Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Conner Chen.
Brain synapses are neuronal structures of the greatest interest. In the brain, the distribution of flat/intended and that of spinal post-synapses are not random. Coverage by flat/intended predominates in dendritic fibers of inhibitory neurons.
- post-synapse
- dendrite
- dendritic fiber
1. Introduction
Brain synapses, main structures of neuronal interactions, operate by two subsequent processes. The first, activated by stimulation of pre-synaptic axon terminals, includes the synthesis and release of specific neurotransmitters; the second, triggered at post-synaptic structures of dendritic fibers, includes the activation of specific neurotransmitter receptors followed by their comprehensive transduction. Ensuing intracellular processes may then participate by widely integrated post-synaptic signaling [1].
Even if integration of pre-synapses and post-synapses was known, interest about them remained indifferent. For several decades knowledge of the first did grow, reaching very high levels, while knowledge of the second remained limited [1]. After 1990, however, interest in post-synapses began to increase, reaching a high level about 10 years ago [1][2][3]. It is therefore time to reconsider the state of post-synaptic processes, focusing especially on recent developments of relevant properties, including their heterogeneity and complexity.
Upon receiving their pre-synaptic messages, post-synaptic structures undergo processing, first by activation of factors associated to their post-synaptic densities (PSDs), including molecular machines directly connected to specific signaling frameworks [4]. In other words, post-synaptic PSDs operate as neuronal antennae, receiving and then converting signaling inputs, finally to adjacent dendritic fibers [4][5][6][7]. The complexity of such signaling does not depend only on the nature of the received pre-synaptic messages. It is also due to the complexity and functionality of the two types of post-synaptic structures, one located at flat or indented areas of dendritic fibers, the other at the spines, small expansions connected to dendritic fibers by their neck [8][9]. Additional types of post-synapses, located not at dendritic fibers but at neuronal bodies [1], will not be considered in the following content.
