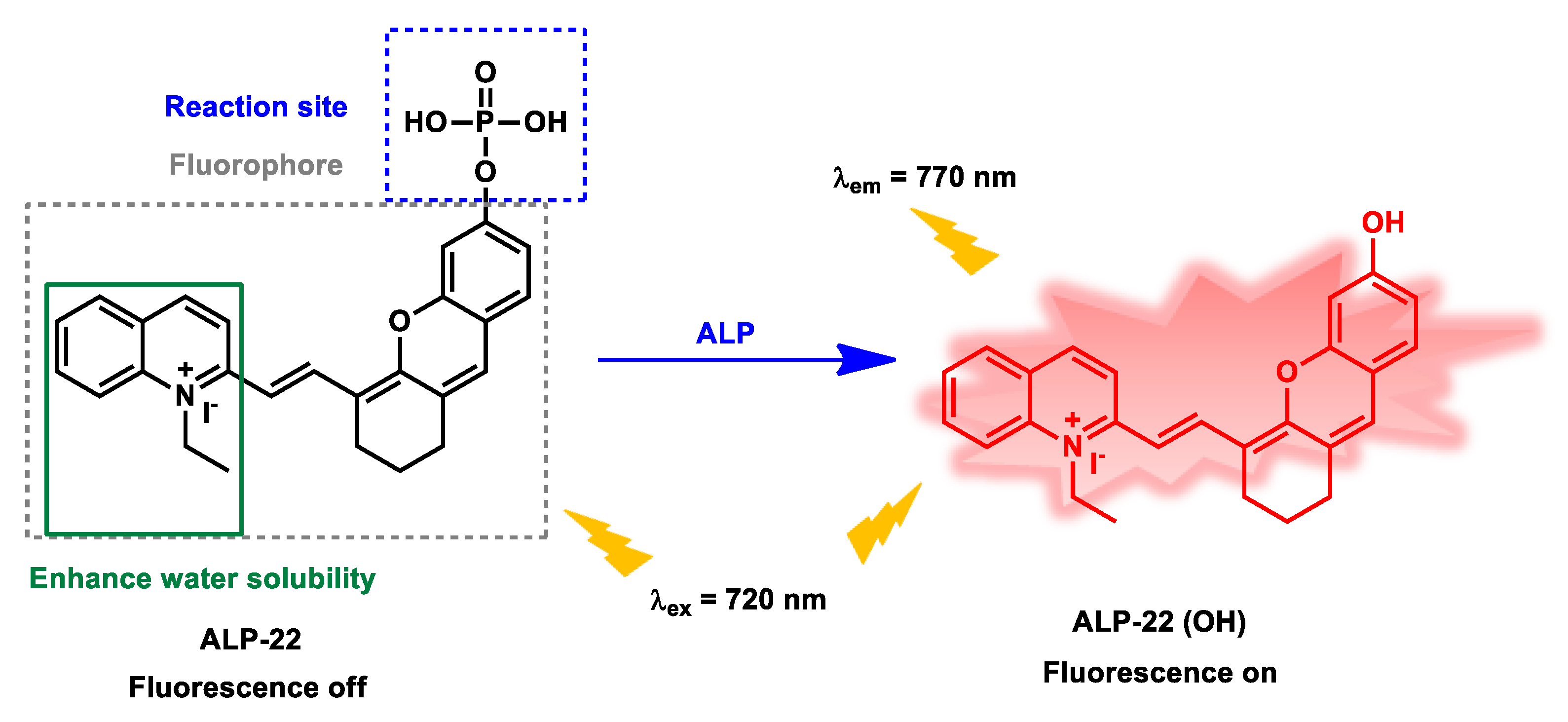
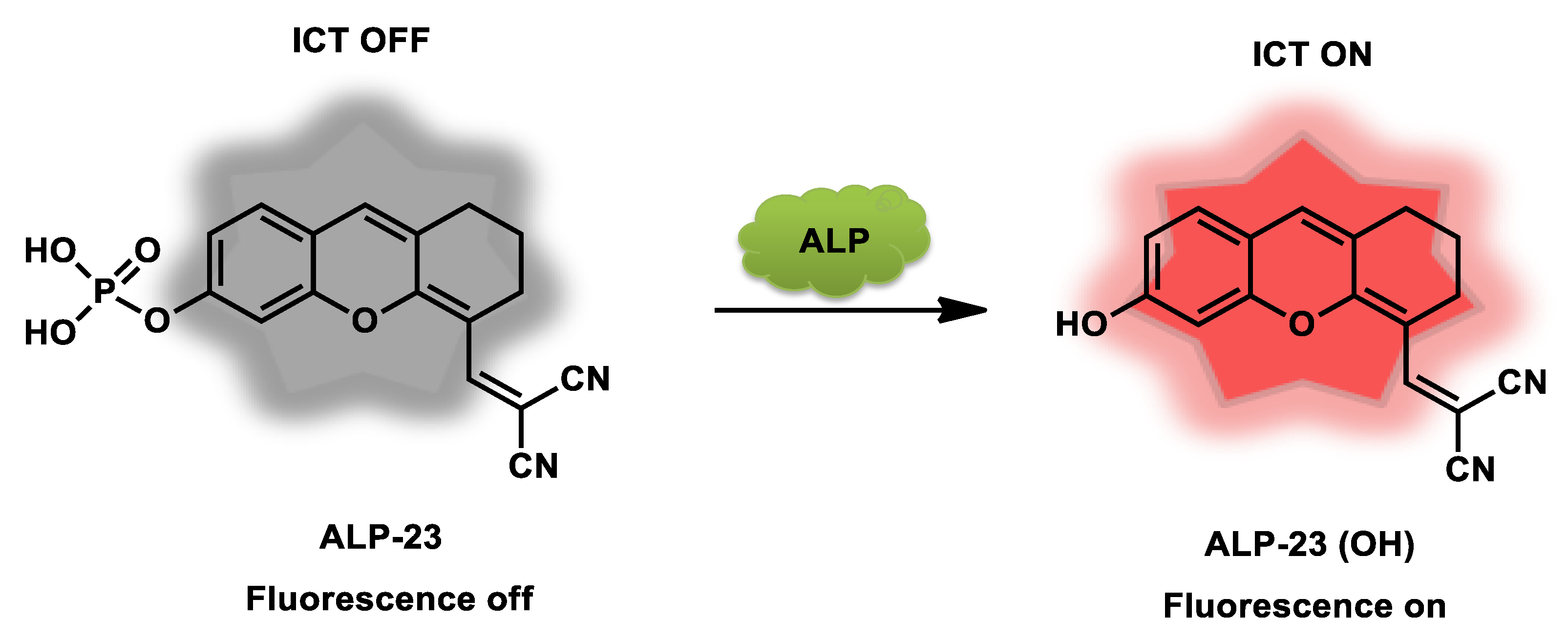 Figure 219.
Figure 219.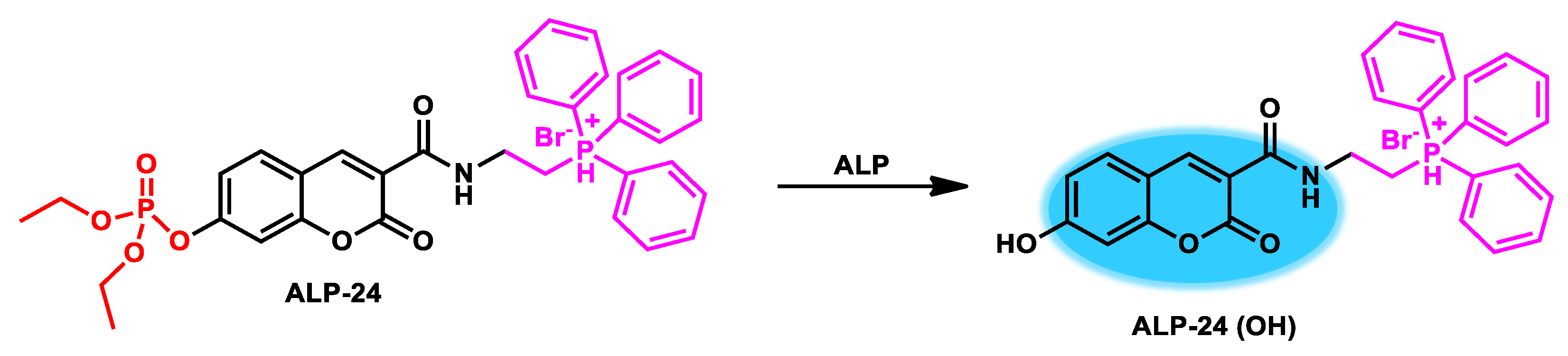
| Probes | Linear Range | kcat/KM Value | Detection Limit |
Response Time |
Stokes Shift |
λex/λem (nm) | Quantum Yield (Φ) |
IC50 Value | Sensing Mode | Application | Cytotoxicity | Cellular Localization |
Ref. |
| ALP-1, ALP-2, ALP-3 |
10–50 mU/mL, 10–40 mU/mL |
/ | / | / | / | 338/469, 337/457, 337/469 |
0.075, 0.118, 0.101 |
/ | Turn-on Fluorescence |
Cellular imaging in living stem cells |
30 µM | Bone marrow mesenchymal stem cells |
[71] |
| ALP-4 | 0–150 U/L | / | 0.15 U/L | / | 211 nm | 430/539, 641 | / | / | Ratiometric | Live cell imaging | 20 µM | Intercellular fluids | [72] |
| ALP-5 | 0.5–10 U/L | / | 0.30 U/L | / | 135 nm | 365/500 | / | 7.39 µM | Turn-on Fluorescence |
In living cells and tissues |
20 µM | Cellular and tissue lysates, cytoplasm |
[73] |
| ALP-6 | 0.01–2.0 U/mL | / | 0.003 U/mL | 20 min | / | 690/738 | / | 141.9 µM | Turn-on Near-Infrared Fluorescence |
In Vivo Cell, tissue and living animal |
30.0 µM | / | [74] |
| ALP-7 | 0–2.8 U/mL | / | 0.06 U/mL | 2 h | / | 465/530 | / | / | Turn-Off Fluorescence |
in Vitro and in Living Cells |
160 µM | / | [75] |
| ALP-8 | 0–0.1 U/mL | 1.01 × 105 M−1 s −1 |
0.07 U/L | 2 min | / | 680/700 | / | 7.51 µM | Off−On Near-infrared Fluorescence |
Living Cells and Mice |
20 µM | / | [76] |
| ALP-9 | 0.5–8.0 U/L | / | 0.072 U/L | / | / | 535/683 | / | / | Rational Turn-on Red-Near-Infrared fluorescence |
In vivo and in vitro |
50 µM | Mitochondria, Lysosome |
[77] |
| ALP-10 | 0–60 mU/mL | / | 0.072 mU/mL |
/ | 260 nm | 390/476, 650 | 0.16 | 10 µM | Ratiometric | living cells and in vivo |
10 µM | Mitochondria | [78] |
| ALP-11, ALP-12 | / | / | / | / | / | 405/500–530 | / | 10 µM | Turn-on Fluorescence |
Living cells and zebrafish model |
10 µM | / | [79] |
| ALP-13 | / | 1.4 × 104 M−1 s −1 |
