Ever since nanotechnology grew into a reckoned field of its own, the implications in medicine and pharmacology became obvious, and are today exploited commercially on many drug formulations. A busy lifestyle, erratic work schedule and oxidative stress, together with genetic and other risk factors have contributed to a surge in cancer incidence throughout the globe, and current estimates predict about 1.26 million deaths for 2022 in the EU due to cancer alone. Conventional approaches come with unavoidable side effects due to systemic exposure and response, and are less effective than directed therapy—a field where MNPs and SPIONs, in particular, have emerged as suitable candidates with more efficient delivery of the anticancer drugs and limited negative effects on neighboring tissues and organs.
Magnetic nanoparticles (MNPs) are at the very core of magnetic delivery systems and they aim to tackle site-specific tumors while ideally affording a controlled-release profile suitable for disease treatment. Their multifunctional dimensionality makes it possible for MNPs to be used in nanomedicine as elective candidates for drug targeting therapy when using an externally applied magnetic field.
With tunable physico-chemical properties and a very high surface-to-volume ratio typical for nanoparticles, MNPs can be engineered into drug-delivery systems with similar sizes to the organism’s own antibodies or proteins for improved biocompatibility, while incorporating therapeutic agents that would otherwise be difficult to deliver to the cancer cells. When superparamagnetic nanoparticles (SPIONs) are coated with biologically compatible polymers of fatty acids, systems with improved colloidal stability and reduced tendency of aggregation are obtained. MNPs were also used as contrast agents in magnetic resonance imaging (MRI). When functionalized with epithelial growth factor receptor antibodies or aptamers, an efficient diagnosis tool is created for many types of cancer or even detection of brain inflammation. MNPs are being used as alternative contrast agents in MR imaging owing to their superparamagnetic properties and high relaxivity, doubled by high biocompatibility upon surface functionalization and low toxicity, unlike the Gd complexes used traditionally as contract agent in MRI, which could potentially release Gd into the bloodstream.
2. Justification and Design Strategies for Magnetic Nanoplatforms
The
curre
are multiple reasons pleading for the use of nt review strategy entailed an examination of search results on specific research topics such as “magnetic nanoparticle” and/or “drug delivery”, “nanomedicine”, “MR imaging” or “hyperthermia”, but is also based on pertinent examples dealing with the above topic without specifically containing the mentioned keywords (for instance, articles dealing with ferrofluids were also included, provided a tentative use in biomedical applications was provided in the original text). Based on the occurrence of research directions found in both original research and review articles published in the past three years (2020–2022), the current table of content was decided and data curation of the 500+ articles identified was carried out around these main topics. Given the exponential expansion of the field, only the last three years were selected, and the inclusion of each article on the reference list was decided upon by reading the abstract and deciding on the suitability of the article for the included review topics.
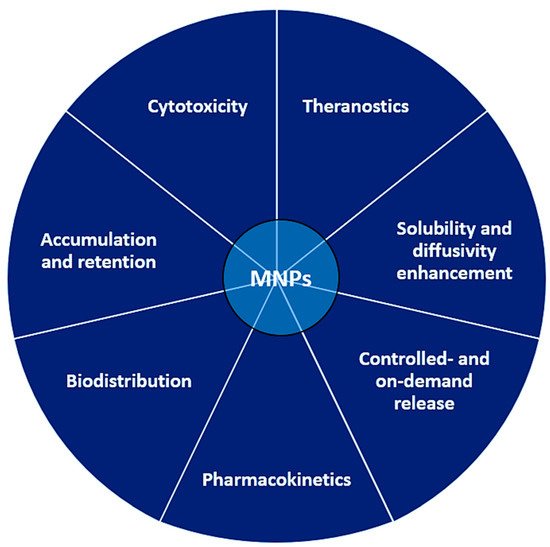
There are multiple reasons pleading for the use of MNPs as carriers for drug and gene delivery, making nanoparticle platforms superior to the traditional administration of the drug alone. Moreover, theranostics are able to integrate today’s MNPs in procedures capable of both diagnosis and disease treatment, which is unachievable via traditional drug administration. Using NPs for drug delivery allows modification of key aspects related to drug solubility, diffusivity, penetration and retention, pharmacokinetics, biodistribution, cytotoxicity, and half-life (controlled- and/or on-demand release).