In the brain, the distribution of flat/intended and that of spinal post-synapses are not random. Coverage by flat/intended predominates in dendritic fibers of inhibitory neurons; coverage by spines (discovered by Santiago Ramon y Cajal at the end of the 19th century) accounts for almost all dendritic fibers from excitatory neurons [8]. Functionally the two types of post-synapses may appear largely analogous. However, in specific effects such as plasticity, relevant for processes including differentiation/de-differentiation, learning and memory, the flat-to-spine prominence is considerable [9][10][11]. Additional properties, such as structures, molecular composition, mechanisms, dynamics and time-dependence of their activity, are more relevant or specific of the spines [3][8][10][11][12].
2. Flat/Intended/Aspine Post-Synapses
This section deals with the synapses established pre-synaptically by axonal terminals integrated with flat post-synapses inserted directly in the dendritic fibers. As already mentioned, these fibers, typically devoid of spines (Figure 1A), largely predominate in inhibitory neurons. Compared to axons, dendritic fibers differ in many respects [11][12]; they are shorter, and their cross sections are thicker and less uniform. Their frequent branching occurs at semi-regular intervals, with typical arbors, starting in the proximity of the cell body and distributed to the whole space. The intense branching of dendrites appears largely controlled by various forms of GTPase also involved in spine generation [2][12]. The flat/indented/aspine post-synapses include small fractions of organelles such as endoplasmic reticulum (ER), Golgi complex and endocytic system, less abundant than those of neuronal bodies. These organelles account for local functions including protein synthesis and transport, generated within or in their proximity [12][13][14][15]. Relevant to this organization and function are the cytoskeletons of both actin and microtubules [16][17]. In addition, post-synaptic areas are important for other functions, including regulation of ionic homeostasis [15].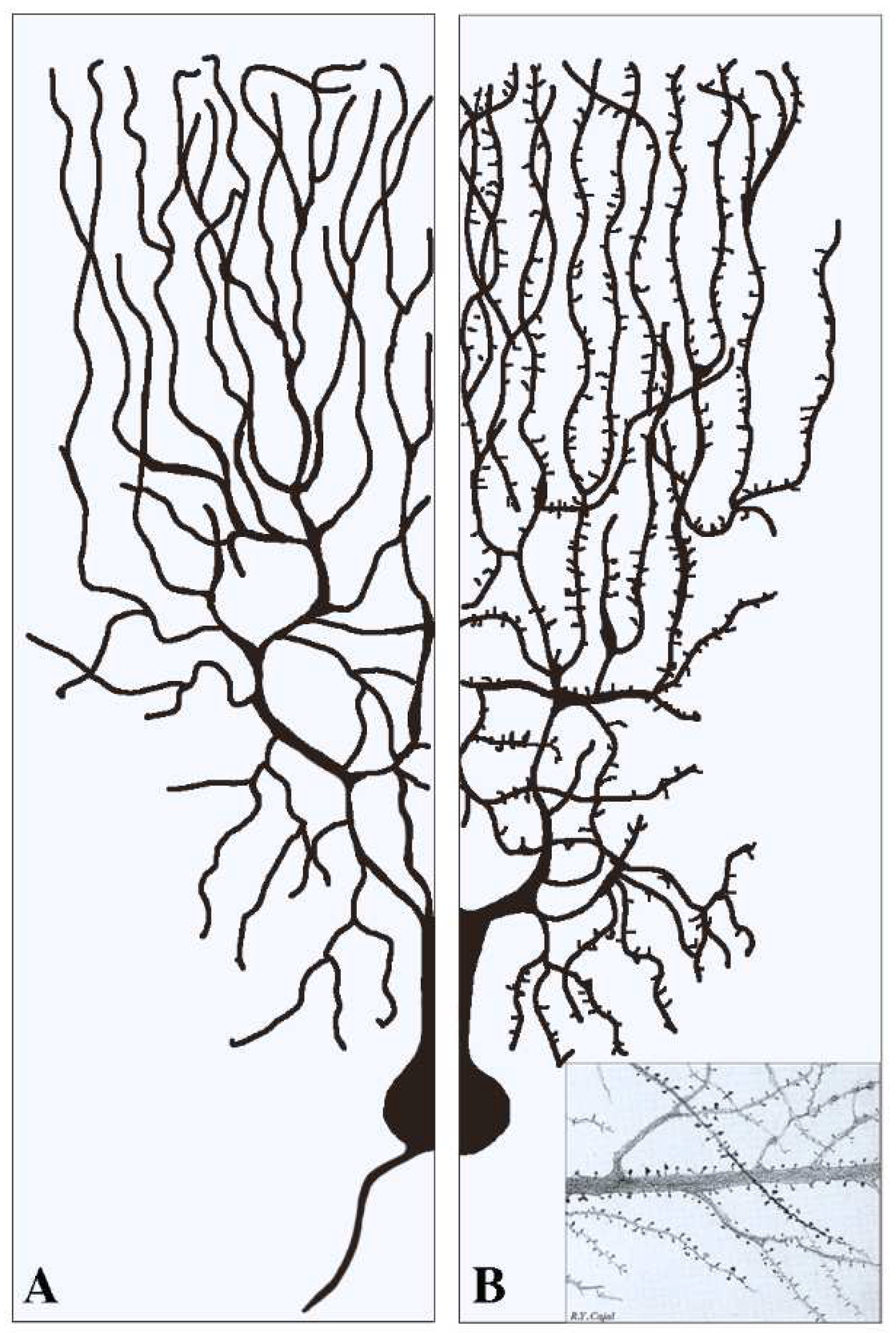
Figure 1. Examples of two dendritic arborizations with flat/intended (A) and spinal (B) post-synapses in brain neurons. In neurons the distribution of the dendritic fibers from the cell body is shown at a side opposite to that of axons (see (A)). Pre-synapses associated with dendritic post-synapses are not shown. In (A) all dendritic fibers appear smooth because their post-synapses, predominant in inhibitory neurons, are flat/intended, i.e., they do not emerge or emerge only marginally from the fiber surface. Dendritic fibers shown in (B), analogous in general shape to those in (A), predominate in stimulatory neurons. Beginning at some distance from the cell body, these fibers are covered by a high density of post-synapses composed by spines. The insertion in (B) is a fraction of an original figure by Santiago Ramon y Cajal (1896), reported as the CAT 024 figure in the book Ciencia y Arte by the Instituto Cajal, Madrid, 2004.
