Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Carlos Amir Carmona and Version 2 by Lindsay Dong.
Approximately 75% of breast cancer (BC) is associated with luminal differentiation expressing endocrine receptors (ER). For ER+ human epidermal growth factor receptor 2 (HER2)− tumors, adjuvant endocrine therapy (ET) is the cornerstone treatment. Although relapse events steadily continue, the ET benefits translate to dramatically lengthen life expectancy with bearable side-effects. This review of ER+ HER2− female BC outlines suitable adjuvant treatment strategies to help guide clinical decision making around appropriate therapy.
- HER2-negative
- endocrine therapy
- breast cancer
- Adjuvant treatment
1. Background
Among females, breast cancer (BC) is the fifth leading cause of cancer-related death worldwide, contributing to almost 12% of all cancer cases [1]. Approximately 75% of BC is associated with luminal differentiation expressing endocrine receptors (ER) [2]. Harboring ER expression is a predictive factor for endocrine therapy (ET) response and has a promising survival outcome with a dramatic risk reduction in local and distant metastases [3][4][3,4]. In contrast, this group typically demonstrates an insufficient chemotherapy response [5]. To ascertain for which patients the magnitude of the adjuvant chemotherapy effect will not be suitable, genomic expression assays help to predict the risk of cancer recurrence and identify those for which ET alone is advantageous [6][7][8][9][6,7,8,9]. ET is distinctly efficacious among the luminal tumors. Regardless of the following factors that affect the ET response, including the level of ER positivity and tumor-infiltrating lymphocyte, cancer morphology, or germline mutation carriage, they are frequently treated as a singular entity [10][11][12][10,11,12]. Interestingly, even within the highly ER positive group, BRCA2 carriers are predictive of poor ET effectiveness [12][13][12,13]. Similarly, a diverse response to ET is seen between the pure ductal and lobular carcinomas versus mixed or hybrid histology [10][14][10,14].
Approximately one out of six women with ER+ and human epidermal growth factor receptor 2 (HER2) negative, with a malignant affected lymph node (LN), will have disease relapse reflecting the high association between LN status and the rates of BC recurrence and mortality [4][15][4,15]. This is compounded by the importance of adequate treatment adherence, as compliance is highly correlated with better outcomes [3].
There are four BC subtypes: ER+ HER2−, ER+ HER2+, ER− HER2+, and triple negative breast cancer, characterized by ER− HER2− [16]. This review of ER+ HER2− female [16]BC will outline suitable treatment strategies in the adjuvant or postoperative setting to help guide clinical decision making around appropriate therapy.
2. Endocrine Status and Adjuvant Endocrine Therapy
Immunohistochemistry (IHC) is an essential assay to determine the expression of endocrine subtype profiling [17][29]. For treatment-making decisions, the challenge lies around determining the ER expression cut off at which patients will benefit from ET. In ER-low positive tumors (1–10% of IHC+) which comprise up to 3% of BC patients, ET is not advantageous [3][17][18][3,29,30]. This is attributed to the heterogeneity of the tumor pathogenesis being more similar to the basal-like, rather than the luminal phenotype [17][29]. With respect to the progesterone receptor (PR) status, for tumors that are ER+, the PR is not predictive of ET efficacy [3]. The role of adjuvant ET is to eradicate potential undetected micrometastatic ER-enriched tumor cells. Evaluating factors such as patient preference, menopausal status, and medical history, as well as pathological tumor features, are decisive to guiding treating physicians towards the breadth of ET selection for each individual case [3]. Determining the risk category helps determine the treatment duration [4].3. Menopausal Status
Premenopausal women contribute to approximately one third of all BC cases [19][20][28,31]. In this population, the main ovarian hormone secreted is 17β-estradiol [2][21][2,32]. In the microenvironment of the breast epithelium and mammary gland, endogenous hormone signaling is mediated by estrogen and progesterone receptors. Through DNA transcription factors, the physiological sex steroidal activity can stimulate stem cells to an eventual development of endocrine enhanced tumors [2][22][2,33]. Within the SERM class, Tamoxifen was a pioneer for ET in BC, and data around its use extends over four decades [23][34]. Numerous other SERMs have been studied, such as Raloxifene, Toremifene, and Endoxifen, but to date, the benefit of Tamoxifen remains unsurpassed within this class of medications [23][24][34,35]. By competitive mechanisms of binding to ERs, Tamoxifen can drive contrasting endogenous activity depending on the targeted cell. Its inhibitory effect on estrogen-regulated pathways leads to suppression of mammary tumor angiogenesis. In addition, as an estrogen agonist, Tamoxifen has a cardioprotective effect, but conversely has an increased risk of venous thromboembolism as well as hyperplasia or tumorigenesis in the endometrium [25][26][36,37]. Regardless of the menopausal status, Tamoxifen is a suitable adjuvant therapy, and continues as the main ET option for premenopausal women with ER+ BC (Figure 1) [3][27][28][3,38,39]. Five years of Tamoxifen therapy can reduce the risk of recurrence by approximately 40% and decrease mortality by a third when compared with no ET, with a carryover benefit extending beyond ten years [3].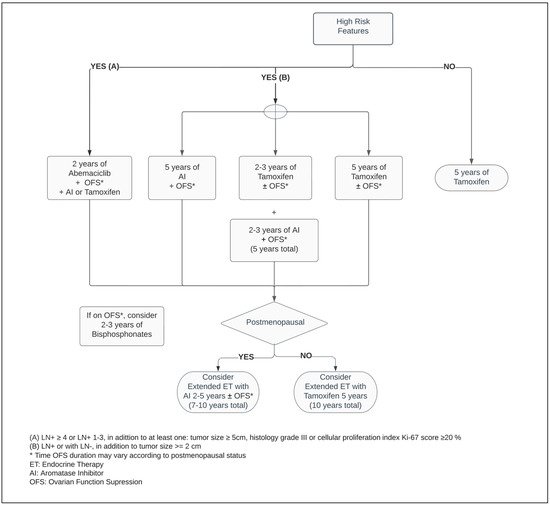
Figure 1.
