Fusion imaging depicts an innovative technique by which previously performed computed tomography/magnetic resonance imaging can be integrated and reconstructed with advanced contrast-enhanced ultrasound using modern ultrasound devices in a real-time manner. Fusion imaging allows for complementing strengths and reducing restrictions of the combined imaging modalities. The visualization of parenchymal and tumoral microperfusion by contrast-enhanced ultrasound can be dynamically fused and assessed with images from previous cross-sectional studies and may help to decipher underlying entities of indeterminate lesions or validate suspicious morphology. The findings from our study demonstrate the benefits of fusion imaging for evaluating focal hepatic and renal lesions. The excellent safety profile, accessibility, repeatability and cost-effectiveness are advantages of fusion imaging which make it a powerful diagnostic tool for the modern radiologist.
- fusion imaging
- ultrasound
- CEUS
- CT
- MRI
- kidney
- RCC
- liver
- HCC
- oncology.
1. Content
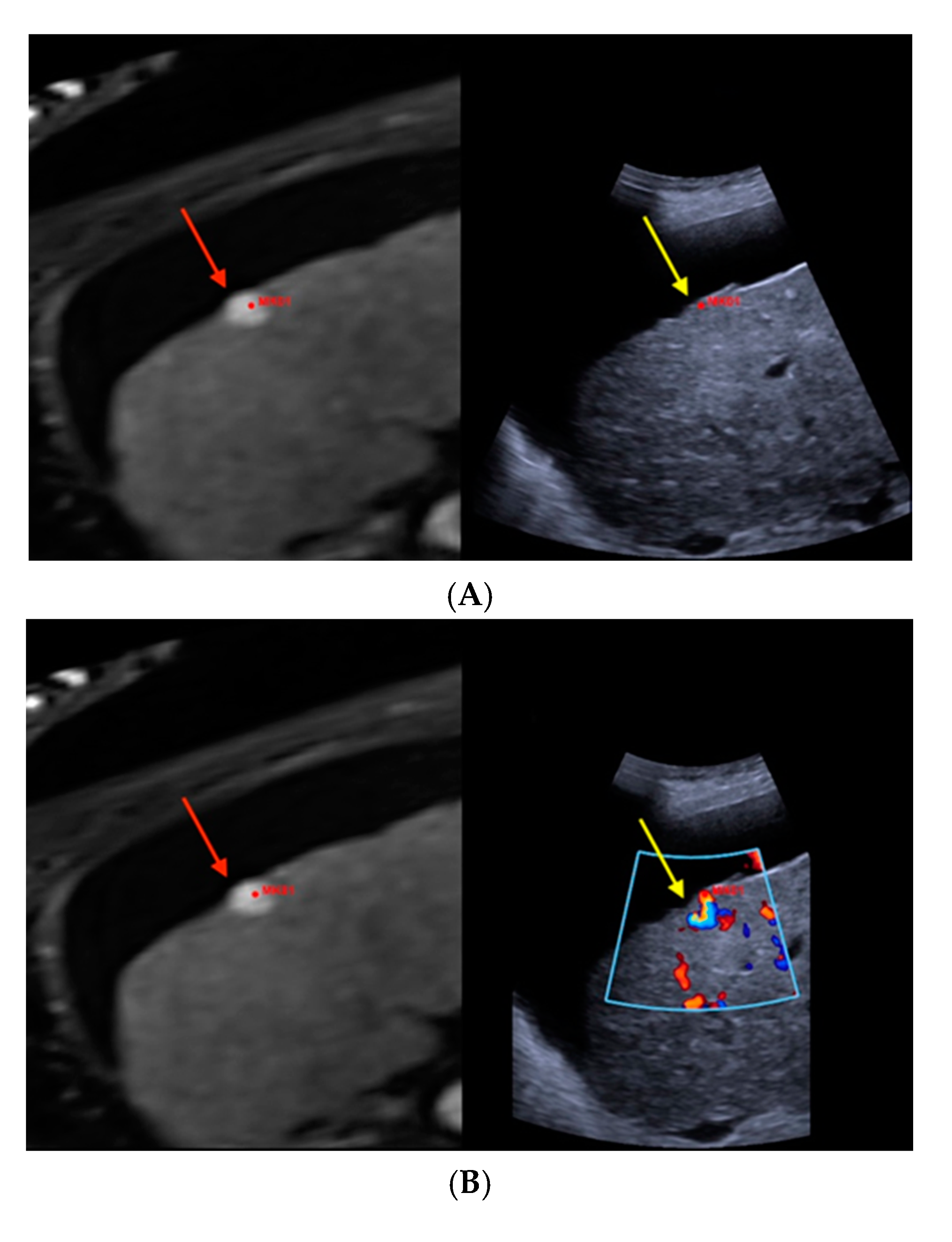
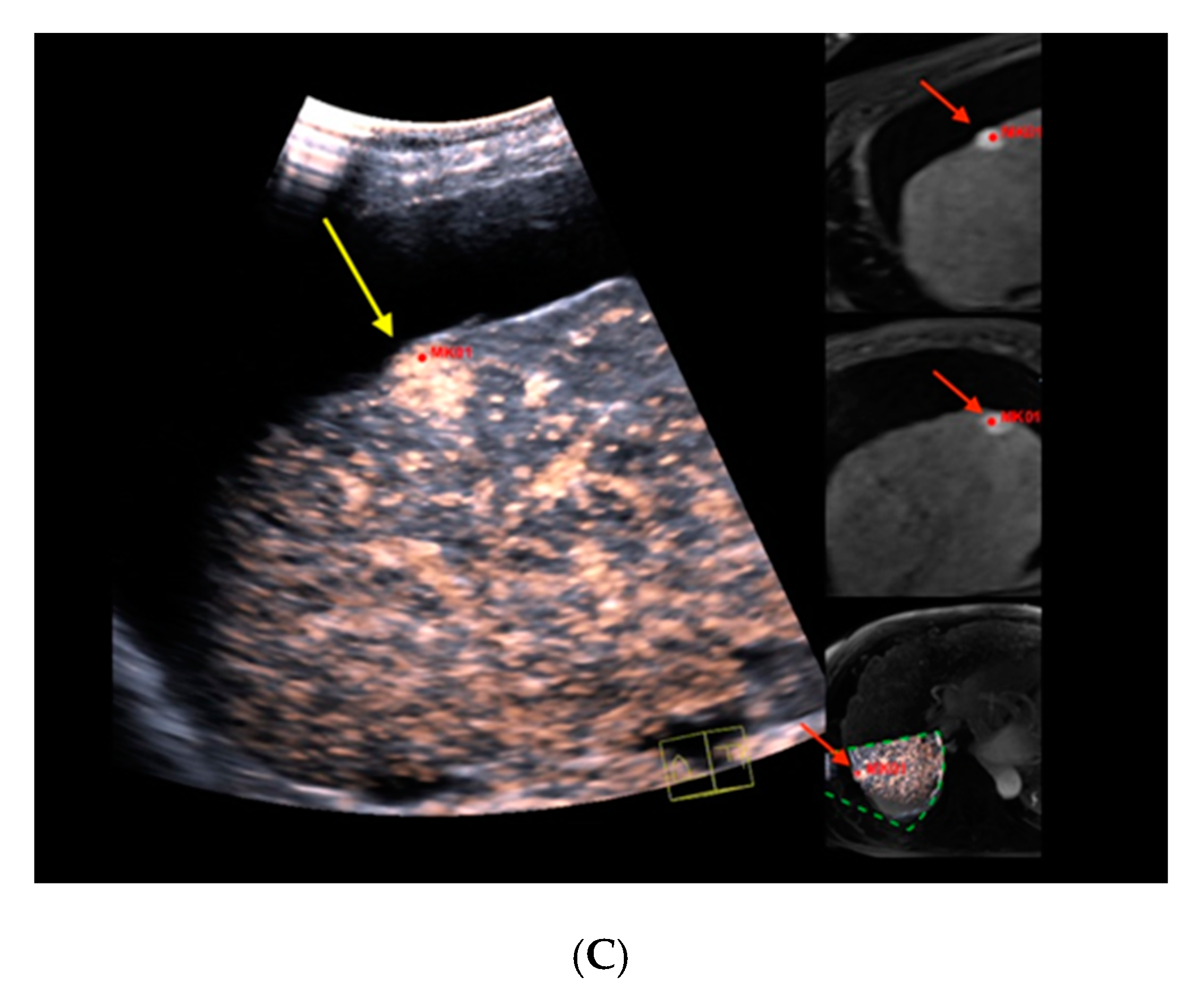
Figure 1. Real-time magnetic resonance imaging-/contrast-enhanced ultrasound (MRI-/CEUS)-fusion imaging of a hypervascularized focal liver lesion in a cirrhotic patient. (A) A hypervascularized subcapsular focal lesion in liver segment 7 was registered in MRI, arterial phase (left), a target lesion (MK01, red) was placed for precise correlation with native B-mode (right) in a side-by-side manner. The focal lesion otherwise was barely detectable by conventional ultrasound. (B) Additional Color Doppler showed livid hypervascularization of the lesion (right), a corresponding plane of MRI, arterial phase (left). (C) Peripheral-to-central contrast-enhancement and, finally, homogeneous contrast enhancement was registered in CEUS (left, maximized), implicating focal nodular hyperplasia. The software interface of the ultrasound device showed four different images: real-time CEUS images (left, maximized), MRI datasets in axial (upper right) and sagittal (middle right) reformation, and a real-time 3D navigation of the MRI-/CEUS-fusion imaging (lower right), (lesion marked by red arrows).