2.1. Structural Properties of Dendritic Post-Synapses
The predominant structures of post-synapses, already mentioned in the Introduction [4][5], include the PSD machineries, composed by adhesion molecules, neurotransmitter receptors, enzymes, and associated scaffolding proteins. PSD is the first place where receptor activation/inhibition starts the signaling, leading to the post-synaptic function. For at least some of their effects, post-synaptic PSDs are compartmentalized into nanodomains, clustered for specialized proteins upon appropriate signaling [11][15][18]. Moreover, the dendritic synapses are regulated by cytoskeletons. Actin fibers, in addition to their distribution and function in dendritic shafts and branches, are spread within the dendritic synapses, with penetration up to the PSDs [17]. Intracellular binding of actin fibers contributes to the nature and structure of scaffolding proteins, and thus to the extracellular tethering of neurotransmitters to their specific receptors, a process of great relevance in the regulation of synaptic gating [17][19]. Both positive and negative controls of these effects are mediated by serum response factors (SRFs), abundant in the synaptic areas together with its MKL/MRTF cofactor [20]. Microtubules, operative from their specific cytoskeleton, are important for both the composition and orientation of dendritic fibers and synapses. At variance with the distal pointing of the plus end, typical of axons, the microtubules of dendrites are of mixed distribution [1][16].2.2. Functions of Dendritic Post-Synapses
In the flat/intended/aspine dendritic post-synapses, the role of actin and microtubule cytoskeletons [16][17] depends on their interaction with receptors. Two types of ionotropic glutamatatergic receptors are included in the post-synaptic PSDs: NMDA, largely stable, and AMPA, widely distributed also in the extra-synaptic plasma membrane. In functional terms, AMPA receptors are involved in the trafficking of endocytic organelles taking place especially upon cell activation [21]. Various actin-associated proteins, such as cofilin, are involved in the establishment of protein interactions, including actin polymerization and NMDA regulation. The function of actin is not limited to excitatory receptors. In fact, actin filaments and their associated proteins operate also regulating inhibitory synapses, by acting on GABA and other receptors [19]. However, the activity of other inhibitory receptors, such as glycine and gephyrin receptors, does not depend on actin. Their direct regulation by microtubules induces their peculiar distribution on PSDs [16][19]. A critical function of post-synapses, largely dependent on dendrites, is plasticity, the capacity of cells to undergo changes including differentiation and de-differentiation. Such functions are related to main critical processes, such as learning and memory. Learning is a process involved in neural network transformations, made up by the synaptic connections of participating neurons; memory, established by reorganization of dendrite dynamics, consists in the maintenance of transformed networks. Both of these processes require structural and functional modifications of post-synapses [22][23]. In other post-synapses, however, the regulation of activity-dependent plasticity is different. In some excitatory synapses such activity depends on the brain-derived neurotrophic factor (BDNF), a neurotrophin critical also for the regulation of long-term potentiation (LTP) [24]. For the opposite process, long-term depression (LTD), recent results in mice have demonstrated the critical role of autophagy in dendritic synapses activated by glutamate receptors. In these experiments, inhibition of autophagy abolishes LTD and triggers neuronal LTP in the hippocampus [25]. Learning and memory of many complex functions of the brain are essential for animal survival [23]. Evidence