Considering how time-consuming (~12 years on average) and extremely expensive (up to USD 2 billion) the drug development process can be, it may be surprising to note that, even when an efficient active component is identified, basic hiccups still plague the process, such as a lower grade in the biopharmaceutical system, which assesses the solubility and permeability of a drug. In other words, a highly efficient drug whose development took years of research may never reach the patient in need due to low solubility/permeability in the biological system. This severe shortcoming can be modulated by drug immobilization on magnetic nanocarriers, that are able to transport insoluble drugs to the target site by smart surface modification (for instance, with antibodies that would bind to molecules overexpressed at the tumoral site). Drug formulations employing stable nanoparticle dispersion of the drug can increase absorption, even when using much lower dosages—this advantage alone can make a drug with low bioavailability and poor penetration/absorption when used alone become suitably efficient for drug-delivery systems, with real prospects of reaching the market. Various groups reported that drug molecules conjugated to MNPs can exhibit efficiencies many times higher than using the drug by itself (
Figure 1).
Figure 1. Advantages of using NP-based carrier platforms compared to classical drug administration protocols; improvements are recorded across all presented segments.
Conventional drug administration is typically carried out via oral, parental, pulmonary or transdermal routes, and usually requires several doses for maximum efficiency. However, a controlled release system based on drug-loaded NPs can bypass these shortcomings, by reducing drug quantities and related toxicity, and also the doses required for treatment. In a typical, conventional drug administration, the drug concentration in biological fluids can vary greatly, from subtherapeutic (no effect) to toxic levels; by contrast, the release profile of a drug in a controlled release system is rather constant, and within the limits required for maximal therapeutic effect. Such systems can be regarded as highly beneficial for patients using anti-inflammatory drugs (especially the elders), or diabetic patients; the former will not experience pain due to constant drug concentration in the blood, while the latter will have better glycemic control because insulin would be released on-demand contingent with blood sugar levels. The release can be prolonged to multiple weeks from a single administration, which would be unheard of in the case of traditional administration.
Another key parameter is the enhanced permeability and retention achievable due to specific hypervascularization at the tumor level (with the formation of epithelial pores), which translates into increased permeability to therapeutic drugs when conjugated to polymeric-coated NPs. For efficient drug accumulation, however, the nanoparticle-based drug formulation must be stable in biological fluids (it should not agglomerate/ precipitate), and should be tailored regarding size and concentration for optimal penetration through cellular membrane and cellular uptake.
2.1. Synthetic Strategies and Feedback-Driven Design
Synthetic methods currently utilized for SPION particle synthesis involve the hydrothermal route (with typically mesoporous NPs as the outcome
[1][2][3][4][5][6][7][8][9][1,2,3,4,5,6,7,8,9]), as opposed to the classical co-precipitation route, which has a number of disadvantages including reproducibility issues regarding morphological parameters with direct influence over the magnetic properties
[10][11][12][10,11,12] or the well-established sol–gel
[13] and (auto)combustion
[14] methods. Other synthetic routes focus on natural extracts as bio-inspired routes to MNPs based on iron oxides, with encouraging therapeutic potential
[15] and other green synthesis strategies
[16]. Other nature-inspired compounds such as magnetic zeolites are also under investigation today
[17].
2.2. Physical Characterization
Physical characterization methods were employed for particle size determination, including analysis of magnetization curves
[18], cation distribution in ferrites by X-ray absorption
[19][20][19,20] and Mossbauer spectroscopy
[21][22][21,22].
Magnetic measurements aim at providing a feedback-driven synthesis route for improving effective magnetic moment
[23][24][25][23,24,25], especially when referring to ferrofluids since these target actual biologic systems
[26][27][28][26,27,28]. Effective quantification of the heating ability of MNPs can be achieved via SAR determination experiments
[29] and novel tools such as small-angle scattering can improve the design of functionalized MNPs
[30].
2.3. MNPs with Improved Magnetic Properties: Substitution/Doping Effect
Doping strategies have modified the therapeutic potential by altering the paramagnetic behavior of maghemite (γ-Fe
2O
3)
[31], metal ferrites
[32], as well as Gd
3+ substitution of magnetite Fe
3O
4 with effect on superparamagnetic NPs
[33].
Various strategies for anisotropy enhancement were explored, including layering of MNPs on amorphous substrates with perpendicular anisotropy
[34].
Ferrites of spinel structure have been synthesized; BaFe
2O
4 [35], Mn–Zn ferrite
[36], CuFe
2O
4 [37][38][37,38], Cu–Ni ferrite
[39], Zn, Cu and Co ferrites
[40][41][40,41], or Ni–Zn–Co ferrites with Gd
3+ substitution
[42].
2.4. Flow Characteristics and Simulated Models for MNPs in Biologic Fluids and High-Performance Ferrofluids
The flow parameters (magnetorheology) were reported recently
[43][44][45][46][47][48][43,44,45,46,47,48], tackling also convection
[49], location
[50] and sedimentation processes
[51][52][51,52] or multi-core MNPs
[26], proper distribution in form of appropriate matrices such as gels
[53], overall tracking efficiency
[54], as well as guidance for cell behavior
[55], and actual flow through artificial blood
[56].
