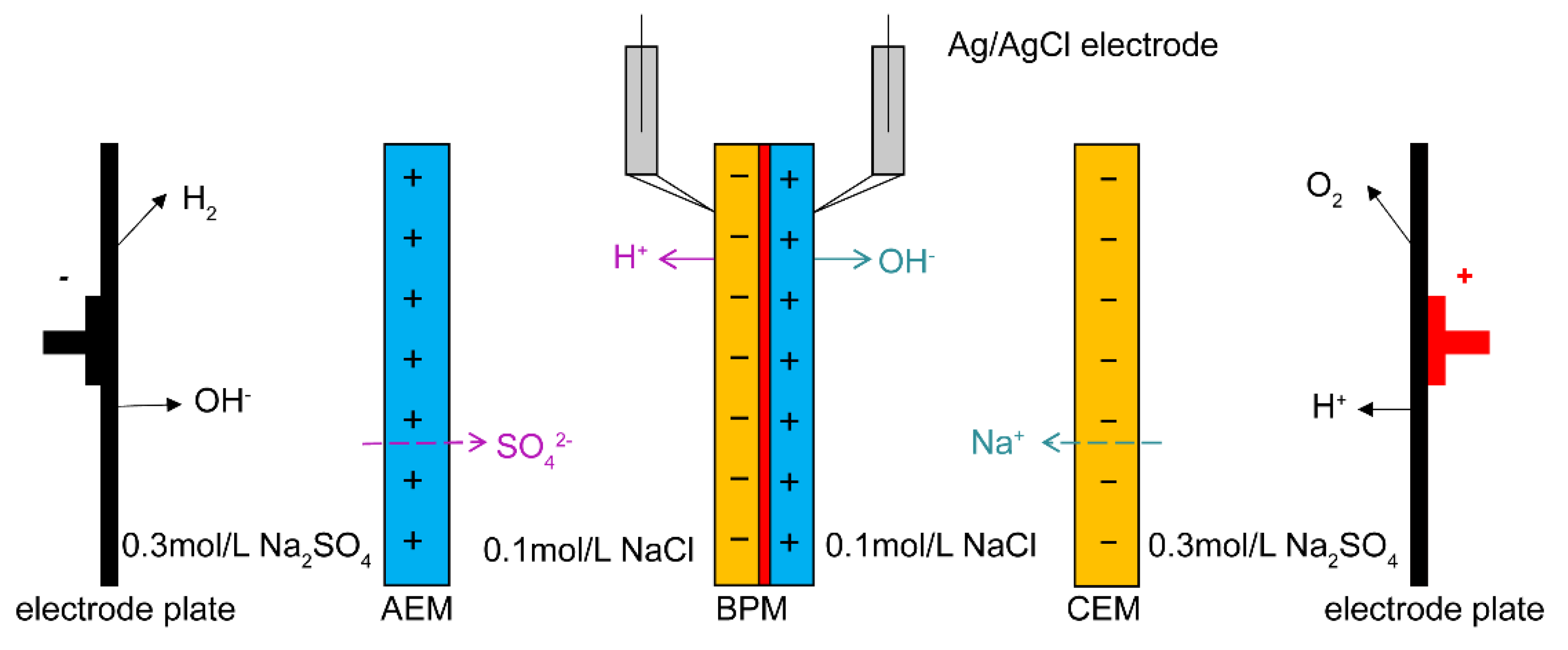
Bipolar membranes, a new type of composite ion exchange membrane, contain an anion exchange layer, a cation exchange layer and an interface layer. The interface layer or junction is the connection between the anion and cation exchange layers. Water is dissociated into protons and hydroxide ions at the junction, which provides solutions to many challenges in the chemical, environmental and energy fields. By combining bipolar membranes with electrodialysis technology, acids and bases could be produced with low cost and high efficiency. The interface layer or junction of bipolar membranes (BPMs) is the connection between the anion and cation exchange layers, which the membrane and interface layer modification are vital for improving the performance of BPMs.