Conventional ultrasound, comprising native B-mode and Color Doppler, is frequently applied as an imaging modality for initial abdominal investigation, including kidney and liver imaging. Ultrasound is applied as a screening tool in patients with chronic diseases, who thus are predisposed to developing cancer, e.g., renal cell carcinoma (RCC) or hepatocellular carcinoma (HCC). It is also used as the imaging instrument of choice when patients present with acute abdominal symptoms. Image acquisition by ultrasound is based on scattering, reflecting, and frequency shifting of ultrasound waves by different tissues. Due to the physics behind imaging acquisition by ultrasound, obesity, a limited acoustic window, or bowel gas depict shortcomings of conventional ultrasound. The administration of intravenous microbubbles for enabling vascular and parenchymal contrast enhancement allows for improved visualization of abdominal pathologies, e.g., demarcate focal liver lesion surrounded by steatotic liver parenchyma. The advantages of contrast-enhanced ultrasound (CEUS) are that it can immediately and repeatably be applied, its cost-effectiveness, and its excellent non-ionizing safety profile. Nevertheless, CEUS cannot overcome all shortcomings of conventional ultrasound. Often, cross-sectional imaging modalities, such as computed tomography (CT) and magnetic resonance imaging (MRI), are critical and inevitable, especially under acute or traumatic circumstances of the patient. Thus, CT or MRI is recommended by the American College of Radiology (ACR) for assessing certain acute abdominal situations. Focal liver or renal lesions are frequently and incidentally registered due to the increasing use of elaborate CT and MRI scans. Because CT and MRI examinations often are performed without adequate protocols that allow for specific scrutinization of the incidentally found parenchymal lesions, their underlying entities often remain indeterminate, and further diagnostic