/ | / | / | 472/542 | 0.002 | 36 µM | Turn-on Fluorescence |
Living cells | 10 µM | / | [80] |
| ALP-14 | / | 1.7 × 104 M−1 s −1 |
1.09 U/L | / | / | 550/585 | 0.0023 | 7.58 µM | Turn-on Fluorescence |
Living cells | / | Cytosol | [81] |
| ALP-15 | 0–10 U/L | / | 0.012 U/L | / | 180 nm | 356/536 | / | / | Turn-on Fluorescence |
Living cells | / | / | [82] |
| ALP-16 | / | / | 0.38 U/L | / | / | 425/554 | / | / | Ratiometric | In vivo | 5 µM | / | [83] |
| ALP-17 | 20–180 U/L | / | 2.3 U/L | / | / | 405/410–460, 470–530 |
0.41 | / | Ratiometric | Living cells | 20 µM | / | [84] |
| ALP-18 | / | 13.6 × 105 M−1 s −1 |
0.011 U/L | / | / | 490/540 | 0.0487 | / | Turn-on Fluorescence |
In vitro, cell imaging and cell cultures |
100.0 µM | Lysosome | [85] |
| ALP-19 | 50–200 U/L | / | 3.8 U/L | 30 min | / | 440/550 | 0.105 | / | Ratiometric | Living cells | 10 µM | / | [86] |
| ALP-20 | / | / | 0.032 U/L | / | / | 400–410/455 | 0.085 | / | Turn-on Fluorescence |
Live cells and serum samples |
50 µM | / | [87] |
| ALP-21 | 1–30 U/L | / | 0.28 U/L | / | / | 680/690–800 | / | / | Turn-on Fluorescence |
In vivo and in vitro |
20 µM | / | [88] |
| ALP-22 | 0.05–1.0 U mL−1 |
/ | 0.017 U mL−1 | / | / | 720/770 | / | 109.6 µM | Turn-on Fluorescence |
Cell imaging, treatment of diabetes |
30 µM | / | [89] |
| ALP-23 | 5–100 U/L | / | 0.28 U/L | / | / | 600/620–850 | / | 141.4 µM | Turn-on Fluorescence |
Living cells and zebrafish |
10 µM | / | [90] |
| ALP-24 | / | 3.12 × 106 M−1 s −1 |
2.921 ng/mL | / | / | 410/450 | 0.567 | / | Ratiometric | Live cells | 50 µM | Mitochondria | [91] |
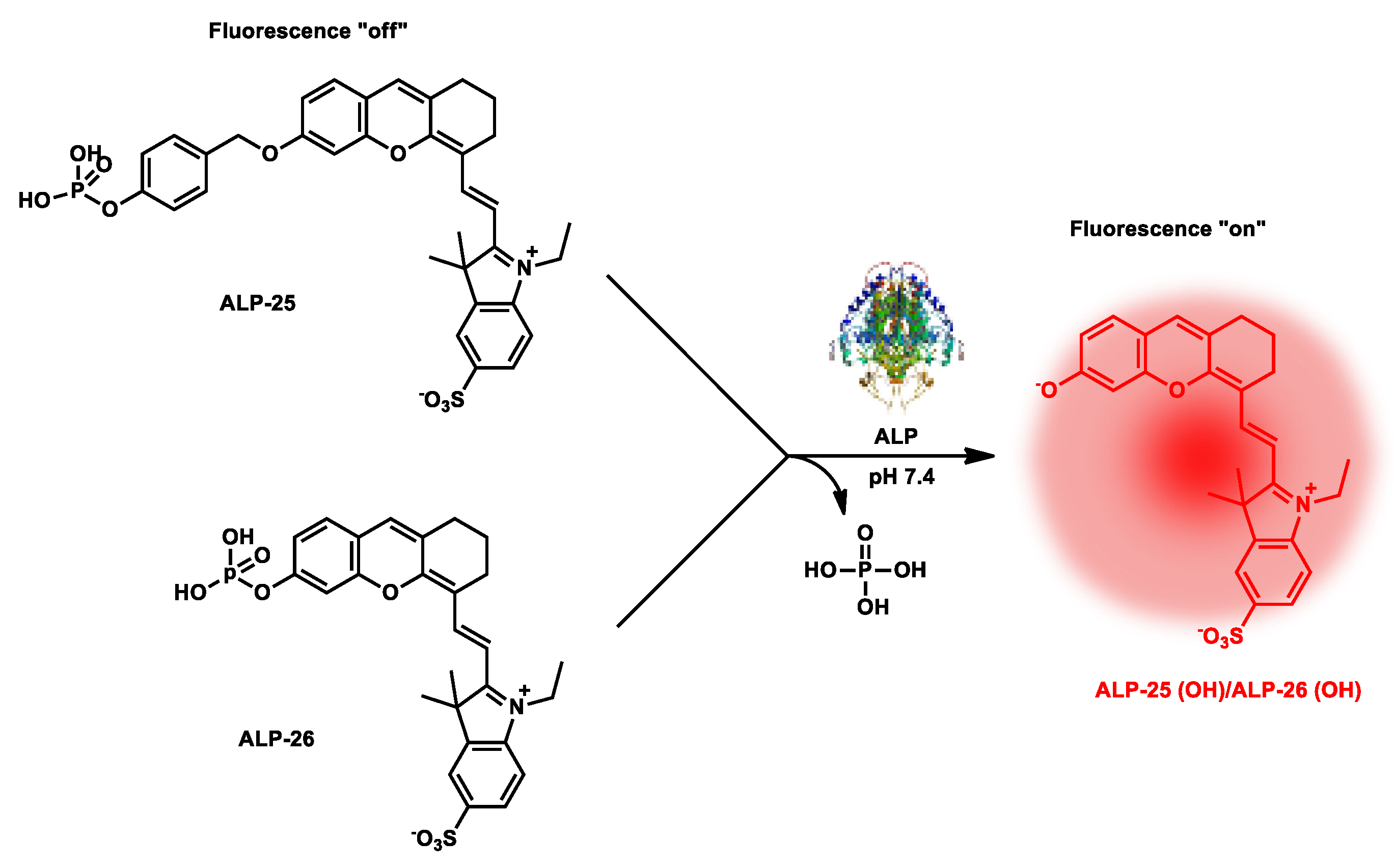
| ALP-25, ALP-26 |
0–1.0 U mL−1 | / | 10−5–10−3 U mL−1 |
1.5 min | / | 685/710 | 0.13 | / | Turn-on fluorescence |
In vivo | 50 µM | / | [92] |
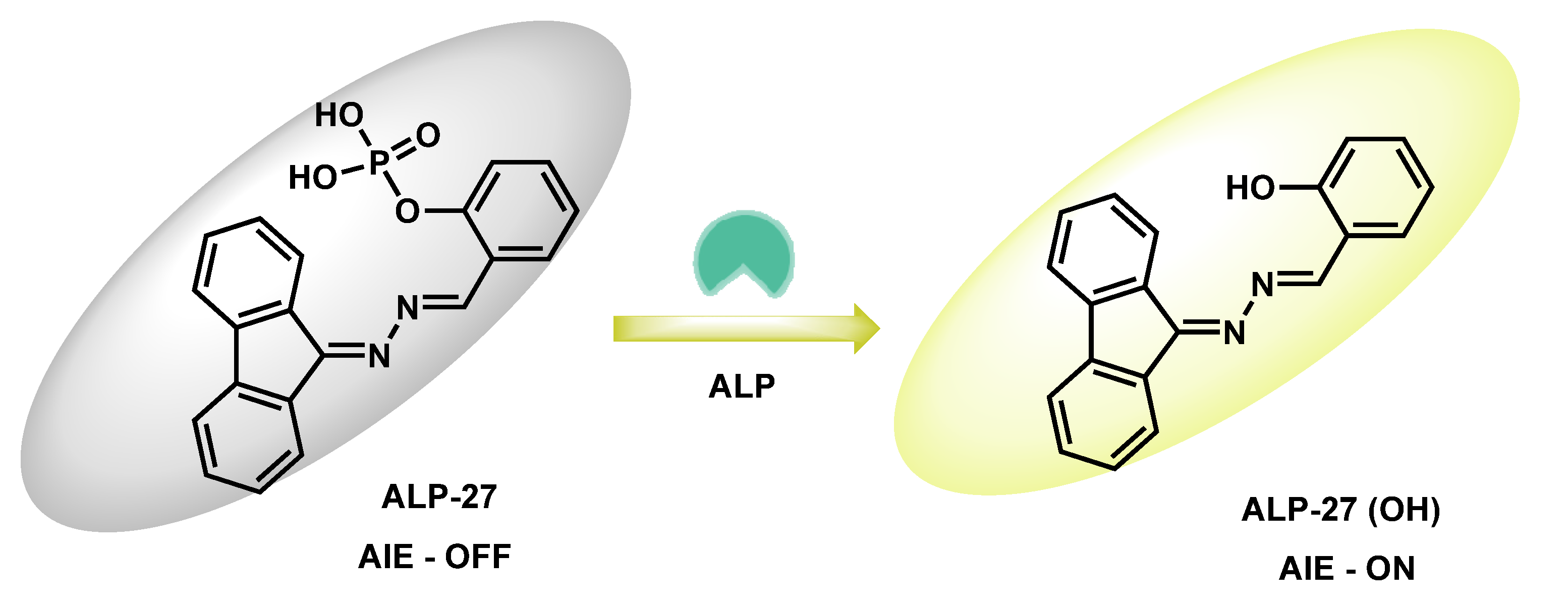
| ALP-27 | 1–100 U/L | / | 0.6 U/L | / | >200 nm | 380/586 | / | / | Turn-on fluorescence |
Living cells | 10 µM | Lipid droplets | [93] |
