Single-wall carbon nanotubes (SWCNTs) have a high aspect ratio, large surface area, good stability and unique metallic or semiconducting electrical conductivity, they are therefore considered a promising candidate for the fabrication of flexible gas sensors that are expected to be used in the Internet of Things and various portable and wearable electronics.
- single-wall carbon nanotubes
- gas sensor
- SWCNT
1. Introduction
2. Working Mechanism of SWCNT-Based Gas Sensors
2.1. SWCNT Structure
The construction of an SWCNT can be conceptualized by rolling a perfect graphene sheet into a cylinder along the chiral vector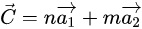 [9] [9]. Three types of SWCNT can be formed according to roll-up vectors (n,m). The (n,0) structure is called “zigzag” and the structure where n = m (n,n) is called “armchair”. The third, where n > m > 0, is called “chiral”. The chirality determines the electrical, mechanical, optical, and other properties of SWCNTs. For example, an SWCNT can be either semiconducting (s-) or metallic (m-) depending on its chirality. Metallic and semi-metallic SWCNTs have roll-up vectors such that n − m=3q (where q is an integer) and semiconducting CNTs have n − m=3q ± 1 . The distinction between semiconducting and metallic SWCNTs is important in the operation of nanotube-based field effect transistor (NTFET) devices [10]. The strong covalent carbon-carbon bonds make SWCNT a superb structural material with an ultrahigh stiffness (up to 1 TPa) and tensile strength (experimentally approaching 80 GPa [11]) in the direction of the tube axis. Meanwhile, the sp2 hybridization gives it fascinating electrical properties that depend on the diameter and helicity [12]. This combination leads to extraordinary mobility [13] and excellent quantum ballistic transport [14]. In addition, the large surface-to-volume ratio of SWCNTs, and their porous structure formed by interconnected tubes (or tube bundles) means that the carbon atoms are exposed to the environment and can be functionalized with abundant [15] and effective binding sites for gas molecules [16]. One of the most attractive features of SWCNT-based gas sensors is their ability to form flexible sensors for various gases [17,18,19][17][18][19], as well as working at room temperature with a low power consumption [20].
[9] [9]. Three types of SWCNT can be formed according to roll-up vectors (n,m). The (n,0) structure is called “zigzag” and the structure where n = m (n,n) is called “armchair”. The third, where n > m > 0, is called “chiral”. The chirality determines the electrical, mechanical, optical, and other properties of SWCNTs. For example, an SWCNT can be either semiconducting (s-) or metallic (m-) depending on its chirality. Metallic and semi-metallic SWCNTs have roll-up vectors such that n − m=3q (where q is an integer) and semiconducting CNTs have n − m=3q ± 1 . The distinction between semiconducting and metallic SWCNTs is important in the operation of nanotube-based field effect transistor (NTFET) devices [10]. The strong covalent carbon-carbon bonds make SWCNT a superb structural material with an ultrahigh stiffness (up to 1 TPa) and tensile strength (experimentally approaching 80 GPa [11]) in the direction of the tube axis. Meanwhile, the sp2 hybridization gives it fascinating electrical properties that depend on the diameter and helicity [12]. This combination leads to extraordinary mobility [13] and excellent quantum ballistic transport [14]. In addition, the large surface-to-volume ratio of SWCNTs, and their porous structure formed by interconnected tubes (or tube bundles) means that the carbon atoms are exposed to the environment and can be functionalized with abundant [15] and effective binding sites for gas molecules [16]. One of the most attractive features of SWCNT-based gas sensors is their ability to form flexible sensors for various gases [17,18,19][17][18][19], as well as working at room temperature with a low power consumption [20].