evaluation is necessary. A thorough evaluation is mandatory before CT and MRI are re-done with optimized protocols. Ionizing radiation, in case of CT, potential renal affection due to iodinated or gadolinium-based contrast agents, potential allergic predisposition to contrast agents, as well as the relevant financial costs, must be considered.
Modern high-end ultrasound devices allow for the integration and adequate reconstruction of previously performed CT or MRI scans, thereby enabling simultaneous and real-time computerized fusion of former cross-sectional studies with live ultrasound images in the same and additional planes. Fusion imaging depicts an innovative technique by which the assets of combined imaging modalities, such as comprehensive field of view and high-contrast resolution of CT/MRI, and high spatial resolution of ultrasound in real-time, can be complemented and restrictions can be minimized, e.g., the limited acoustic window of ultrasound is extended by the wide field of view of CT and MRI. Of note, fusion imaging can be conducted with native B-mode, Color Doppler, CEUS, and elastography, which facilitates thorough and dynamic scrutiny of focal parenchymal lesions of interest. The visualization of tissue and tumoral microperfusion by CEUS can be dynamically fused and analyzed with images from previous cross-sectional studies, thereby further elevating the confidence of the observer. Of note, compared to elaborate cross-sectional imaging modalities, CEUS and advanced fusion imaging are easily accessible and repeatable, comparably inexpensive, and have an excellent safety profile. By using fusion imaging, the ultrasound examination depends less on the capability of the observer to mentally fuse findings from previous CT/MRI scans with recent sonographic findings. The advantage of using fusion imaging to ablate liver lesions and to monitor subsequent therapeutic outcome was already described. Furthermore, fusion imaging of CEUS and CT was shown to help the placement of endovascular aortic repair (EVAR) and further improve the visualization of graft endoleaks.