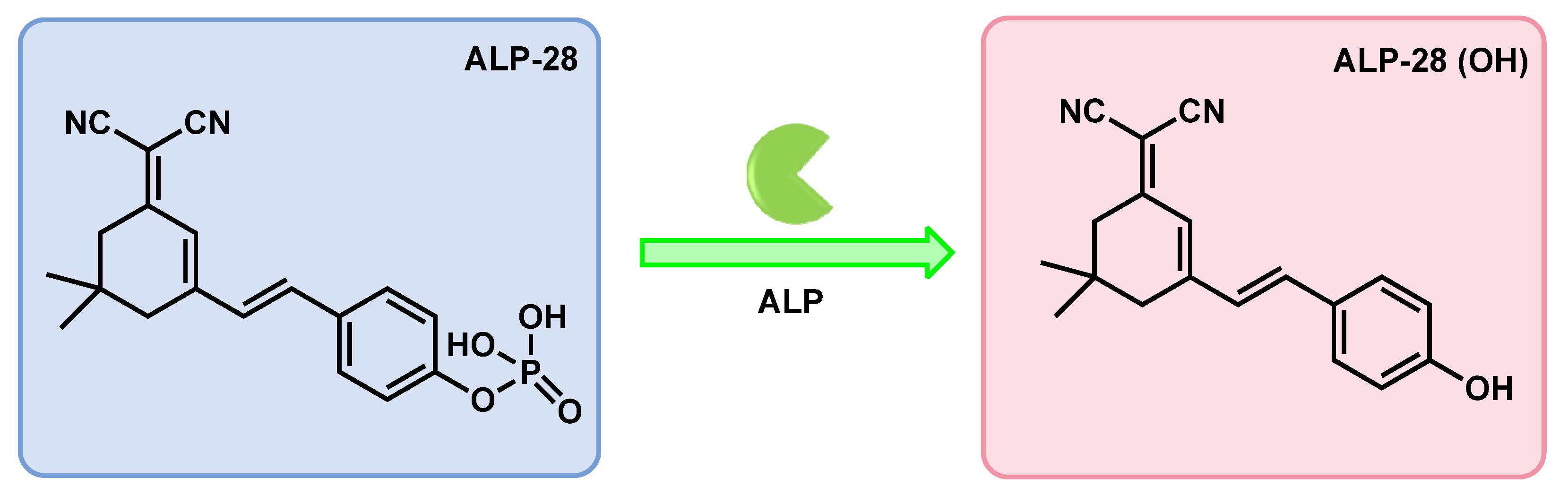
| ALP-28 | / | / | 0.088 U/L | 6 min | / | 440/570 | / | / | Turn-on Fluorescence |
Living cells | 10 µM | / | [94] |
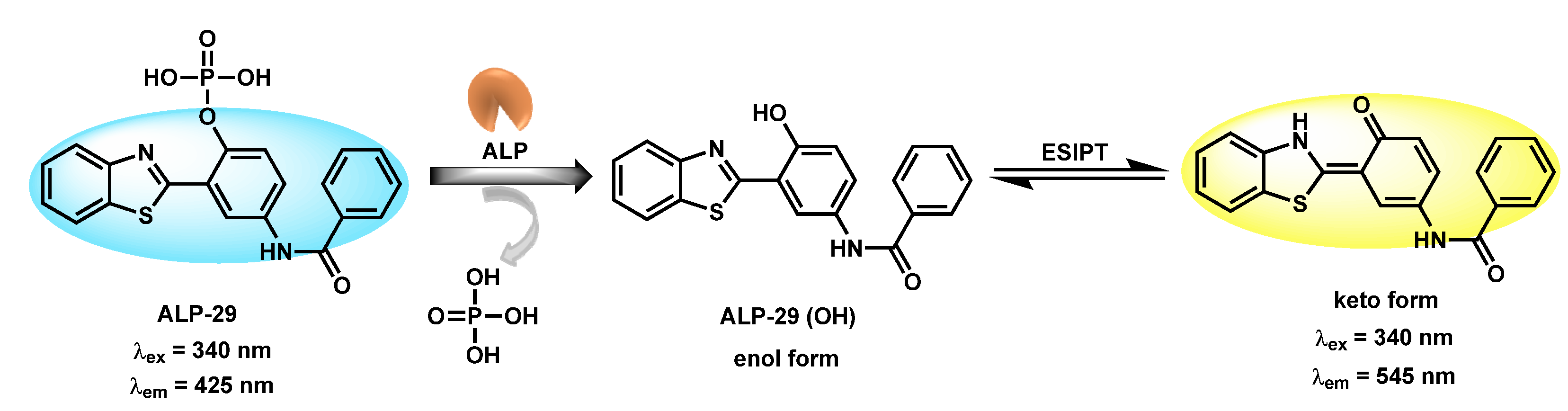
| ALP-29 | / | / | 0.004 mU/mL |
35 min | / | 340/545 | 0.028 | / | Ratiometric | Living cells | 20 µM | / | [95] |
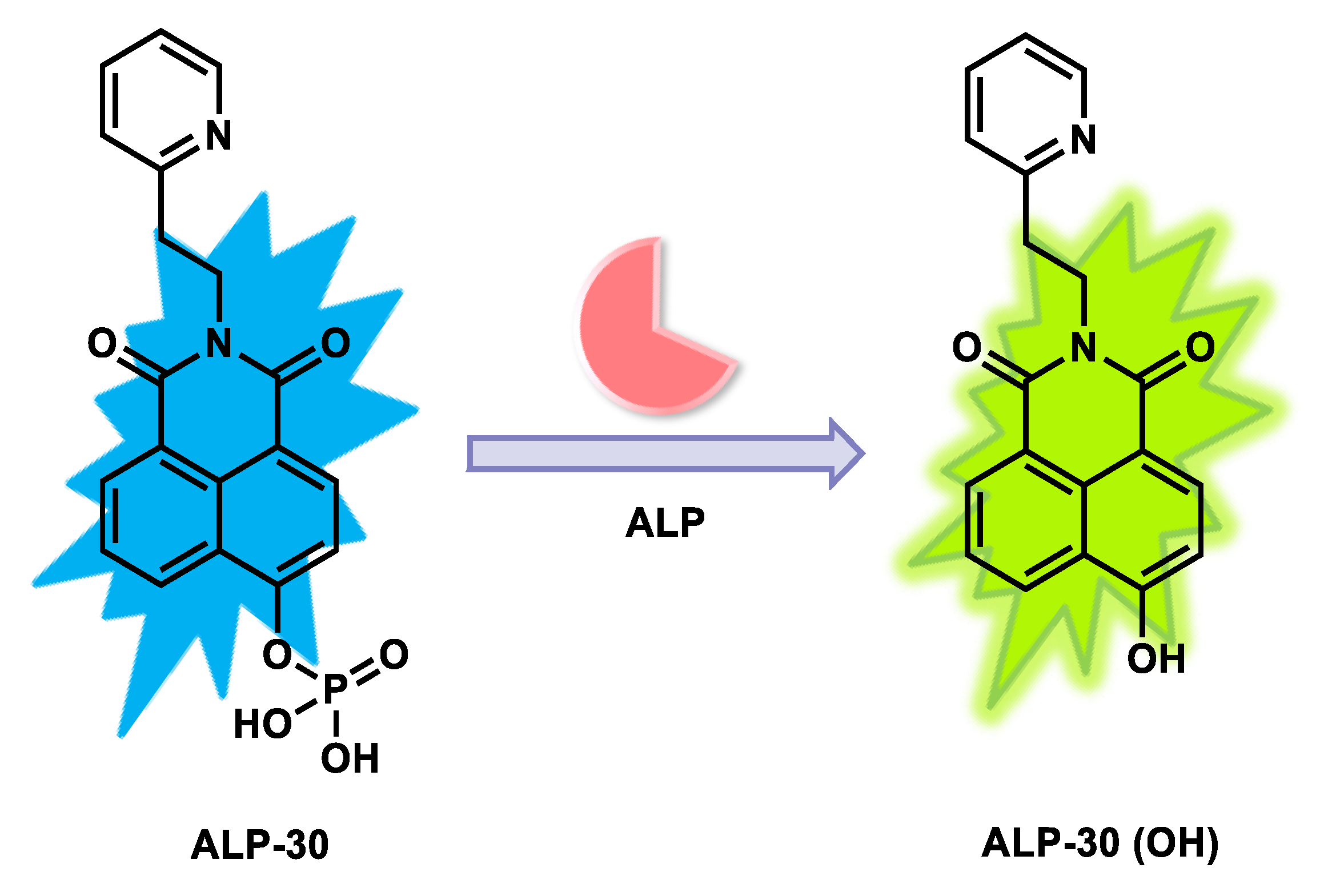
| ALP-30 | 0–200 U/L | / | 0.25 U/L | / | / | 425/472 | / | / | Ratiometric | Living cells | / | / | [96] |
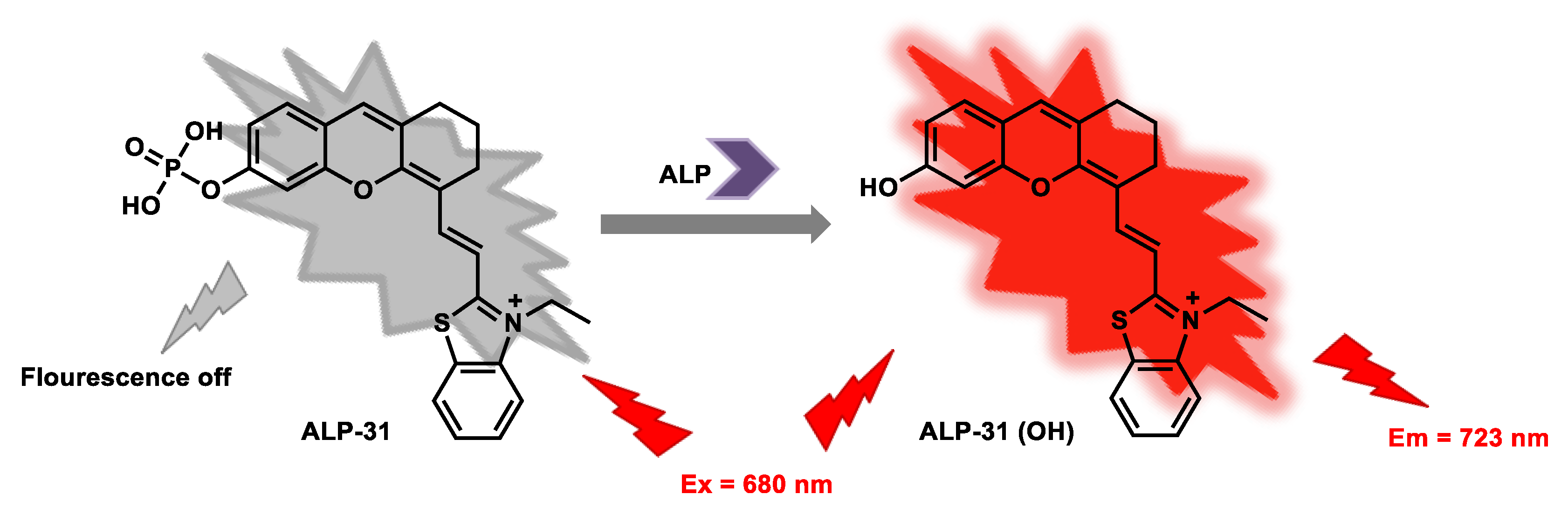
| ALP-31 | 0–8 U/L | / | 0.042 U/L | / | 43 nm | 680/723 | 0.15 | 23.98 µM | Turn-on Near-Infrared Fluorescence |
Living cells | 20 µM | / | [97] |
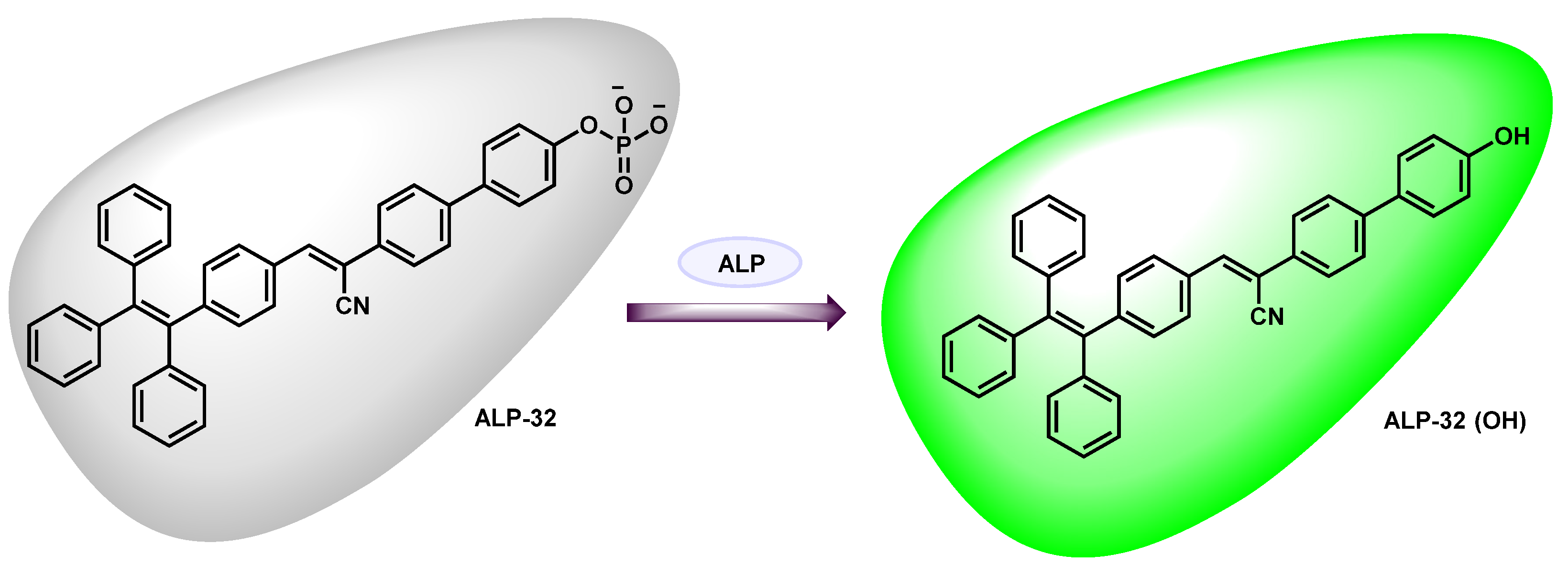
| ALP-32 | / | / | 14.2 U/L | / | / | 440/543 | / | / | Turn-on Fluorescence |
Live cells | 50 µM | / | [98] |
| ALP-33 | 0.05–1.05 U/mL |
/ | 0.012 U/mL | / | / | 377/572 | 0.0392 | / | Colorimetric and fluorescent on-off-on |
Over expressed cancer cells & normal salivary gland cells |
25 µM | / | [99] |
| ALP-34 | / | / | / | / | / | / | / | / | Turn-on fluorescence (NIR fluorescence) |
In vitro and live cells |
/ | Osteoblast | [100] |
| ALP-35 | 0.01–2.5 U/mL | / | 0.0033 U/mL | / | / | 685/718 | / | 148.6 µM | Turn-on fluorescence (NIR fluorescence) |
In vivo | / | / | [101] |
4. Organo-Metallic-Based Fluorescent Probes
Seo et al. reported the fluorescein/hydrazine-based probe ALP-33 (Figure 2830) to investigate ALP activity in cellular systems by measuring the consumption of trace amounts of PPi in the intracellular matrix [9699]. With this indirect strategy, the green emissive probe ALP-33 was designed to detect Cu2+/Ppi under physiological conditions in a sequential manner. In this sequential analysis, on–off–on switching properties were observed upon sequential addition of Cu2+ and PPi ions. In addition, dramatic color transitions occurred from colorless → yellow → colorless. ALP-33 showed a characteristic absorption maximum at 370 nm in the UV–Vis absorption spectrum. However, upon addition of Cu2+ ions, a new band at 446 nm was generated, making the solution an intense yellow color.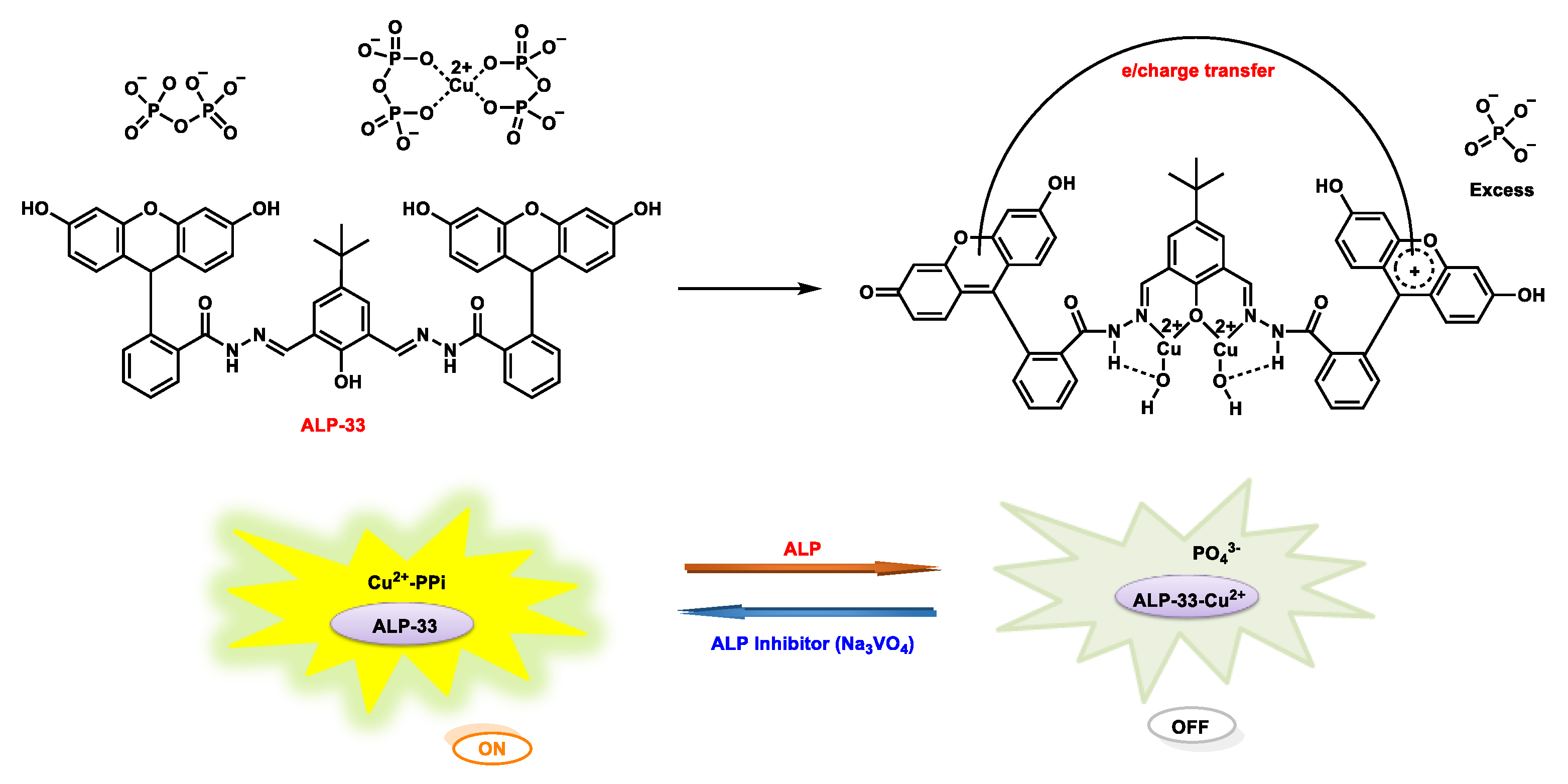