The effect of viscosity of the medium as a means to counteract the sedimentation tendency of MNPs was investigated
[57], while the dispersibility of NPs was shown to be an efficient way to obtain stable ferrofluids and magnetic therapeutic fluids
[58][59][58,59].
Simulation models of MNPs in the actual blood flow were reported very recently
[60][61][62][60,61,62], on the magnetic susceptibility vs. frequency for MNPs
[63][64][63,64], suitability of poly(vinyl) alcohol PVA coating of MNPs for drug delivery
[65] or on the possible replacement of dopamine, the go-to drug used to treat Parkinson’s disease, by magnetite Fe
3O
4 NPs
[66]. Alzheimer’s is also among the diseases targeted by functionalized SPIONs when conjugated with NIR dyes
[67].
2.5. Morphology—Role of Size and Shape
Size control of MNPs
[68] was been recently achieved in a green synthesis, bioinspired strategy by employing lysine in the synthesis of SPION magnetite materials
[69]. Studies have shown that, for particle sizes higher than 100 nm, the MNPs are subject to macrophage phagocytosis by the spleen and liver, hence the requirements to design particles below 100 nm.
Size and shape effects on hyperthermia performance were also studied
[70][71][72][70,71,72]. Results point to relatively lower toxicity when spherical nanoparticles are used (such as those obtained by the polyol method), rather than irregular/polyhedral-shaped NPs. I. Craciunescu et al. have investigated hydrophobic (oleic acid coating)/hydrophilic (azelaic acid-coated) magnetite (Fe
3O
4) and ferrites (
MFe2O4, M = Mn, Zn) produced by the polyol method, of different shapes: spherical, cubic, hexagonal, octahedral and sizes (10–100 nm) with interesting findings linking shape and size of MNPs to the hyperthermia procedure. MFH (magnetic fluid hyperthermia) is a technique currently investigated which allows MNP-mediated conversion of alternating magnetic field energy into heat; moreover, this heat release event can be doubled by drug release at the tumor site which enhances the therapeutic chances of success in tumor treatment and should respect a maximal exposure criterion f × H ≤ 5 × 109 Hz × A/m for applicability in biological systems [70]. The SAR (specific absorption rate) is a key parameter quantifying the energy conversion process and is dependent on AC magnetic field amplitude, frequency and MNPs relaxation mechanisms. The magnetization at saturation increases to 90 emu/g in the case of cubic shapes and MNPs of 100 nm average size (Fe3O4 and MnFe2O4) and lower values (50–70 emu/g) for zinc ferrite nanoparticles [70]. Large-sized MNPs are expected to transfer heat in hyperthermia applications by means of hysteretic losses due to magnetic wall displacements, hence of prime interest parameters are magnetization at saturation MS and magnetic susceptibility in low AC magnetic fields—information that can be deduced by analysis of hysteresis loops [70]. Additional information regarding NPs size and size distribution, interparticle interactions and magnetic domain structure can be deduced by analysis of FC–ZFC curves (field-cooled–zero-field-cooled) or Mössbauer spectroscopy (for anisotropy energy determination, KV: K is the magnetic anisotropy constant, and V is the nanoparticle magnetic volume). It is worth noting that among the three types of samples analyzed (Fe3O4, MnFe2O4 and ZnFe2O4), the highest heating efficiency was that of the soft magnetic ZnFe2O4@azelaic acid (AZA) with a SAR of 175 W/g, more than double that of Fe3O4. AZA (SAR = 85 W/g), although it has the lowest saturation field among them. Increasing the heat efficiency is possible when using MNPs in a magnetically frozen regime at room temperature, which will not allow for the formation of moving magnetic domain walls [70].
Manganese ferrite MnFe
2O
4 was investigated extensively due to tunable magnetic properties, high biocompatibility and chemical stability
[70][71][70,71]. Besides the essential role of hysteresis losses mentioned above (where it was the dominant heat transfer mechanism), two other mechanisms describe the heat transferred by NPs to the surroundings (SPL, specific power losses): Neel and Brownian relaxation
[71]. SPL is also strongly influenced by particle size because this parameter alters the shape anisotropy. Chitosan-coated MnFe
2O
4 were obtained by co-precipitation of FeCl
3 and MnCl
2.4H
2O with NH
4OH, producing stable, functionalized MNPs with potential applications as positive/negative MRI contrast agents in the rat model
[71]. A theoretical investigation of various sized Fe
3O
4 NPs (25, 50, 100 and 200 nm) showed that NPs with lower sizes produced a higher heat gradient in the tumor mesh (61, 49, 42 and 41, respectively), while those in the 50–100 nm size ranges were found to be the most promising candidates for hyperthermia and cellular uptake
[72]. Considering the heat produced by hysteresis per volume unit P = μ
0 f ∫H dM, one can expect better results when the alternating magnetic field frequency f is increased, within the biologically safe limit
[72][73][72,73]. Theoretical simulations for correlating size with potential hyperthermia applications were also reported
[73].