A thorough evaluation is critical before CT and MRI examinations are performed. The ionizing radiation in the case of CT results in an elevated risk of radiation-related cancers, potential allergic predisposition against, as well as affections of the renal and thyroid function due to iodinated contrast agents, must also be considered. Limited availability, higher financial costs, restricted applicability of contrast-agents in patients with kidney failure or allergic predisposition, as well as limited usage in case of corporeal metallic medical devices, are detrimental aspects of MRI. Although possible long-term clinical effects have not been reported so far, the recently reported potential deposition of gadolinium-based contrast agents within the basal nuclei requires deliberation. In sharp contrast, CEUS and its innovative integration in the context of fusion imaging are cost-effective, directly accessible, and repeatable with fewer hesitations at an excellent safety profile. Once cross-sectional studies are performed, fusion imaging can be safely conducted in patients with renal or thyroidal disorders, allergic predispositions to iodinated or gadolinium-based contrast agents, as well as in pregnant patients and children. Of importance, fusion imaging allows for the visualization of tissue and tumor microperfusion at higher temporal and spatial resolutions in a real-time manner compared to CT/MRI alone. The limited diagnostic performance of static cross-sectional imaging compared to fusion imaging as a reference modality in our cohorts emphasizes the associated diagnostic benefits of using advanced and real-time fusion imaging. The depicted advantages of fusion imaging plausibly enhance the confidence of the observer. Of course, performing fusion imaging highly depends on the skills of the observer and is susceptible to moving artifacts, e.g., due to breathing.
Frequently oncologic patients do have relevant comorbidities, which may limit their transfer to the Radiology Department and, therefore, the use of CT or MRI, e.g., invasive ventilation or catecholamine therapy. Fusion imaging can easily be performed at the patients’ bedside, therefore, sparing potential anxiety of the patient due to delayed reporting, and the patient can immediately be informed about the findings. Doubtless, fusion imaging is not capable of replacing cross-sectional studies in terms of oncological staging. Its excellent safety profile, its accessibility and repeatability, its applicability in oncologic patients with kidney or thyroid affections, as well as in pregnant and young patients, and its cost-effectiveness are assets of fusion imaging, which, therefore, should be integrated into the daily routine of the modern radiologist.


Figure 1. Real-time magnetic resonance imaging-/contrast-enhanced ultrasound (MRI-/CEUS)-fusion imaging of a hypervascularized focal liver lesion in a cirrhotic patient. (A) A hypervascularized subcapsular focal lesion in liver segment 7 was registered in MRI, arterial phase (left), a target lesion (MK01, red) was placed for precise correlation with native B-mode (right) in a side-by-side manner. The focal lesion otherwise was barely detectable by conventional ultrasound. (B) Additional Color Doppler showed livid hypervascularization of the lesion (right), a corresponding plane of MRI, arterial phase (left). (C) Peripheral-to-central contrast-enhancement and, finally, homogeneous contrast enhancement was registered in CEUS (left, maximized), implicating focal nodular hyperplasia. The software interface of the ultrasound device showed four different images: real-time CEUS images (left, maximized), MRI datasets in axial (upper right) and sagittal (middle right) reformation, and a real-time 3D navigation of the MRI-/CEUS-fusion imaging (lower right), (lesion marked by red arrows).