5. Nanomaterial-Based Fluorescent Probes
Lim et al. developed a new type of nanocomposite by formulating the doping of FDA-approved indocyanine green (IcyG), a fluorescent material, onto hydroxyapatite, followed by its surface modification through gold nanoparticles. This composite, ALP-34 [97100], was made for the recognition of ALP activity during the osteoblast differentiation process (Figure 2931).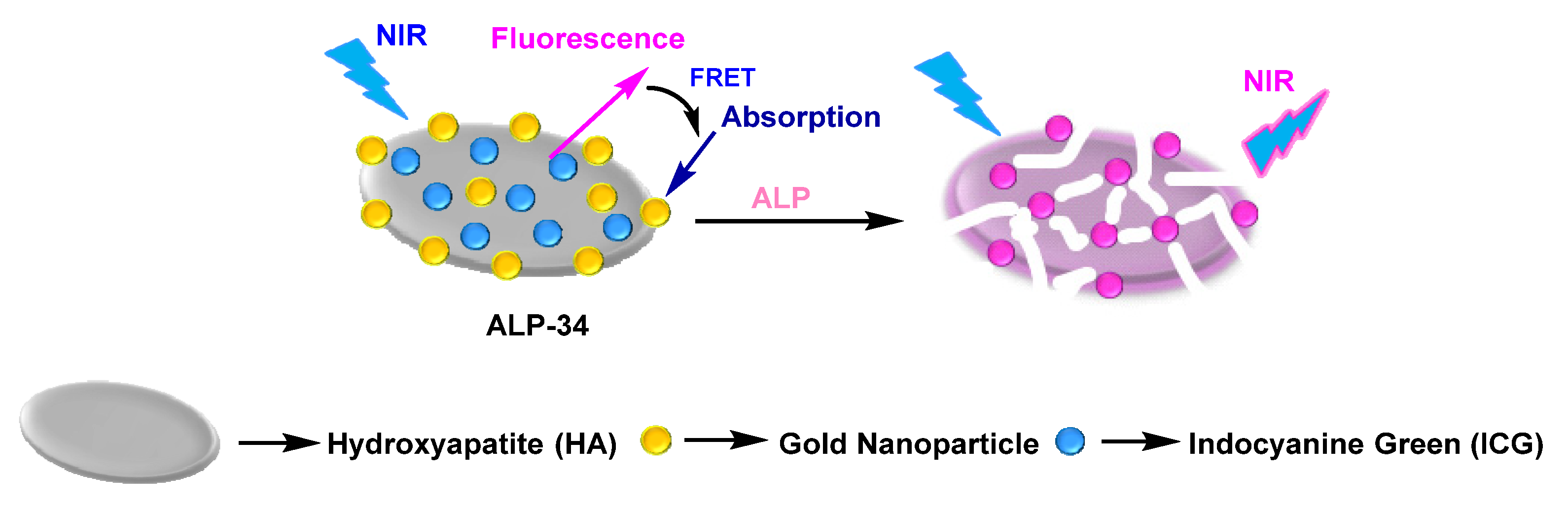
6. Miscellaneous
Ou-Yang et al. reported an NIR-based infinite coordination polymer nanoparticle (ALP-35) (Figure 302) for endogenous ALP activity detection in in cellulo and in vivo models [98101]. The rationally designed nanoprobe was constructed based on doping of fluorescent dye into a metal-ion-driven co-ordinational supramolecular nano-assembly. Accordingly, NIR emissive hemicyanine dyes were doped into Tb3+-GMP, an infinite nano-co-ordinational polymer. Due to the proximity induced quenching in confined spaces in the metal nano-coordination polymer, NIR emissive behavior was diminished. However, upon dephosphorylation, self-assembled metal nanostructures gradually dissolved by releasing NIR dye. The nanoprobe showed a sharp absorption band at ca. 695 nm and weak emission centered at 738 nm upon excitation at 685 nm. Paradoxically, upon ALP-catalyzed dephosphorylation of GMP, there was bright NIR emission centered at 738 nm (~8-fold) and substantial hyperchromicity in the absorption spectrum.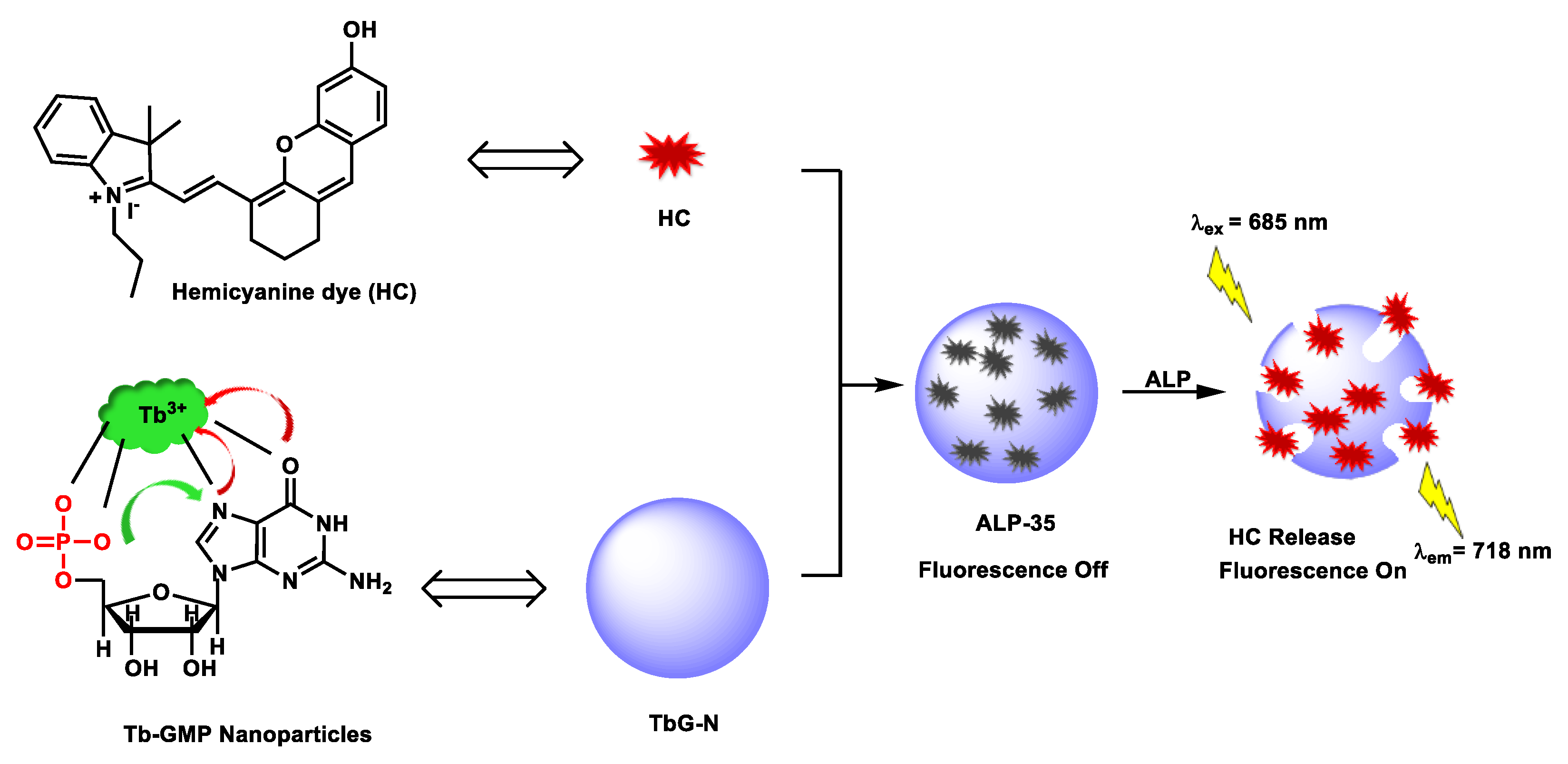
References
- Liang, J.; Kwok, R.T.K.; Shi, H.; Tang, B.Z.; Liu, B. Fluorescent Light-up Probe with Aggregation-Induced Emission Characteristics for Alkaline Phosphatase Sensing and Activity Study. ACS Appl. Mater. Interfaces 2013, 5, 8784–8789.