of the last years has demonstrated the role of RNAs for the plasticity and many other functions. Non-coding, activity-dependent RNAs (ncRNAs), abundant in dendrites, are highly enriched at post-synapses where they appear critical for the plasticity of responses, including learning and memory [26]. Also critical for learning and memory of dendritic post-synapses are various microRNAs (miRs) involved in the regulated expression of numerous mRNAs involved in the translational synthesis of proteins [27]. The role of plasticity has remained unclear for decades. The advent of high-resolution time-lapse imaging, employed in conjunction with fluorescent biosensors and actuators, has enabled researchers to monitor and manipulate the structure and function of synapses, both in vitro and in vivo. The study of molecular signals associated with neuronal activities, including the activation of early genes, have led to the identification of the roles of RNAs in neuronal populations involved in memory coding [26][27].2.3. Local Depolarizations and Action Potentials
The potentials produced at single dendritic post-synapses, no matter what their distance from the cell body, are too small to generate action potentials, which require summation of multiple synaptic potentials generated within short time intervals [1][2][28]. At first approximation, however, a dendritic branch resembles a leaky cable. In these conditions, therefore, synaptic potentials are expected to become smaller and smaller due to current leakage at multiple sites of branch organization. During the last decade the investigation of post-synapses has been strengthened, on the one hand, by probing the dendritic role of Ca2+ imaging [29]; on the other hand, by clarifying the involvement of various types of voltage-gated channels [29][30]. In dendrites the variable distance of synapses from the cell body is compensated by their size, increasing with distance from the cell body and by the ion gradients established along the dendrites [28][31].References
- Geshorn, M.D.; Schwartz, H.D.; Kandel, E.R. Synapses in the central nervous system have diverse morphologies. In Principles of Neural Sciences, 6th ed.; Kandel, E.R., Loester, J.D., Mack, S.H., Siegelbaum, A., Eds.; McGraw-Hill Education: New York, NY, USA, 2021; p. 140.
- Jan, Y.N.; Jan, L.Y. Dendrites. Genes Dev. 2001, 15, 2627–2641.
- Kulkarni, V.A.; Firestein, B.L. The dendritic tree and brain disorders. Mol. Cell. Neurosci. 2012, 50, 10–20.
- Kennedy, M.B. Signal processing mechanisms at the postsynaptic density. Science 2000, 290, 750–754.
- Hausser, M.; Spruston, N.; Stuart, G.J. Diversity and dynamics of dendritic signaling. Science 2000, 290, 739–744.
- Schaefer, A.T.; Larkum, M.E.; Sakmann, B.; Roth, A. Coincidence detection in pyramidal neurons is tuned by their dendritic branching pattern. J. Neurophysiol. 2003, 89, 3143–3154.
- Dall’Oglio, A.; Gehlen, G.; Achaval, M.; Rasia-Filho, A.A. Dendritic branching features of postero-dorsal medial amygdala neurons male and female rats. Neurosci. Lett. 2008, 430, 151–156.
- Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Hediyal, T.A.; Raj, P.; Rathiptiya, A.G.; Ooronfleh, M.W.; Essa, M.M.; Chidambaram, S.B. Impact of pharmacological and non-pharmacological modulators on dendritic spines structure and functions in brain. Cells 2021, 10, 3405.
- Poirazi, P.; Mel, B.W. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron 2001, 29, 779–796.
- Holthoff, K.; Kovalchuk, Y.; Konnerth, A. Dendritic spikes and activity-dependent synaptic plasticity. Cell Tissue Res. 2006, 326, 369–377.
- Pulikkottil, V.V.; Somashekar, B.P.; Bhalla, U.S. Computation, wiring, and plasticity in synaptic clusters. Curr. Opin. Neurobiol. 2021, 70, 101–112.
- Parekh, R.; Ascoli, G.A. Quantitative investigations of axonal and dendritic arbors: Development, structure, function, and pathology. Neuroscientist 2015, 21, 241–254.