- bipolar membrane
- water dissociation
- interface layer
- catalyst
- organic material
- inorganic materials
1. Overview of Bipolar Membranes
2. Application of Bipolar Membranes
3. Study of the Interface Layer Catalyst
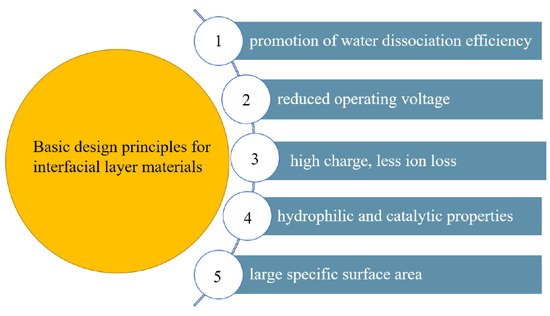
The interfacial layer is the main place where water dissociation occurs in bipolar membranes. Assuming that this phenomenon was explained by the usual water dissociation mechanism, according to Simons’ theory [44], the calculated fluxes of H+ and OH− were about 2 × 10−9 mol/m2 s, while the actual fluxes in bipolar membranes were as high as 10−2 mol/m2 s, the calculated value was inconsistent with the experimental value, indicating that the water dissociation of the bipolar membrane is not the same as the water dissociation in the normal state. Therefore, there is an effect of promoting water dissociation. According to the studies of the water dissociation behavior of a bipolar membrane by many researchers, there are two main theoretical models to explain this phenomenon. The second Wien effect is a theoretical model based on the hydrolysis of weak electrolytes under high electric fields [45]. The theory holds that when the applied electric field is high enough, the conductivity of electrolyte increases rapidly with the electric field, causing Ohm’s law to fail. Applying the second Wien effect to bipolar membranes, water dissociation occurs in a sharp depletion region between the anion and cation exchange layers, where water is broken down into H+ and OH− as a weak electrolyte. In Onsager’s experiments, the electric field strength in the depletion region was as high as 108 V/m, and the water dissociation rate constant was 107 times higher than when no applied voltage was applied. Surprisingly, the theory cannot continue to be implemented in the experiment for the electric field intensity higher than 108 V/m. To investigate the cause, other effects that high electric fields may bring are ignored. Since then, Simons had conducted numerous experiments to investigate functional groups and proposed that the hydrolysis of water molecules into H+ and OH− generated a protonation and deprotonation reaction model by interacting with the membrane’s fixed charge groups [44[44][46],46], i.e., the chemical reaction theoretical model (CHR). According to the CHR hypothesis, water dissociation is primarily driven by active group catalysis, i.e., protonation and deprotonation of the active group. The application of this model to the water dissociation mechanism of the bipolar membrane interface layer showed that the water dissociation depends on the properties of charged groups, and not all functional groups made a significant contribution to water dissociation. Other studies had found that protonation and deprotonation reactions were not limited to membrane fixed charge groups [47], and the role between other active points in the membrane (such as metal oxides, heavy metal impurities, and metal complexes) and water molecules might also be applicable. The bipolar membranes currently used could not bear high temperatures with low mechanical strength and poor conductivity. New bipolar membranes which have good permeability, high permeaselectivity and good stability are urgently needed. Selectivity is an important parameter in the BMED process, if it is necessary to electrolyze ion solutions containing different valence states to produce a specific substance. Bipolar membranes with high selectivity improve the purity of the product. On the contrary, it