- McComb, R.B.; Bowers, G.N., Jr.; Posen, S. Alkaline Phosphatase, 1st ed.; Springer: New York, NY, USA; Plenum Press: New York, NY, USA, 1979.
- Yu, L.; Feng, L.; Xiong, L.; Li, S.; Xu, Q.; Pan, X.; Xiao, Y. Rational design of dual-emission lanthanide metal-organic framework for visual alkaline phosphatase activity assay. ACS Appl. Mater. Interfaces 2021, 13, 11646–11656.
- Ma, F.; Liu, M.; Zhang, C.-Y. Ligase amplification reaction-catalyzed assembly of a single quantum dot-based nanosensor for sensitive detection of alkaline phosphatase. Chem. Commun. 2019, 55, 8963–8966.
- Haarhaus, M.; Brandenburg, V.; Kalantar-Zadeh, K.; Stenvinkel, P.; Magnusson, P. Alkaline phosphatase: A novel treatment target for cardiovascular disease in CKD. Nat. Rev. Nephrol. 2017, 13, 429–442.
- Zhang, W.; Gao, Y.; Li, Y.; Zhang, Q.; Hu, Z.; Zhang, Y.; Hussain, E.; Yang, X.; Yu, D.; Yu, C. Polyphosphoric acid-induced perylene probe self-assembly and label-free fluorescence turn-on detection of alkaline phosphatase. Anal. Bioanal. Chem. 2017, 409, 1031–1036.
- Yang, D.; Guo, Z.; Tang, Y.; Miao, P. Poly(thymine)-templated selective formation of copper nanoparticles for alkaline phosphatase analysis aided by alkyne-azide cycloaddition “Click” reaction. ACS Appl. Nano Mater. 2018, 1, 168–174.
- Liu, X.-G.; Xing, X.-J.; Li, B.; Guo, Y.-M.; Zhang, Y.-Z.; Yang, Y.; Zhang, L.-F. Fluorescent assay for alkaline phosphatase activity based on graphene oxide integrating with λ exonuclease. Biosens. Bioelectron. 2016, 81, 460–464.
- Hu, X.-L.; Wu, X.-M.; Fang, X.; Li, Z.-J.; Wang, G.-L. Switchable fluorescence of gold nanoclusters for probing the activity of alkaline phosphatase and its application immunoassay. Biosens. Bioelectron. 2016, 77, 666–672.
- Liu, S.; Pang, S.; Na, W.; Su, X. Near-infrared fluorescence probe for the determination of alkaline phosphatase. Biosens. Bioelectron. 2014, 55, 249–254.
- Halawa, M.I.; Gao, W.; Saqib, M.; Kitte, S.A.; Wu, F.; Xu, G. Sensitive detection of alkaline phosphatase by switching on gold nanoclusters fluorescence quenched by pyridoxal phosphate. Biosens. Bioelectron. 2017, 95, 8–14.
- Rao, G.M.M.; Morghom, L.O. Correlation between serum alkaline phosphatase activity and blood glucose levels. Enzyme 1986, 35, 57–59.
- Sharma, U.; Pal, D.; Prasad, R. Alkaline Phosphatase: An Overview. Ind. J. Clin. Biochem. 2014, 29, 269–278.
- Wang, X.; Zhang, Z.; Ma, X.; Wen, J.; Geng, Z.; Wang, Z. Real-time fluorescence assays of alkaline phosphatase and ATP sulfurylase activities based on a novel PPi fluorescent probe. Talanta 2015, 137, 156–160.
- Deng, J.; Yu, P.; Wang, Y.; Mao, L. Real-time ratiometric fluorescent assay for alkaline phosphatase activity with stimulus responsive infinite coordination polymer nanoparticles. Anal. Chem. 2015, 87, 3080–3086.
- Zheng, F.; Guo, S.; Zeng, F.; Li, J.; Wu, S. Ratiometric fluorescent probe for alkaline phosphatase based on betaine-modified polyethylenimine via excimer/monomer conversion. Anal. Chem. 2014, 86, 9873–9879.
- Zhang, L.; Zhao, J.; Duan, M.; Zhang, H.; Jiang, J.; Yu, R. Inhibition of dsDNA-templated copper nanoparticles by pyrophosphate as a label-free fluorescent strategy for alkaline phosphatase assay. Anal. Chem. 2013, 85, 3797–3801.
- Wang, H.B.; Li, Y.; Chen, Y.; Zhang, Z.P.; Gan, T.; Liu, Y.M. Determination of the activity of alkaline phosphatase by using nanoclusters composed of flowerlike cobalt oxyhydroxide and copper nanoclusters as fluorescent probes. Microchim. Acta 2018, 185, 102.
- Li, G.; Huili Fu, H.; Chen, X.; Gong, P.; Chen, G.; Xia, L.; Wang, H.; You, J.; Wu, Y. Facile and sensitive fluorescence sensing of alkaline phosphatase activity with photoluminescent carbon dots based on inner filter effect. Anal. Chem. 2016, 88, 2720–2726.
- Cox, W.G.; Singer, V.L. A High-resolution, fluorescence-based method for localization, of endogenous alkaline phosphatase activity. J. Histochem. Cytochem. 1999, 47, 1443–1455.
- Butterworth, P.J. Biochemistry of alkaline phosphatases. Cell Biochem. Funct. Biochem. Data 1983, 1, 66–69.
- Štefková, K.; Procházková, J.; Pacherník, J. Alkaline phosphatase in stem cells. Stem Cells Int. 2015, 2015, 628368.
- Millan, J.L. Alkaline Phosphatases Structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2006, 2, 335–341.
- Chen, S.L.; Liao, R.Z. Phosphate monoester hydrolysis by trinuclear alkaline phosphatase; DFT study of transition states and reaction mechanism. ChemPhysChem 2014, 15, 2321–2330.
- Holtz, K.M.; Kantrowitz, E.R. The mechanism of the alkaline phosphatase reaction: Insights from NMR, crystallography and site-specific mutagenesis. FEBS Lett. 1999, 462, 7–11.
- Holtz, K.M.; Stec, B.; Kantrowitz, E.R. A model of the transition state in the alkaline phosphatase reaction. J. Biol. Chem. 1999, 274, 8351–8354.
- Krupaa, R.J.; Hariharan, R.; Babu, N.A.; Masthan, K.M.K. Alkaline phosphatase and its clinical importance-A review. Eur. J. Mol. Clin. Med. 2020, 7, 1409–1413. Chaudhuri, G.; Selvaraj, U.; Babu, V.; Thilagaraj, R.W.. Recent Trends in Phosphatase-Mediated Bioremediation; In Phosphoric Acid Industry Problems and Solutions; IntechOpen: London, UK, 2017; pp. 27–46.
- Thanih Balbaied, T.; Moore, E. Overview of optical and electrochemical alkaline phosphatase (ALP) biosensors: Recent approaches in cells culture techniques. Biosensors 2019, 9, 102. W Meyer-Sabellek; P Sinha; E Köttgen; Alkaline phosphatase. Laboratory and clinical implications.. Journal of chromatography 1988, 429, 419-44.
- Nsabimana, A.; Lan, Y.; Du, F.; Wang, C.; Zhang, W.; Xu, G. Alkaline phosphatase-based electrochemical sensors for health applications. Anal. Methods 2019, 11, 1996–2006. Krupaa, R.J.; Hariharan, R.; Babu, N.A.; Masthan, K.M.K. Alkaline phosphatase and its clinical importance-A review. Eur. J. Mol. Clin. Med. 2020, 7, 1409–1413.
- Goggins, S.; Naz, C.; Marsh, B.J.; Frost, C.G. Ratiometric electrochemical detection of alkaline phosphatase. Chem. Commun. 2015, 51, 561–564. Thanih Balbaied, T.; Moore, E. Overview of optical and electrochemical alkaline phosphatase (ALP) biosensors: Recent approaches in cells culture techniques. Biosensors 2019, 9, 102.
- Miao, P.; Ning, L.; Li, X.; Shu, Y.; Li, G. An electrochemical alkaline phosphatase biosensor fabricated with two DNA probes coupled with lambda exonuclease. Biosens. Bioelectron. 2011, 27, 178–182. Nsabimana, A.; Lan, Y.; Du, F.; Wang, C.; Zhang, W.; Xu, G. Alkaline phosphatase-based electrochemical sensors for health applications. Anal. Methods 2019, 11, 1996–2006.
- Kim, H.-U.; Kim, H.Y.; Kulkarni, A.; Ahn, C.; Jin, Y.; Kim, Y.; Lee, K.-N.; Lee, M.-H.; Kim, T. A sensitive electrochemical sensor for in vitro detection of parathyroid hormone based on a MoS2-graphene composite. Sci. Rep. 2016, 6, 34587. Goggins, S.; Naz, C.; Marsh, B.J.; Frost, C.G. Ratiometric electrochemical detection of alkaline phosphatase. Chem. Commun. 2015, 51, 561–564.
- Sun, D.; Xu, W.; Liang, C.; Shi, W.; Xu, S. Smart surface-enhanced resonance Raman scattering nanoprobe for monitoring cellular alkaline phosphatase activity during osteogenic differentiation. ACS Sens. 2020, 5, 1758–1767. Miao, P.; Ning, L.; Li, X.; Shu, Y.; Li, G. An electrochemical alkaline phosphatase biosensor fabricated with two DNA probes coupled with lambda exonuclease. Biosens. Bioelectron. 2011, 27, 178–182.
- Dai, X.; Lu, L.; Zhang, X.; Song, Z.-L.; Song, W.; Chao, Q.; Li, Q.; Wang, W.; Chen, J.; Fan, G.C.; et al. MnO2 shell-isolated SERS nanoprobe for the quantitative detection of ALP activity in trace serum: Relying on the enzyme-triggered etching of MnO2 shell to regulate the signal. Sens. Actuators B Chem. 2021, 334, 129605. Kim, H.-U.; Kim, H.Y.; Kulkarni, A.; Ahn, C.; Jin, Y.; Kim, Y.; Lee, K.-N.; Lee, M.-H.; Kim, T. A sensitive electrochemical sensor for in vitro detection of parathyroid hormone based on a MoS2-graphene composite. Sci. Rep. 2016, 6, 34587.
- Yi-Wei, T.; Charles, W.S. Advanced Techniques in Diagnostic Microbiology, 2nd ed.; Springer: New York, NY, USA, 2013. Sun, D.; Xu, W.; Liang, C.; Shi, W.; Xu, S. Smart surface-enhanced resonance Raman scattering nanoprobe for monitoring cellular alkaline phosphatase activity during osteogenic differentiation. ACS Sens. 2020, 5, 1758–1767.