- Kulik, Y.D.; Watson, D.J.; Cao, G.; Kuwajima, M.; Harris, K.M. Structural plasticity of dendritic secretory compartments during LTP-induced synaptogenesis. Shaft SER remained more abundant in spiny than aspiny dendritic regions. eLife 2019, 8, e46356.
- Conde, C.; Cáceres, A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009, 10, 319–332.
- Chazeau, A.; Giannone, C. Organization and dynamics of the actin cytoskeleton during dendritic spine morphological remodeling. Cell. Mol. Life Sci. 2016, 73, 3053–3073.
- Weiler, S.; Guggiana Nilo, D.; Bonhoeffer, T.; Hubener, M.; Rose, T.; Scheuss, V. Orientation and direction tuning align with dendritic morphology and special connectivity in mouse visual cortex. Curr. Biol. 2022, 32, 1743–1753.e7.
- Steward, O.; Schumn, E.M. Protein synthesis of synaptic sites on dendrites. Annu. Rev. Neurosci. 2001, 24, 299–325.
- Valles, A.S.; Barrantes, F.J. Nanoscale sub-compartmentalisation of dendritic spine compartments. Biomolecules 2021, 11, 1697.
- Gentile, J.E.; Carrizales, M.G.; Koleske, A.J. Control of synapse structure and function by actin and its regulators. Cells 2022, 11, 603.
- Tabuchi, A.; Ihara, D. Regulation of dendritic synaptic morphology and transcription by the SRF cofactor MKL/MRTF. Front. Mol. Neurosci. 2021, 14, 76.
- Hanley, J.G. Actin-dependent mechanisms in AMPA receptor trafficking. Front. Cell. Neurosci. 2014, 8, 381.
- Rolotti, S.V.; Blockus, H.; Sparks, F.T.; Prestley, J.B.; Losonczy, A. Reorganization of CA1 dendritic dynamics by hippocampal sharp-wave ripples during learning. Neuron 2022, 110, 977–991.
- Ma, S.; Zuo, Y. Synaptic modifications in learning and memory. A dendritic spine story. Semin. Cell Dev. Biol. 2022, 125, 84–90.
- Leal, G.; Afonso, P.M.; Salazar, I.L.; Duarte, G.B. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015, 1621, 82–101.
- Kallergi, E.; Daskalaki, A.D.; Kolaxi, A.; Camus, C.; Ioannou, E.; Mercaldo, V.; Haberkant, P.; Stein, F.; Sidiropoulou, K.; Dalezios, Y.; et al. Dendritic autophagy degrades postsynaptic proteins and is required for long-term synaptic depression in mice. Nat. Commun. 2022, 13, 680.
- Smalheiser, N.P. The RNA-centered view of the synapse: Non-coding RNAs and synaptic plasticity. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130504.
- Bicker, S.; Lackinger, M.; Weiss, K.; Schratt, G. MicroRNA-132, -134 and -138: A microRNA troika roles in neuronal dendrites. Cell. Mol. Life Sci. 2014, 71, 3987–4005.
- Grienberger, C.; Chen, X.; Konnert, A. Dendritic function in vivo. Trends Neurosci. 2015, 38, 45–54.
- Siegel, F.; Lohmann, C. Probing synaptic function in dendrites with Ca2+ imaging. Exp. Neurol. 2013, 242, 27–32.
- Murphy, J.G.; Gutzmann, J.J.; Lin, L.; Hu, J.; Petralia, R.S.; Wang, Y.X.; Hoffman, D.A. R-type voltage-gated Ca2+ channels mediate A-type K+ current regulation of synaptic input in hippocampal dendrites. Cell Rep. 2022, 38, 110264.
- Hausser, M. Synaptic function: Dendritic democracy. Curr. Biol. 2001, 11, R10–R12.
More