Adjuvant endocrine therapy for premenopausal women with ER+ HER2− breast cancer.
In postmenopausal women, the main source of estrogen comes from extragonadal tissues and is mediated by aromatase, a crucial enzyme responsible for a cascade of steroid synthesis and regulation. The AIs substantially reduce the circulating estrogen within plasma levels by suppressing its conversion from androgens, predominantly in adipose tissues. Hence, it leads to vasomotor symptoms such as hot flashes and vaginal dryness, as well as arthralgia, lipid metabolism dysregulation and bone mineral loss [26][29][37,40].
Five years of adjuvant treatment with AI in postmenopausal women has a similar efficacy and safety profile among Anastrozole, Letrozole and Exemestane [30][31][32][41,42,43]. When compared with Tamoxifen, the AIs have shown to be superior in postmenopausal patients, reducing the risk of mortality by approximately 15% and distant and local recurrence by 14% and 26%, respectively, at ten years (Figure 2) [33][44].
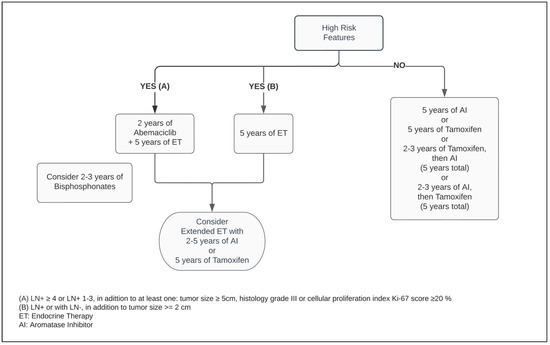

Figure 2.
Adjuvant endocrine therapy for postmenopausal women with ER+ HER2− breast cancer.
4. Ovarian Function Suppressors (OFS)
Definitive and effective transitory methods can be employed to decrease the production of sex hormones to postmenopausal range values. The first consists of a bilateral oophorectomy or directed radiation to the ovaries [34][35][48,49]. The second is through a transient drug effect induced by OFS such as the luteinizing hormone (LH)-releasing hormone (LHRH) analogs [36][50]. As an initial effect of chemical castration, the serum estradiol and progesterone levels are increased. Its regular administration promotes downstream inhibitory cascades in the hypothalamic–pituitary axis to the gonadotropic hormones, decreasing the secretion of the follicle stimulating hormone (FSH) and LH, hence suppressing the gonadal estrogen levels [36][37][38][39][21,22,50,51].
A high-certainty evidence-based systematic review which included studies such as SOFT and TEXT, comprised more than eleven thousand premenopausal patients. Thereby, it demonstrated that regardless of the ET of choice for premenopausal BC, the addition of OFS agents, administered monthly to adjuvant ET, reduced the risk of mortality by 14%, as well as disease-free survival (DFS) and contralateral BC by 17% and 25%, respectively, when compared with ET alone. While the adjunct administration of OFS between one and three years resulted in a mortality reduction, its prolonged use for over three years enhanced the DFS endpoint. However, there is insufficient randomized data evidence around OFS in the extended adjuvant setting beyond five years. In patients who did not receive chemotherapy, combining OFS to ET did not improve survival or decrease recurrence rates [40][52]. Considering previous exposure to chemotherapy as an acceptable surrogate from which an overall risk assessment demonstrates a higher risk for cancer recurrence, this suggests that only a select group of patients may benefit from OFS in the adjuvant setting. This inference is reinforced by the pathologic feature of LN involvement being a predictive factor for a superior efficacy of the ET with OFS, significantly improving OS and DFS outcomes (Figure 1) [40][41][42][52,53,54].