- Roelofs, H.; Manes, T.; Janszen, T.; Millan, J.L.; Oosterhuis, J.W.; Looijenga, L.H.J. Heterogeneity in alkaline phosphatase isozyme expression in human testicular germ cell tumours: An enzyme-/immunohistochemical and molecular analysis. J. Pathol. 1999, 189, 236–244. Dai, X.; Lu, L.; Zhang, X.; Song, Z.-L.; Song, W.; Chao, Q.; Li, Q.; Wang, W.; Chen, J.; Fan, G.C.; et al. MnO2 shell-isolated SERS nanoprobe for the quantitative detection of ALP activity in trace serum: Relying on the enzyme-triggered etching of MnO2 shell to regulate the signal. Sens. Actuators B Chem. 2021, 334, 129605.
- Mano, H.; Furuhashi, Y.; Morikawa, Y.; Hattori, S.E.; Goto, S.; Tomoda, Y. Radioimmunoassay of placental alkaline-phosphatase in ovarian-cancer sera and tissues. Obstet. Gynecol. 1986, 68, 759–764. Yi-Wei, T.; Charles, W.S. Advanced Techniques in Diagnostic Microbiology, 2nd ed.; Springer: New York, NY, USA, 2013.
- Degroote, G.; Dewaele, P.; Vandevoorde, A.; Debroe, M.; Fiers, W. Use of monoclonal-antibodies to detect human placental alkaline-phosphatase. Clin. Chem. 1983, 29, 115–119. Roelofs, H.; Manes, T.; Janszen, T.; Millan, J.L.; Oosterhuis, J.W.; Looijenga, L.H.J. Heterogeneity in alkaline phosphatase isozyme expression in human testicular germ cell tumours: An enzyme-/immunohistochemical and molecular analysis. J. Pathol. 1999, 189, 236–244.
- Sekar, S.; Giermanska, J.; Chapel, J.P. Reusable and recyclable quartz crystal microbalance sensors. Sens. Actuators B Chem. 2015, 212, 196–199. Mano, H.; Furuhashi, Y.; Morikawa, Y.; Hattori, S.E.; Goto, S.; Tomoda, Y. Radioimmunoassay of placental alkaline-phosphatase in ovarian-cancer sera and tissues. Obstet. Gynecol. 1986, 68, 759–764.
- Kacar, T.; Zin, M.T.; So, C.; Wilson, B.; Ma, H.; Gul-Karaguler, N.; Tamerler, C. Directed self-immobilization of alkaline phosphatase on micro-patterned substrates via genetically fused metal-binding peptide. Biotechnol. Bioeng. 2009, 103, 696–705. Degroote, G.; Dewaele, P.; Vandevoorde, A.; Debroe, M.; Fiers, W. Use of monoclonal-antibodies to detect human placental alkaline-phosphatase. Clin. Chem. 1983, 29, 115–119.
- Jang, H.J.; Ahn, J.; Kim, M.G.; Shin, Y.B.; Jeun, M.; Cho, W.J.; Lee, K.H. Electrical signaling of enzyme-linked immunosorbent assays with an ion-sensitive field-effect transistor. Biosens. Bioelectron. 2015, 64, 318–323. Sekar, S.; Giermanska, J.; Chapel, J.P. Reusable and recyclable quartz crystal microbalance sensors. Sens. Actuators B Chem. 2015, 212, 196–199.
- Li, C.M.; Zhen, S.J.; Wang, J.; Li, Y.F.; Huang, C.Z. A gold nanoparticles-based colorimetric assay for alkaline phosphatase detection with tunable dynamic range. Biosens. Bioelectron. 2013, 43, 366–371. Kacar, T.; Zin, M.T.; So, C.; Wilson, B.; Ma, H.; Gul-Karaguler, N.; Tamerler, C. Directed self-immobilization of alkaline phosphatase on micro-patterned substrates via genetically fused metal-binding peptide. Biotechnol. Bioeng. 2009, 103, 696–705.
- Hu, Q.; He, M.; Mei, Y.; Feng, W.; Jing, S.; Kong, J.; Zhang, X. Sensitive and selective colorimetric assay of alkaline phosphatase activity with Cu(II)-phenanthroline complex. Talanta 2017, 163, 146–152. Jang, H.J.; Ahn, J.; Kim, M.G.; Shin, Y.B.; Jeun, M.; Cho, W.J.; Lee, K.H. Electrical signaling of enzyme-linked immunosorbent assays with an ion-sensitive field-effect transistor. Biosens. Bioelectron. 2015, 64, 318–323.
- Hu, X.; Sun, C.; Shi, Y.; Long, Y.; Zheng, H. Colorimetric sensing of alkaline phosphatase and a-fetoprotein based on the photoinduced oxidase activity of fluorescein. New J. Chem. 2019, 43, 4525–4530. Li, C.M.; Zhen, S.J.; Wang, J.; Li, Y.F.; Huang, C.Z. A gold nanoparticles-based colorimetric assay for alkaline phosphatase detection with tunable dynamic range. Biosens. Bioelectron. 2013, 43, 366–371.
- Upadhyay, Y.; Kumar, R.; Sahoo, S.K. Developing a cost-effective bioassay to detect alkaline phosphatase activity and generating white light emission from a single nano-assembly by conjugating Vitamin B6 cofactors with lysozyme-stabilized fluorescent gold nanoclusters. ACS Sustain. Chem. Eng. 2020, 8, 4107–4113. Hu, Q.; He, M.; Mei, Y.; Feng, W.; Jing, S.; Kong, J.; Zhang, X. Sensitive and selective colorimetric assay of alkaline phosphatase activity with Cu(II)-phenanthroline complex. Talanta 2017, 163, 146–152.
- Westmeyer, G.G.; Emer, Y.; Lintelmann, J.; Jasanoff, A. MRI-based detection of alkaline phosphatase gene reporter activity using a porphyrin solubility switch. Chem. Biol. 2014, 21, 422–429. Hu, X.; Sun, C.; Shi, Y.; Long, Y.; Zheng, H. Colorimetric sensing of alkaline phosphatase and a-fetoprotein based on the photoinduced oxidase activity of fluorescein. New J. Chem. 2019, 43, 4525–4530.
- Zhang, Q.; Li, S.; Fu, C.; Xiao, Y.; Zhang, P.; Ding, C. Near-infrared mito-specific fluorescent probe for ratiometric detection and imaging of alkaline phosphatase activity with high sensitivity. J. Mater. Chem. B 2019, 7, 443–445. Upadhyay, Y.; Kumar, R.; Sahoo, S.K. Developing a cost-effective bioassay to detect alkaline phosphatase activity and generating white light emission from a single nano-assembly by conjugating Vitamin B6 cofactors with lysozyme-stabilized fluorescent gold nanoclusters. ACS Sustain. Chem. Eng. 2020, 8, 4107–4113.
- Pandith, A.; Seo, Y.J. Label-free sensing platform for miRNA-146a based on chromo-fluorogenic pyrophosphate recognition. J. Inorg. Biochem. 2020, 203, 110867. Westmeyer, G.G.; Emer, Y.; Lintelmann, J.; Jasanoff, A. MRI-based detection of alkaline phosphatase gene reporter activity using a porphyrin solubility switch. Chem. Biol. 2014, 21, 422–429.
- Pandith, A.; Koo, J.; Seo, Y.J. Daphnetin: A novel blue-green photonic switch for disodium phosphates that allows monitoring of polymerase chain reactions. Spectrochim. Acta Part A 2018, 204, 620–628. Zhang, Q.; Li, S.; Fu, C.; Xiao, Y.; Zhang, P.; Ding, C. Near-infrared mito-specific fluorescent probe for ratiometric detection and imaging of alkaline phosphatase activity with high sensitivity. J. Mater. Chem. B 2019, 7, 443–445.
- Pandith, A.; Siddappa, R.G.; Seo, Y.J. Recent developments in novel blue/green/red/NIR small fluorescent probes for in cellulo tracking of RNA/DNA G-quadruplexes. J. Photochem. Photobiol. C Rev. 2019, 40, 81–116. Pandith, A.; Seo, Y.J. Label-free sensing platform for miRNA-146a based on chromo-fluorogenic pyrophosphate recognition. J. Inorg. Biochem. 2020, 203, 110867.
- Zhang, H.; Ju, Q.; Pang, S.; Wei, N.; Zhang, Y. Recent progress of fluorescent probes for the detection of alkaline phosphatase (ALP): A review. Dyes Pigments 2021, 194, 109569. Pandith, A.; Koo, J.; Seo, Y.J. Daphnetin: A novel blue-green photonic switch for disodium phosphates that allows monitoring of polymerase chain reactions. Spectrochim. Acta Part A 2018, 204, 620–628.
- Wang, K.; Wang, W.; Zhang, X.Y.; Jiang, A.Q.; Yang, Y.S.; Zhu, H.L. Fluorescent probes for the detection of alkaline phosphatase in biological systems: Recent advances and future prospects. Trends Anal. Chem. 2021, 136, 116189. Pandith, A.; Siddappa, R.G.; Seo, Y.J. Recent developments in novel blue/green/red/NIR small fluorescent probes for in cellulo tracking of RNA/DNA G-quadruplexes. J. Photochem. Photobiol. C Rev. 2019, 40, 81–116.
- Gwynne, L.; Sedgwick, A.C.; Gardiner, J.E.; Williams, G.T.; Kim, G.; Lowe, J.P.; Maillard, J.Y.; Jenkins, A.T.A.; Bull, S.D.; Sessler, J.L.; et al. Long wavelength TCF-based fluorescent probe for the detection of alkaline phosphatase in live cells. Front. Chem. 2019, 7, 255. Zhang, H.; Ju, Q.; Pang, S.; Wei, N.; Zhang, Y. Recent progress of fluorescent probes for the detection of alkaline phosphatase (ALP): A review. Dyes Pigments 2021, 194, 109569.
- Jidong, Z.; Hongze, L.; Li, M. Research Progress in the Fluorescent Probes for Alkaline Phosphatase. Chin. J. Org. Chem. 2019, 39, 3132–3144. Wang, K.; Wang, W.; Zhang, X.Y.; Jiang, A.Q.; Yang, Y.S.; Zhu, H.L. Fluorescent probes for the detection of alkaline phosphatase in biological systems: Recent advances and future prospects. Trends Anal. Chem. 2021, 136, 116189.
- Li, M.; Gurram, B.; Lei, S.; Blum, N.T.; Huang, P.; Lin, J. Recent advances in fluorescence imaging of alkaline phosphatase. Chin. Chem. Lett. 2021, 32, 1316–1330. Gwynne, L.; Sedgwick, A.C.; Gardiner, J.E.; Williams, G.T.; Kim, G.; Lowe, J.P.; Maillard, J.Y.; Jenkins, A.T.A.; Bull, S.D.; Sessler, J.L.; et al. Long wavelength TCF-based fluorescent probe for the detection of alkaline phosphatase in live cells. Front. Chem. 2019, 7, 255.
- Liu, H.W.; Chen, L.; Xu, C.; Li, Z.; Zhang, H.; Zhang, X.B.; Tan, W. Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging. Chem. Soc. Rev. 2018, 47, 7140–7180. Jidong, Z.; Hongze, L.; Li, M. Research Progress in the Fluorescent Probes for Alkaline Phosphatase. Chin. J. Org. Chem. 2019, 39, 3132–3144.
- Niu, X.; Ye, K.; Wang, L.; Lin, Y.; Du, D. A review on emerging principles and strategies for colorimetric and fluorescent detection of alkaline phosphatase activity. Anal. Chim. Acta 2019, 1086, 29–45. Li, M.; Gurram, B.; Lei, S.; Blum, N.T.; Huang, P.; Lin, J. Recent advances in fluorescence imaging of alkaline phosphatase. Chin. Chem. Lett. 2021, 32, 1316–1330.
- Han, Y.; Chen, J.; Li, Z.; Chen, H.; Qiu, H. Recent progress and prospects of alkaline phosphatase biosensor based on fluorescence strategy. Biosens. Bioelectron. 2020, 148, 111811. Liu, H.W.; Chen, L.; Xu, C.; Li, Z.; Zhang, H.; Zhang, X.B.; Tan, W. Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging. Chem. Soc. Rev. 2018, 47, 7140–7180.