2.2. Sensing Principle
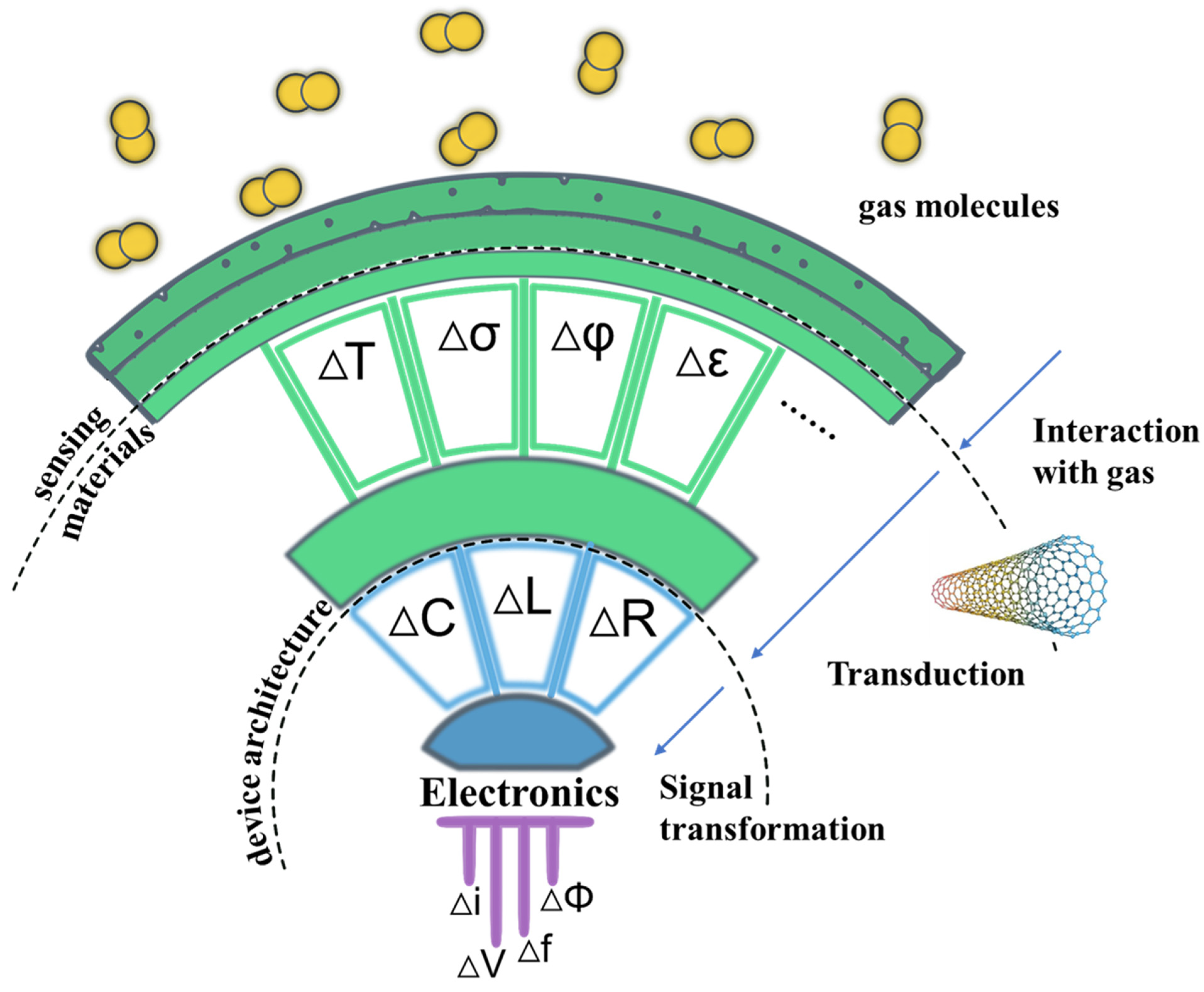
According to the International Union of Pure and Applied Chemistry, a chemical sensor is defined as a device that transforms chemical information, ranging from the concentration of a specific sample component to total composition analysis, into an analytically useful signal [21]. Such devices are logically made up of two main components: the sensing material (or receptor) and the transducer (Figure 21 in Molecules-10.3390/molecules27175381). Pure SWCNTs alone act as both the sensing material and the transducer, directly recognizing gases or vapors such as NO2, NH3, benzene and benzene derivatives with high affinity and transducing them into measurable signals. In addition, SWCNTs are a superb medium for functionalization that can detect insensitive gases towards pristine CNTs. SWCNT-based gas sensors can be classified according to the type of signal they produce, either electrical, optical [22[22][23][24],23,24], capacitive [25] and acoustic [26]. Among these, sensors that produce electrical signals are preferred due to their simplicity, portability, compatibility with standard electronics, and ability to be continuously monitored [27].2.3. Important Figure of Merit of Gas Sensors
Figure 1. Logical structure of a gas sensor. Adapted with permission from American Chemical Society https://doi.org/10.1021/acs.chemrev.6b00361 (accessed on 4 August 2022) [28]. Analytes interact with the sensing material (CNTs or functional active sites on CNTs) changing some of its physical properties (e.g., temperature, ΔT; conductivity, Δσ; work function, ΔΦ; and permittivity, Δε). Transduction converts one of these physical quantities into a change in an electrical parameter (capacitance, inductance, and resistance are mentioned). Finally, the circuit connected to the sensor gives rise to a signal, usually a current or voltage change that can be measured.