not only reduces the product purity but also increases the energy consumption in the electrodialysis process [48]. Since water molecules in the interface layer are split to generate an acid-base solution, it is further required that the bipolar membranes need to have great resistance ability for acids and bases. The methods to improve the performance of bipolar membranes are mainly divided into the modification of the anion and cation exchange layers and the introduction of catalysts in the interface layer. Figure 2 depicts the development process of the interfacial layer materials. The transmembrane voltage of bipolar membranes is an important factor affecting the performance of bipolar membranes, which mainly depends on the voltage drop of the interface layer. Catalysts are added to the interface layer to promote the water dissociation, reduce the activation energy of the water dissociation reaction, increase the water dissociation rate, reduce the membrane impedance, and make the BPM process more energy efficient. The basic principles for the selection of the interfacial layer materials are included in Figure 3.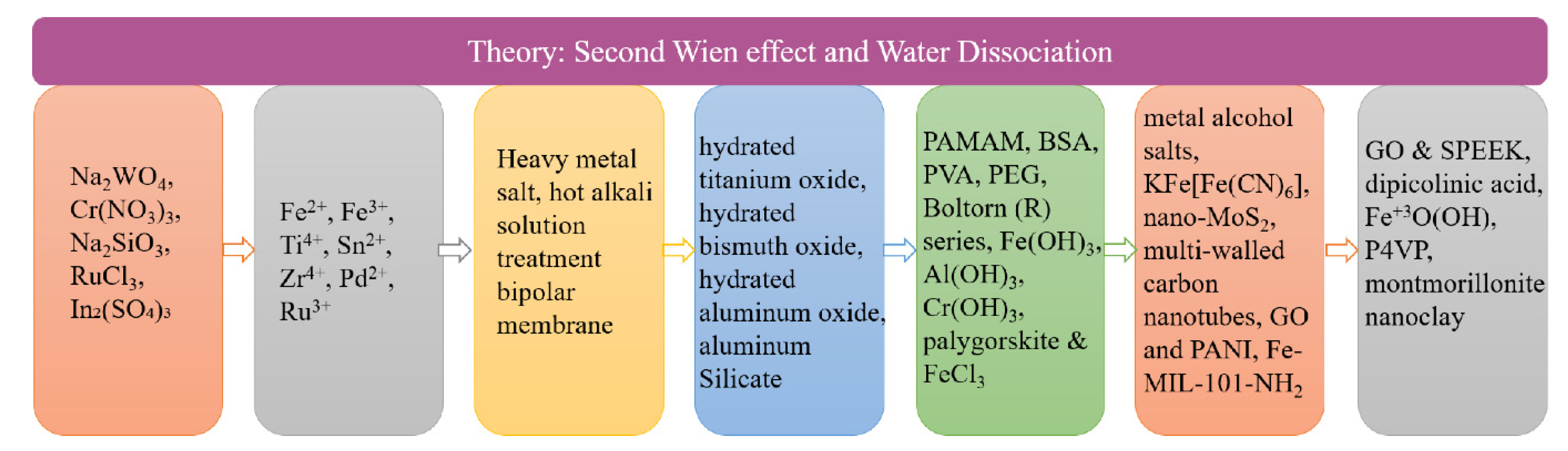
References
- McDonald, M.B.; Freund, M.S. Graphene Oxide as a Water Dissociation Catalyst in the Bipolar Membrane Interfacial Layer. ACS Appl. Mater. Interfaces 2014, 6, 13790–13797.
- Balster, J.; Srinkantharajah, S.; Sumbharaju, R.; Punt, I.; Lammertink, R.G.H.; Stamatialis, D.F.; Wessling, M. Tailoring the interface layer of the bipolar membrane. J. Membr. Sci. 2010, 365, 389–398.
- Abdu, S.; Sricharoen, K.; Wong, J.E.; Muljadi, E.S.; Melin, T.; Wessling, M. Catalytic Polyelectrolyte Multilayers at the Bipolar Membrane Interface. ACS Appl. Mater. Interfaces 2013, 5, 10445–10455.
- Balster, J.; Sumbharaju, R.; Srikantharajah, S.; Punt, I.; Stamatialis, D.F.; Jordan, V.; Wessling, M. Asymmetric bipolar membrane: A tool to improve product purity. J. Membr. Sci. 2007, 287, 246–256.
- Parnamae, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021, 617, 25.
- Eswaraswamy, B.; Goel, P.; Mandal, P.; Chandra, A.; Chattopadhyay, S. Nanocomposite interface coupled with thickness optimization promoting water dissociation in heterogeneous bipolar membrane. Polym. Adv. Technol. 2022, 33, 353–367.
- Eswaraswamy, B.; Suhag, A.; Goel, P.; Mandal, P.; Chattopadhyay, S. Potential of montmorillonite nanoclay as water dissociation catalyst at the interface of bipolar membrane. Sep. Purif. Technol. 2022, 295, 121257.
- Celik, A.; Hasar, H. Fabrication and implementation of extensively dense bipolar membrane using FeCl3 as a junction catalyst. Polym. Bull. 2022, 79, 6815–6825.
- Rathod, N.H.; Sharma, J.; Raj, S.K.; Yadav, V.; Rajput, A.; Kulshrestha, V. Fabrication of a Stable and Efficient Bipolar Membrane by Incorporation of Nano-MoS2 Interfacial Layer for Conversion of Salt into Corresponding Acid and Alkali by Water Dissociation Using Electrodialysis. ACS Sustain. Chem. Eng. 2020, 8, 13019–13029.
- Fu, L.L.; Gao, X.L.; Yang, Y.; Fan, A.Y.; Hao, H.W.; Gao, C.J. Preparation of succinic acid using bipolar membrane electrodialysis. Sep. Purif. Technol. 2014, 127, 212–218.
- Szczygielda, M.; Antczak, J.; Prochaska, K. Separation and concentration of succinic acid from post-fermentation broth by bipolar membrane electrodialysis (EDBM). Sep. Purif. Technol. 2017, 181, 53–59.
- Liu, X.H.; Li, Q.H.; Jiang, C.X.; Lin, X.C.; Xu, T.W. Bipolar membrane electrodialysis in aqua-ethanol medium: Production of salicylic acid. J. Membr. Sci. 2015, 482, 76–82.