- Hu, L.; Zhang, Q.; Gan, X.; Yin, W.; Fu, W. Switchable fluorescence of MoS2 quantum dots: A multifunctional probe for sensing of chromium (VI), ascorbic acid, and alkaline phosphatase activity. Anal. Bioanal. Chem. 2018, 410, 7551–7557. Niu, X.; Ye, K.; Wang, L.; Lin, Y.; Du, D. A review on emerging principles and strategies for colorimetric and fluorescent detection of alkaline phosphatase activity. Anal. Chim. Acta 2019, 1086, 29–45.
- Syahir, A.; Usui, K.; Tomizaki, K.Y.; Kajikawa, K.; Mihara, H. Label and label-free detection techniques for protein microarrays. Microarrays 2015, 4, 228–244. Han, Y.; Chen, J.; Li, Z.; Chen, H.; Qiu, H. Recent progress and prospects of alkaline phosphatase biosensor based on fluorescence strategy. Biosens. Bioelectron. 2020, 148, 111811.
- Boaro, A.; Ageitos, L.; Torres, M.; Bartoloni, F.H.; de la Fuente-Nunez, C. Light-emitting probes for labeling peptides. Cell Rep. Phys. Sci. 2020, 1, 100257. Hu, L.; Zhang, Q.; Gan, X.; Yin, W.; Fu, W. Switchable fluorescence of MoS2 quantum dots: A multifunctional probe for sensing of chromium (VI), ascorbic acid, and alkaline phosphatase activity. Anal. Bioanal. Chem. 2018, 410, 7551–7557.
- Upadhyay, Y.; Kumar, R.; Sk, A.K.; Sahoo, S.K. Vitamin B6 cofactors conjugated ovalbumin-stabilized gold nanoclusters: Application in alkaline phosphatase activity detection and generating white-light emission. Microchem. J. 2020, 156, 104859. Syahir, A.; Usui, K.; Tomizaki, K.Y.; Kajikawa, K.; Mihara, H. Label and label-free detection techniques for protein microarrays. Microarrays 2015, 4, 228–244.
- Upadhyay, Y.; Bothra, S.; Kumar, R.; Sk, A.K.; Sahoo, S.K. Mimicking biological process to detect alkaline phosphatase activity using the vitamin B6 cofactor conjugated bovine serum albumin capped CdS quantum dots. Colloids Surf. B Biointerfaces 2020, 185, 110624. Boaro, A.; Ageitos, L.; Torres, M.; Bartoloni, F.H.; de la Fuente-Nunez, C. Light-emitting probes for labeling peptides. Cell Rep. Phys. Sci. 2020, 1, 100257.
- Wang, J.H.; Wang, K.; Bartling, B.; Liu, C.C. The detection of alkaline phosphatase using an electrochemical biosensor in a single-step approach. Sensors 2009, 9, 8709–8721. Upadhyay, Y.; Kumar, R.; Sk, A.K.; Sahoo, S.K. Vitamin B6 cofactors conjugated ovalbumin-stabilized gold nanoclusters: Application in alkaline phosphatase activity detection and generating white-light emission. Microchem. J. 2020, 156, 104859.
- Li, X.; Wang, X.; Guo, W.; Wang, Y.; Hua, Q.; Tang, F.; Luan, F.; Tian, C.; Zhuang, X.; Zhao, L. Selective Detection of Alkaline Phosphatase Activity in Environmental Water Samples by Copper Nanoclusters Doped Lanthanide Coordination Polymer Nanocomposites as the Ratiometric Fluorescent Probe. Biosensors 2022, 12, 372. Upadhyay, Y.; Bothra, S.; Kumar, R.; Sk, A.K.; Sahoo, S.K. Mimicking biological process to detect alkaline phosphatase activity using the vitamin B6 cofactor conjugated bovine serum albumin capped CdS quantum dots. Colloids Surf. B Biointerfaces 2020, 185, 110624.
- Cao, Y.; Yu, X.; Sun, C.; Cui, J. Theoretical Investigation on the ESIPT Process and Detection Mechanism for Dual-Proton Type Fluorescent Probe. Int. J. Mol. Sci. 2022, 23, 2132. Wang, J.H.; Wang, K.; Bartling, B.; Liu, C.C. The detection of alkaline phosphatase using an electrochemical biosensor in a single-step approach. Sensors 2009, 9, 8709–8721.
- Sun, T.; Xia, N.; Liu, L. A graphene oxide-based fluorescent platform for probing of phosphatase activity. Nanomaterials 2016, 6, 20. Li, X.; Wang, X.; Guo, W.; Wang, Y.; Hua, Q.; Tang, F.; Luan, F.; Tian, C.; Zhuang, X.; Zhao, L. Selective Detection of Alkaline Phosphatase Activity in Environmental Water Samples by Copper Nanoclusters Doped Lanthanide Coordination Polymer Nanocomposites as the Ratiometric Fluorescent Probe. Biosensors 2022, 12, 372.
- David, C.I.; Prabakaran, G.; Nandhakumar, R. Recent approaches of 2HN derived fluorophores on recognition of Al3+ ions: A review for future outlook. Microchem. J. 2021, 169, 106590. Cao, Y.; Yu, X.; Sun, C.; Cui, J. Theoretical Investigation on the ESIPT Process and Detection Mechanism for Dual-Proton Type Fluorescent Probe. Int. J. Mol. Sci. 2022, 23, 2132.
- Cao, F.Y.; Long, Y.; Wang, S.B.; Li, B.; Fan, J.X.; Zeng, X.; Zhang, X.Z. Fluorescence light-up AIE probe for monitoring cellular alkaline phosphatase activity and detecting osteogenic differentiation. J. Mater. Chem. B 2016, 4, 4534–4541. Sun, T.; Xia, N.; Liu, L. A graphene oxide-based fluorescent platform for probing of phosphatase activity. Nanomaterials 2016, 6, 20.
- Song, Z.; Kwok, R.T.; Zhao, E.; He, Z.; Hong, Y.; Lam, J.W.; Liu, B.; Tang, B.Z. A ratiometric fluorescent probe based on ESIPT and AIE processes for alkaline phosphatase activity assay and visualization in living cells. ACS Appl. Mater. Interfaces 2014, 6, 17245–17254. David, C.I.; Prabakaran, G.; Nandhakumar, R. Recent approaches of 2HN derived fluorophores on recognition of Al3+ ions: A review for future outlook. Microchem. J. 2021, 169, 106590.
- Zhang, H.; Xiao, P.; Wong, Y.T.; Shen, W.; Chhabra, M.; Peltier, R.; Jiang, Y.; He, Y.; He, J.; Tan, Y.; et al. Construction of an alkaline phosphatase-specific two-photon probe and its imaging application in living cells and tissues. Biomaterials 2017, 140, 220–229. Cao, F.Y.; Long, Y.; Wang, S.B.; Li, B.; Fan, J.X.; Zeng, X.; Zhang, X.Z. Fluorescence light-up AIE probe for monitoring cellular alkaline phosphatase activity and detecting osteogenic differentiation. J. Mater. Chem. B 2016, 4, 4534–4541.
- Li, S.J.; Li, C.Y.; Li, Y.F.; Fei, J.; Wu, P.; Yang, B.; Ou-Yang, J.; Nie, S.X. Facile and sensitive near-infrared fluorescence probe for the detection of endogenous alkaline phosphatase activity in vivo. Anal. Chem. 2017, 89, 6854–6860. Song, Z.; Kwok, R.T.; Zhao, E.; He, Z.; Hong, Y.; Lam, J.W.; Liu, B.; Tang, B.Z. A ratiometric fluorescent probe based on ESIPT and AIE processes for alkaline phosphatase activity assay and visualization in living cells. ACS Appl. Mater. Interfaces 2014, 6, 17245–17254.
- Dong, L.; Miao, Q.; Hai, Z.; Yuan, Y.; Liang, G. Enzymatic hydrogelation-induced fluorescence turn-off for sensing alkaline phosphatase in vitro and in living cells. Anal. Chem. 2015, 87, 6475–6478. Zhang, H.; Xiao, P.; Wong, Y.T.; Shen, W.; Chhabra, M.; Peltier, R.; Jiang, Y.; He, Y.; He, J.; Tan, Y.; et al. Construction of an alkaline phosphatase-specific two-photon probe and its imaging application in living cells and tissues. Biomaterials 2017, 140, 220–229.
- Tan, Y.; Zhang, L.; Man, K.H.; Peltier, R.; Chen, G.; Zhang, H.; Zhou, L.; Wang, F.; Ho, D.; Yao, S.Q.; et al. Reaction-based off–on near-infrared fluorescent probe for imaging alkaline phosphatase activity in living cells and mice. ACS Appl. Mater. Interfaces 2017, 9, 6796–6803. Li, S.J.; Li, C.Y.; Li, Y.F.; Fei, J.; Wu, P.; Yang, B.; Ou-Yang, J.; Nie, S.X. Facile and sensitive near-infrared fluorescence probe for the detection of endogenous alkaline phosphatase activity in vivo. Anal. Chem. 2017, 89, 6854–6860.
- Jie, X.; Wu, M.; Yang, H.; Wei, W. Red–Near-Infrared Fluorescent Probe for Time-Resolved in Vivo Alkaline Phosphatase Detection with the Assistance of a Photoresponsive Nanocontainer. Anal. Chem. 2019, 91, 13174–13182. Dong, L.; Miao, Q.; Hai, Z.; Yuan, Y.; Liang, G. Enzymatic hydrogelation-induced fluorescence turn-off for sensing alkaline phosphatase in vitro and in living cells. Anal. Chem. 2015, 87, 6475–6478.
- Zhang, P.; Fu, C.; Zhang, Q.; Li, S.; Ding, C. Ratiometric fluorescent strategy for localizing alkaline phosphatase activity in mitochondria based on the ESIPT process. Anal. Chem. 2019, 91, 12377–12383. Tan, Y.; Zhang, L.; Man, K.H.; Peltier, R.; Chen, G.; Zhang, H.; Zhou, L.; Wang, F.; Ho, D.; Yao, S.Q.; et al. Reaction-based off–on near-infrared fluorescent probe for imaging alkaline phosphatase activity in living cells and mice. ACS Appl. Mater. Interfaces 2017, 9, 6796–6803.
- Jia, Y.; Li, P.; Han, K. AMP/GMP analogs as affinity ESIPT probes for highly selective sensing of alkaline phosphatase activity in living systems. Chem. Asian J. 2015, 10, 2444–2451. Jie, X.; Wu, M.; Yang, H.; Wei, W. Red–Near-Infrared Fluorescent Probe for Time-Resolved in Vivo Alkaline Phosphatase Detection with the Assistance of a Photoresponsive Nanocontainer. Anal. Chem. 2019, 91, 13174–13182.
- Kim, T.I.; Kim, H.; Choi, Y.; Kim, Y. A fluorescent turn-on probe for the detection of alkaline phosphatase activity in living cells. Chem. Commun. 2011, 47, 9825–9827. Zhang, P.; Fu, C.; Zhang, Q.; Li, S.; Ding, C. Ratiometric fluorescent strategy for localizing alkaline phosphatase activity in mitochondria based on the ESIPT process. Anal. Chem. 2019, 91, 12377–12383.
- Zhang, H.; Xu, C.; Liu, J.; Li, X.; Guo, L.; Li, X. An enzyme-activatable probe with a self-immolative linker for rapid and sensitive alkaline phosphatase detection and cell imaging through a cascade reaction. Chem. Commun. 2015, 51, 7031–7034. Jia, Y.; Li, P.; Han, K. AMP/GMP analogs as affinity ESIPT probes for highly selective sensing of alkaline phosphatase activity in living systems. Chem. Asian J. 2015, 10, 2444–2451.