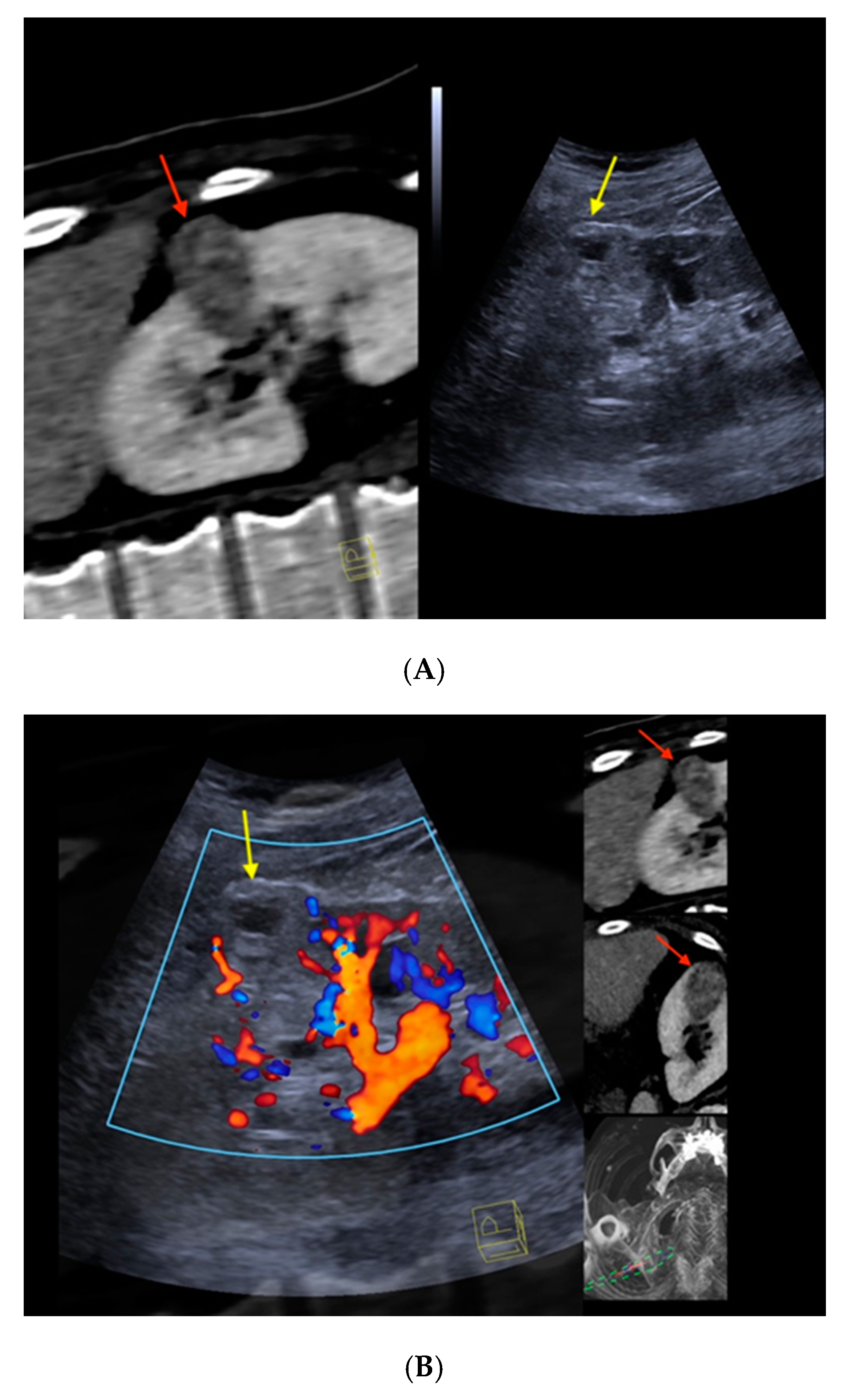
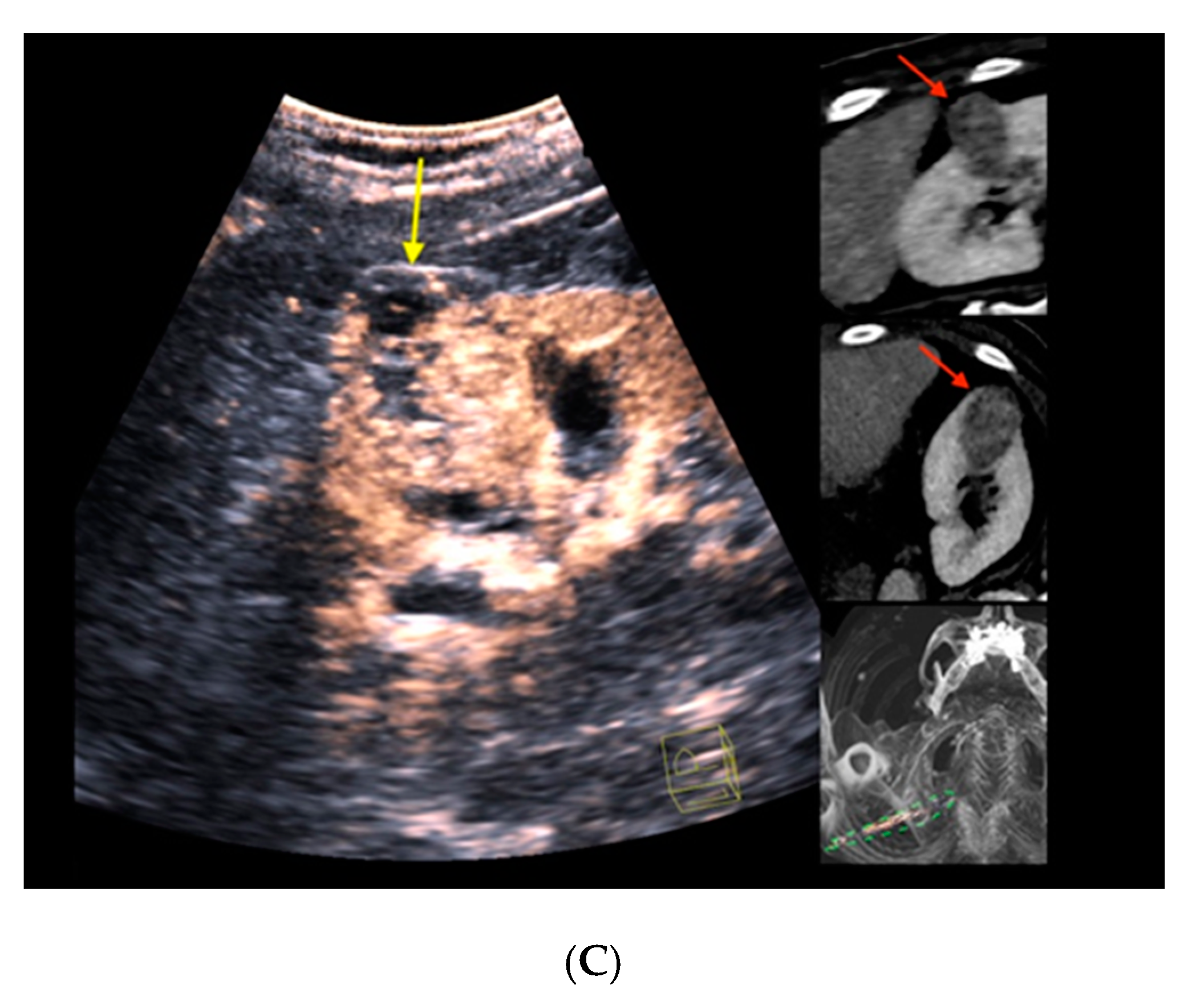
Figure 2. Real-time CT-/CEUS-fusion imaging of a complex renal cystic lesion. (A) Known complex renal cystic lesion with indicated septations and solid components in the right kidney from previous contrast-enhanced CT (left, red arrow) is displayed in a side-by-side mode with native B-mode (right, yellow arrow) by a high-end ultrasound system. (B) Additional Color Doppler did not reveal hypervascularization of the lesion (yellow arrow). The software interface of the ultrasound device showed four different images: the real-time Color Doppler image (left, maximized), the CT imaging dataset in sagittal (upper right) and axial (middle right) reformation (lesion marked by red arrows), and a real-time 3D navigation of the Fusion Imaging (lower right). (C) Contrast-enhanced ultrasound allowed for visualization of early arterial microperfusion of solid components of the lesion, implicating malignancy (left, maximized), (lesion marked by red arrows in corresponding CT images). The patient underwent partial nephrectomy. Histopathology, finally, revealed underlying clear-cell renal cell carcinoma.