- Rottiers, T.; Bruggen, V.B.; Pinoy, L. Production of salicylic acid in a three compartment bipolar membrane electrodialysis configuration. J. Ind. Eng. Chem. 2017, 54, 190–199.
- Wang, X.L.; Wang, Y.M.; Zhang, X.; Feng, H.Y.; Xu, T.W. In-situ combination of fermentation and electrodialysis with bipolar membranes for the production of lactic acid: Continuous operation. Bioresour. Technol. 2013, 147, 442–448.
- Wang, X.L.; Wang, Y.M.; Zhang, X.; Xu, T.W. In situ combination of fermentation and electrodialysis with bipolar membranes for the production of lactic acid: Operational compatibility and uniformity. Bioresour. Technol. 2012, 125, 165–171.
- Grib, H.; Bonnal, L.; Sandeaux, J.; Sandeaux, R.; Gavach, C.; Mameri, N. Extraction of amphoteric amino acids by an electromembrane process. pH and electrical state control by electrodialysis with bipolar membranes. J. Chem. Technol. Biotechnol. 1998, 73, 64–70.
- Li, Y.; Shi, S.Y.; Cao, H.B.; Wu, X.M.; Zhao, Z.J.; Wang, L.Y. Bipolar membrane electrodialysis for generation of hydrochloric acid and ammonia from simulated ammonium chloride wastewater. Water Res. 2016, 89, 201–209.
- Kroupa, J.; Kincl, J.; Cakl, J. Recovery of H2SO4 and NaOH from Na2SO4 by electrodialysis with heterogeneous bipolar membrane. Desalin. Water Treat. 2015, 56, 3238–3246.
- Zhang, X.; Ren, W.L.H.; Cong, W. Sulfuric acid and ammonia generation by bipolar membranes electrodialysis: Transport rate model for ion and water through anion exchange membrane. Chem. Biochem. Eng. Q. 2008, 22, 1–8.
- Trivedi, G.S.; Shah, B.G.; Adhikary, S.K.; Rangarajan, R. Studies on bipolar membranes—Part III: Conversion of sodium phosphate to phosphoric acid and sodium hydroxide. React. Funct. Polym. 1999, 39, 91–97.
- Yao, L.; Qiu, Y.B.; Zhao, Y.; Tang, C.; Shen, J.N. A continuous mode operation of bipolar membrane electrodialysis (BMED) for the production of high-pure choline hydroxide from choline chloride. Sep. Purif. Technol. 2020, 233, 9.
- Sheldeshov, N.V.; Zabolotsky, V.I.; Kovalev, N.V.; Karpenko, T.V. Electrochemical characteristics of heterogeneous bipolar membranes and electromembrane process of recovery of nitric acid and sodium hydroxide from sodium nitrate solution. Sep. Purif. Technol. 2020, 241, 11.
- Venugopal, K.; Dharmalingam, S. Evaluation of the efficiency of brackish desalination ion exchange membranes using electrodialysis process. RSC Adv. 2015, 5, 73901–73913.
- Berkessa, Y.W.; Lang, Q.L.; Yan, B.H.; Kuang, S.P.; Mao, D.B.; Shu, L.; Zhang, Y. Anion exchange membrane organic fouling and mitigation in salt valorization process from high salinity textile wastewater by bipolar membrane electrodialysis. Desalination 2019, 465, 94–103.
- Tian, W.D.; Wang, X.; Fan, C.Y.; Cui, Z. Optimal Treatment of Hypersaline Industrial Wastewater via Bipolar Membrane Electrodialysis. ACS Sustain. Chem. Eng. 2019, 7, 12358–12368.
- Sun, Y.; Wang, Y.Y.; Peng, Z.; Liu, Y. Treatment of high salinity sulfanilic acid wastewater by bipolar membrane electrodialysis. Sep. Purif. Technol. 2022, 281, 9.
- Liu, G.L.; Luo, H.P.; Wang, H.H.; Wang, B.W.; Zhang, R.D.; Chen, S.S. Malic acid production using a biological electrodialysis with bipolar membrane. J. Membr. Sci. 2014, 471, 179–184.
- Gutierrez, L.F.; Bazinet, L.; Hamoudi, S.; Belkacemi, K. Production of lactobionic acid by means of a process comprising the catalytic oxidation of lactose and bipolar membrane electrodialysis. Sep. Purif. Technol. 2013, 109, 23–32.
- Mikhaylin, S.; Nikonenko, V.; Pourcelly, G.; Bazinet, L. Hybrid bipolar membrane electrodialysis/ultrafiltration technology assisted by a pulsed electric field for casein production. Green Chem. 2016, 18, 307–314.