- He, Y.; Yu, J.; Hu, X.; Huang, S.; Cai, L.; Yang, L.; Zhang, H.; Jiang, Y.; Jia, Y.; Sun, H. An activity-based fluorescent probe and its application for differentiating alkaline phosphatase activity in different cell lines. Chem. Commun. 2020, 56, 13323–13326. Kim, T.I.; Kim, H.; Choi, Y.; Kim, Y. A fluorescent turn-on probe for the detection of alkaline phosphatase activity in living cells. Chem. Commun. 2011, 47, 9825–9827.
- Hou, X.; Yu, Q.; Zeng, F.; Ye, J.; Wu, S. A ratiometric fluorescent probe for in vivo tracking of alkaline phosphatase level variation resulting from drug-induced organ damage. J. Mater. Chem. B 2015, 3, 1042–1048. Zhang, H.; Xu, C.; Liu, J.; Li, X.; Guo, L.; Li, X. An enzyme-activatable probe with a self-immolative linker for rapid and sensitive alkaline phosphatase detection and cell imaging through a cascade reaction. Chem. Commun. 2015, 51, 7031–7034.
- Podder, A.; Senapati, S.; Maiti, P.; Kamalraj, D.; Jaffer, S.S.; Khatun, S.; Bhuniya, S. A ‘turn-on’ fluorescent probe for lysosomal phosphatase: A comparative study for labeling of cancer cells. J. Mater. Chem. B 2018, 6, 4514–4521. He, Y.; Yu, J.; Hu, X.; Huang, S.; Cai, L.; Yang, L.; Zhang, H.; Jiang, Y.; Jia, Y.; Sun, H. An activity-based fluorescent probe and its application for differentiating alkaline phosphatase activity in different cell lines. Chem. Commun. 2020, 56, 13323–13326.
- Lu, Z.; Wu, J.; Liu, W.; Zhang, G.; Wang, P. A ratiometric fluorescent probe for quantification of alkaline phosphatase in living cells. RSC Adv. 2016, 6, 32046–32051. Hou, X.; Yu, Q.; Zeng, F.; Ye, J.; Wu, S. A ratiometric fluorescent probe for in vivo tracking of alkaline phosphatase level variation resulting from drug-induced organ damage. J. Mater. Chem. B 2015, 3, 1042–1048.
- Hu, Q.; Zeng, F.; Yu, C.; Wu, S. A fluorescent probe for alkaline phosphatase via excited state intramolecular proton transfer. Sens. Actuators B Chem. 2015, 220, 720–726. Xiaohong Zhou; Yuren Jiang; Xiongjie Zhao; Yao Zhu; A New Two-Photon Ratiometric Fluorescent Probe for Detecting Alkaline Phosphatase in Living Cells. Molecules 2016, 21, 1619, 10.3390/molecules21121619.
- Liu, H.W.; Hu, X.X.; Zhu, L.; Li, K.; Rong, Q.; Yuan, L.; Zhang, X.B.; Tan, W. In vivo imaging of alkaline phosphatase in tumor-bearing mouse model by a promising near-infrared fluorescent probe. Talanta 2017, 175, 421–426. Podder, A.; Senapati, S.; Maiti, P.; Kamalraj, D.; Jaffer, S.S.; Khatun, S.; Bhuniya, S. A ‘turn-on’ fluorescent probe for lysosomal phosphatase: A comparative study for labeling of cancer cells. J. Mater. Chem. B 2018, 6, 4514–4521.
- Wang, W.X.; Jiang, W.L.; Guo, H.; Li, Y.; Li, C.Y. Real-time imaging of alkaline phosphatase activity of diabetes in mice via a near-infrared fluorescent probe. Chem. Commun. 2021, 57, 480–483. Lu, Z.; Wu, J.; Liu, W.; Zhang, G.; Wang, P. A ratiometric fluorescent probe for quantification of alkaline phosphatase in living cells. RSC Adv. 2016, 6, 32046–32051.
- Pang, X.; Li, Y.; Lu, Q.; Ni, Z.; Zhou, Z.; Xie, R.; Wu, C.; Li, H.; Zhang, Y. A turn-on near-infrared fluorescent probe for visualization of endogenous alkaline phosphatase activity in living cells and zebrafish. Analyst 2021, 146, 521–528. Hu, Q.; Zeng, F.; Yu, C.; Wu, S. A fluorescent probe for alkaline phosphatase via excited state intramolecular proton transfer. Sens. Actuators B Chem. 2015, 220, 720–726.
- Khatun, S.; Biswas, S.; Mahanta, A.K.; Joseph, M.M.; Vidyalekshmi, M.S.; Podder, A.; Maiti, P.; Maiti, K.K.; Bhuniya, S. Biocompatible fluorescent probe for detecting mitochondrial alkaline phosphatase activity in live cells. J. Photochem. Photobiol. B Biol. 2020, 212, 112043. Liu, H.W.; Hu, X.X.; Zhu, L.; Li, K.; Rong, Q.; Yuan, L.; Zhang, X.B.; Tan, W. In vivo imaging of alkaline phosphatase in tumor-bearing mouse model by a promising near-infrared fluorescent probe. Talanta 2017, 175, 421–426.
- Park, C.S.; Ha, T.H.; Kim, M.; Raja, N.; Yun, H.S.; Sung, M.J.; Kwon, O.S.; Yoon, H.; Lee, C.S. Fast and sensitive near-infrared fluorescent probes for ALP detection and 3d printed calcium phosphate scaffold imaging in vivo. Biosens. Bioelectron. 2018, 105, 151–158. Wang, W.X.; Jiang, W.L.; Guo, H.; Li, Y.; Li, C.Y. Real-time imaging of alkaline phosphatase activity of diabetes in mice via a near-infrared fluorescent probe. Chem. Commun. 2021, 57, 480–483.
- Li, Y.; Xie, R.; Pang, X.; Zhou, Z.; Xu, H.; Gu, B.; Wu, C.; Li, H.; Zhang, Y. Aggregation-induced emission fluorescent probe for monitoring endogenous alkaline phosphatase in living cells. Talanta 2019, 205, 120143. Pang, X.; Li, Y.; Lu, Q.; Ni, Z.; Zhou, Z.; Xie, R.; Wu, C.; Li, H.; Zhang, Y. A turn-on near-infrared fluorescent probe for visualization of endogenous alkaline phosphatase activity in living cells and zebrafish. Analyst 2021, 146, 521–528.
- Li, J.; Huo, F.; Wen, Z.; Yin, C. A fluorescent turn-on probe based on isophorone for the rapid detection of alkaline phosphatase and its application in bioimaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 221, 117156. Khatun, S.; Biswas, S.; Mahanta, A.K.; Joseph, M.M.; Vidyalekshmi, M.S.; Podder, A.; Maiti, P.; Maiti, K.K.; Bhuniya, S. Biocompatible fluorescent probe for detecting mitochondrial alkaline phosphatase activity in live cells. J. Photochem. Photobiol. B Biol. 2020, 212, 112043.
- Yangyang, Y.; Chen, Z.; Rizhao, P.; Shiwei, Z.; Shengtao, Y.; Yao, T.; Weilong, Z.; Liyue, W.; Weiping, Z.; Yufang, X.; et al. A ratiometric fluorescent probe for alkaline phosphatase with high sensitivity. Chin. Chem. Lett. 2020, 31, 125–128. Park, C.S.; Ha, T.H.; Kim, M.; Raja, N.; Yun, H.S.; Sung, M.J.; Kwon, O.S.; Yoon, H.; Lee, C.S. Fast and sensitive near-infrared fluorescent probes for ALP detection and 3d printed calcium phosphate scaffold imaging in vivo. Biosens. Bioelectron. 2018, 105, 151–158.
- Gao, C.; Zang, S.; Nie, L.; Tian, Y.; Zhang, R.; Jing, J.; Zhang, X. A sensitive ratiometric fluorescent probe for quantitative detection and imaging of alkaline phosphatase in living cells. Anal. Chim. Acta 2019, 1066, 131–135. Li, Y.; Xie, R.; Pang, X.; Zhou, Z.; Xu, H.; Gu, B.; Wu, C.; Li, H.; Zhang, Y. Aggregation-induced emission fluorescent probe for monitoring endogenous alkaline phosphatase in living cells. Talanta 2019, 205, 120143.
- Xu, L.; He, X.; Huang, Y.; Ma, P.; Jiang, Y.; Liu, X.; Tao, S.; Sun, Y.; Song, D.; Wang, X. A novel near-infrared fluorescent probe for detecting intracellular alkaline phosphatase and imaging of living cells. J. Mater. Chem. B 2019, 7, 1284–1291. Li, J.; Huo, F.; Wen, Z.; Yin, C. A fluorescent turn-on probe based on isophorone for the rapid detection of alkaline phosphatase and its application in bioimaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 221, 117156.
- Lin, M.; Huang, J.; Zeng, F.; Wu, S. A fluorescent probe with aggregation-induced emission for detecting alkaline phosphatase and cell imaging. Chem. Asian J. 2019, 14, 802–808. Yangyang, Y.; Chen, Z.; Rizhao, P.; Shiwei, Z.; Shengtao, Y.; Yao, T.; Weilong, Z.; Liyue, W.; Weiping, Z.; Yufang, X.; et al. A ratiometric fluorescent probe for alkaline phosphatase with high sensitivity. Chin. Chem. Lett. 2020, 31, 125–128.
- Pandith, A.; Bhattarai, K.R.; Siddappa, R.K.G.; Chae, H.J.; Seo, Y.J. Novel fluorescent C2-symmetric sequential on-off-on switch for Cu2+ and pyrophosphate and its application in monitoring of endogenous alkaline phosphatase activity. Sens. Actuators B Chem. 2019, 282, 730–742. Gao, C.; Zang, S.; Nie, L.; Tian, Y.; Zhang, R.; Jing, J.; Zhang, X. A sensitive ratiometric fluorescent probe for quantitative detection and imaging of alkaline phosphatase in living cells. Anal. Chim. Acta 2019, 1066, 131–135.
- Lim, E.K.; Keem, J.O.; Yun, H.S.; Jung, J.; Chung, B.H. Smart nanoprobes for the detection of alkaline phosphatase activity during osteoblast differentiation. Chem. Commun. 2015, 51, 3270–3272. Xu, L.; He, X.; Huang, Y.; Ma, P.; Jiang, Y.; Liu, X.; Tao, S.; Sun, Y.; Song, D.; Wang, X. A novel near-infrared fluorescent probe for detecting intracellular alkaline phosphatase and imaging of living cells. J. Mater. Chem. B 2019, 7, 1284–1291.
- Ou-Yang, J.; Li, C.Y.; Li, Y.F.; Yang, B.; Li, S.J. An infinite coordination polymer nanoparticles-based near-infrared fluorescent probe with high photostability for endogenous alkaline phosphatase in vivo. Sens. Actuators B Chem. 2018, 255, 3355–3363. Lin, M.; Huang, J.; Zeng, F.; Wu, S. A fluorescent probe with aggregation-induced emission for detecting alkaline phosphatase and cell imaging. Chem. Asian J. 2019, 14, 802–808.
- Pandith, A.; Bhattarai, K.R.; Siddappa, R.K.G.; Chae, H.J.; Seo, Y.J. Novel fluorescent C2-symmetric sequential on-off-on switch for Cu2+ and pyrophosphate and its application in monitoring of endogenous alkaline phosphatase activity. Sens. Actuators B Chem. 2019, 282, 730–742.
- Lim, E.K.; Keem, J.O.; Yun, H.S.; Jung, J.; Chung, B.H. Smart nanoprobes for the detection of alkaline phosphatase activity during osteoblast differentiation. Chem. Commun. 2015, 51, 3270–3272.
- Juan Ou-Yang; Chun-Yan Li; Yong-Fei Li; Bin Yang; Song-Jiao Li; An infinite coordination polymer nanoparticles-based near-infrared fluorescent probe with high photostability for endogenous alkaline phosphatase in vivo. Sensors and Actuators B: Chemical 2018, 255, 3355-3363, 10.1016/j.snb.2017.09.162.