2.6. Intra- and Interparticle Interactions: Colloidal Stability and Size Differentiation
Interparticle interaction has long been seen as a possible cause of further aggregation
[74]. R. Das et al. have shown the effect of shape (NRs nanorings of 55 nm length vs. NTs nanotubes of 470 nm length) on the MFH performance corresponding to Fe
3O
4 nanoparticles, while also raising the question of inter- and intraparticle interaction
[74]. The morphology of NPs was controlled by the amount of NaH
2PO
4.2H
2O used in the first precipitation step (higher concentration leads to NRs, lower concentration favors NTs). The iron oxide nanotubes NTs showed higher effective anisotropy and M
S, but lower SAR value (80 W/g at 400 Oe and 300 Hz) than nanorings NRs featuring weaker intraparticle interactions (110 W/g). It becomes apparent that MFH is positively influenced by using MNPs of lower volume and weaker intraparticle interactions
[74].
Colloidal stability is a key parameter to preventing undesired NP accumulation before ever reaching the target organ
[75][76][77][78][79][80][75,76,77,78,79,80]. For instance, optical tracking of iron oxide ferrofluids at 10 T and a 100 T/m gradient has shown that aqueous ferrofluids are best investigated in high fields, which offer a reliable estimation of their behavior under lower, practical fields
[75]: 0.25 vol% iron oxide as stabilized dispersion of citrate-coated maghemite nanoparticles (γ-Fe
2O
3), and commercial Fe
3O
4 ferrofluids
[75]. Depending on the magnetic field strength (0.3–0.5 T and ~20 T/m for a neodymium magnet, 10 T and 100 T/m for a Bitter magnet), citrate-coated maghemite remains separately dispersed. However, when MNPs of higher polydispersity are used, the largest NPs separate rapidly from the solution while smaller NPs remain dispersed because of their low dipolar coupling energies
[75].
V. Pilati et al. synthesized aqueous ferrofluids using the electric double-layer (EDL) strategy to maintain their solution stability
[76]. These systems were based on biomagnetic core-shell ZnMn mixed ferrite@ maghemite shell out of which two specific compositions were further investigated, namely Zn
δMn
1 − δFe
2O
4@γ-Fe
2O
3 (δ = 0.2 and 0.5). The surface was further covered by a maghemite layer by exposing the Zn
δMn
1 − δFe
2O
4 core (co-precipitation) to HNO
3 washing followed by hydrothermal treatment with Fe(NO
3)
3 0.5 M. The electrostatically stabilized ferrofluid was achieved by peptization of as-synthesized ferrite NPs in a dialysis bag, using HNO
3 with pH fine tuning (final pH = 2.0) and solution ionic strength adjustment by means of NaNO
3 formation
[76]. Interestingly, dynamic light scattering (DLS) and small-angle X-ray scattering (SAXS) revealed that changing the NPs concentration from dilute to >25 mg/mL is accompanied by a change in global interaction forces from attractive (diluted) to repulsive (concentrated)
[76]. L. L. e Castro et al. extended the applicability of EDL repulsive interactions by using Monte Carlo simulations to surfacted MNPs, where the charge is located typically at the extremities of the surfactant molecule (at the organic functionality, such as amino, carboxyl, etc.). They ran the simulations on spherically shaped magnetic NPs using a model proposed by Schnitzer and Morozov—an improvement over the traditional DLVO model traditionally used for modeling colloid stability
[77].
J.C. Riedl et al. used maghemite (γ-Fe
2O
3) NPs dispersed in ionic liquids (ILs) based on ethylmethylimidazolium bistriflimide (EMIM TFSI) in a pursuit to obtain colloids stable from room temperature up to 200 °C; the dispersion of maghemite at concentrations up to 12 vol% was shown to be stable for several days at 200 °C
[78]. M. Boskovic et al. synthesized Fe
3 − xGd
xO
4 (
x = 0, 0.1, 0.2) NPs of diameter ~8 nm by the coprecipitation method and by coating with citric acid (CA) with improved colloidal stability; the sample Fe
2.
80Gd
0.
20O
4@CA embedded in human serum albumin afforded magnetic microspheres (MMS) as suitable carriers for drug-delivery applications
[79]. Polymeric coatings of iron oxide nanoparticles such as silica-coated Fe
3O
4 NPs (diblock copolymers obtained by living cationic polymerization, PEO-b-PMAA) are oftentimes used because they lower Gibbs free energy of magnetic nanoparticles in solution, hence maintaining colloidal stability and preventing agglomeration
[80].