- Xu, T.W.; Huang, C.H. Electrodialysis-Based Separation Technologies: A Critical Review. Aiche J. 2008, 54, 3147–3159.
- Ramdin, M.; Morrison, A.R.T.; de Groen, M.; van Haperen, R.; de Kler, R.; van den Broeke, L.J.P.; Trusler, J.P.M.; de Jong, W.; Vlugt, T.J.H. High Pressure Electrochemical Reduction of CO2 to Formic Acid/Formate: A Comparison between Bipolar Membranes and Cation Exchange Membranes. Ind. Eng. Chem. Res. 2019, 58, 1834–1847.
- Li, Y.G.C.; Yan, Z.F.; Hitt, J.; Wycisk, R.; Pintauro, P.N.; Mallouk, T.E. Bipolar Membranes Inhibit Product Crossover in CO2 Electrolysis Cells. Adv. Sustain. Syst. 2018, 2, 5.
- Liu, X.; Jian, X.; Yang, H.M.; Song, X.L.; Liang, Z.H. A photocatalytic graphene quantum dots-Cu2O/bipolar membrane as a separator for water splitting. New J. Chem. 2016, 40, 3075–3079.
- Iizuka, A.; Hashimoto, K.; Nagasawa, H.; Kumagai, K.; Yanagisawa, Y.; Yamasaki, A. Carbon dioxide recovery from carbonate solutions using bipolar membrane electrodialysis. Sep. Purif. Technol. 2012, 101, 49–59.
- Eisaman, M.D.; Alvarado, L.; Larner, D.; Wang, P.; Garg, B.; Littau, K.A. CO2 separation using bipolar membrane electrodialysis. Energy Environ. Sci. 2011, 4, 1319–1328.
- Valluri, S.; Kawatra, S.K. Reduced reagent regeneration energy for CO2 capture with bipolar membrane electrodialysis. Fuel Process. Technol. 2021, 213, 7.
- Jiang, C.X.; Zhang, Y.L.; Feng, H.Y.; Wang, Q.Y.; Wang, Y.M.; Xu, T.W. Simultaneous CO2 capture and amino acid production using bipolar membrane electrodialysis (BMED). J. Membr. Sci. 2017, 542, 264–271.
- Wang, Q.Y.; Jiang, C.X.; Wang, Y.M.; Yang, Z.J.; Xu, T.W. Reclamation of Aniline Wastewater and CO2 Capture Using Bipolar Membrane Electrodialysis. ACS Sustain. Chem. Eng. 2016, 4, 5743–5751.
- Xue, S.; Wu, C.M.; Wu, Y.H.; Zhang, C.Y. An optimized process for treating sodium acetate waste residue: Coupling of diffusion dialysis or electrodialysis with bipolar membrane electrodialysis. Chem. Eng. Res. Des. 2018, 129, 237–247.
- Xu, T.W. Electrodialysis processes with bipolar membranes (EDBM) in environmental protection—A review. Resour. Conserv. Recycl. 2002, 37, 1–22.
- Wei, X.L.; Wang, Y.M.; Yan, H.Y.; Jiang, C.X.; Xu, T.W. A sustainable valorization of neopentyl glycol salt waste containing sodium formate via bipolar membrane electrodialysis. Sep. Purif. Technol. 2021, 254, 8.
- Noguchi, M.; Nakamura, Y.; Shoji, T.; Iizuka, A.; Yamasaki, A. Simultaneous removal and recovery of boron from waste water by multi-step bipolar membrane electrodialysis. J. Water Process. Eng. 2018, 23, 299–305.
- Lameloise, M.L.; Lewandowski, R. Recovering L-malic acid from a beverage industry waste water: Experimental study of the conversion stage using bipolar membrane electrodialysis. J. Membr. Sci. 2012, 403, 196–202.
- Simons, R. Electric field effects on proton transfer between ionizable groups and water in ion exchange membranes. Electrochim. Acta 1984, 29, 151–158.
- Onsager, L. Deviations from Ohm’s Law in Weak Electrolytes. J. Chem. Phys. 1934, 2, 599–615.
- Simons, R. Strong electric field effects on proton transfer between membrane-bound amines and water. Nature 1979, 280, 824–826.
- Helfferich, F.G.; Dranoff, J.S. Ion Exchange; McGraw-Hill: New York, NY, USA, 1962.
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146.
- Simons, R. Preparation of a high performance bipolar membrane. J. Membr. Sci. 1993, 78, 13–23.