2.3. Important Figure of Merit of Gas Sensors
An ideal gas sensor needs to have the following features: (i) high sensitivity to low gas concentrations, (ii) rapid response, (iii) reversible operating ability, (iv) good selectivity to different gases of interest, (v) low-manufacturing cost, (vi) stable operation over many cycles of use, and (vii) low power consumption during operation [29]. Sensitivity is defined as the ability to discriminate small differences in the concentration of the analyte gas, and it can be calculated by the relative changes in the signal measured by the sensors, including resistance, current, conductance, capacitance, and power gain, depending on the type of sensor. The response and recovery times are important factors when evaluating the performance of a gas sensor. The response time is defined as the time for the sensor to reach 90% of its steady state or maximum value on exposure to a given concentration of the analyte, while the recovery time is the time taken to recover 90% of its peak value [32][30]. The response time is strongly dependent on the device structure, recognition components, and analytical techniques used to generate the signal [30][31]. A fast response time is desired for the real-time and continuous detection of gases and their monitoring [33,34][32][33]. LOD is the lowest concentration of target gas which can be reliably distinguished with a specified precision and reproducibility (typically with a 99% confidence level) [27]. The LOD of a sensor can be influenced by receptor–analyte interactions, surface area, functionalization, and signal amplification [37][34], and is closely tied to high sensitivity. The higher the affinity between target gas and SWCNTs (or functionalized SWCNTs), the lower the LOD, the faster the gas sensing response, and the harder the recovery of the sensors. Drift is the slow, non-random change of signal with time while the concentration of the measured analyte remains constant. Although drift can be addressed either by in-device recalibration or algorithms during data processing and/or workup, many applications cannot sustain intensive computational solutions to sensor drift [37][34]. Drift is undesirable for practical sensors and remains a challenge to be solved for CNT-based sensors. Selectivity is the ability of a sensor to identify the target gas present in a sample containing several other interfering chemicals [21]. Although the selectivity of pristine SWCNTs is usually poor due to their robust and stable C-C covalent bonding, some CNT-based sensors have demonstrated a satisfactory selectivity with the help of functionalization [17,27,42,43][17][27][35][36]. A field-effect transistor (FET) using SWCNT(s) as the active channel is a versatile sensor platform. The simplest FET consists of two electrodes (the source and the drain), connected by a semiconductor as the channel, and a gate electrode located typically at the back of an insulating gate oxide substrate that applies a gate voltage to modulate the channel current, which provides additional means to control the current response in the channel material when it interacts with a target gas. The amplification effect of FETs makes them easy to be used as gas sensors that can detect weak signals caused by trace amounts of gases, and are expected to blaze a novel trail in the field of trace gas detection [36][37]. FET-structure SWCNT-based sensors commonly have a better sensitivity [30][31] and provide more data for sensing analysis than a resistive sensor. The most common structure is a resistive sensor, where only two electrodes are used. In the absence of the gate electrode, this structure is simpler than transistors, leading to a low cost, making it available for widespread use. The sensors show changes in conductivity when exposed to target gases but are not suitable for further investigation of working mechanisms. Sometimes, although a sensor is fabricated with the configuration of FET, it has resistive behavior [44][38] due to the metal decoration on SWCNTs.2.4. The Origin of the Sensing Response
The frontier orbitals of SWCNTs described using the band structure are better at predicting or describing the sensing mechanisms [32][30], according to solid-state physics. The responses of the SWCNT-based sensors are attributed to effects resulting from (a) contact between the tubes and the electrodes (Schottky barrier modulations); (b) the sidewall or the length of the tubes (intra-SWCNT); or (c) contact points between nanotubes (inter-SWCNT). To some extent, all these sites can be regarded as effective sites. For sensor devices consisting of a network of SWCNTs, responses at the interfaces between nanotubes may be significant to the electronic properties of the overall network, because the distance between tubes might be changed by the gas absorption [45][39]. The active sites that dominate the response may differ with the analyte, the type of SWCNT, and the device structure [46][40]. Electrical measurements on resistive sensors cannot provide sufficient information to elucidate their gas sensing behaviors, but I–V testing of SWCNT FETs can distinguish the different sensing mechanisms. When a metal contacts SWCNTs, a potential barrier arises due to the difference in their work functions, as a result of which the junction may exhibit rectifying characteristics, which is called a Schottky barrier. Under a constant bias voltage, the conductance of semiconducting SWCNTs can be changed by changing the gate voltage (VG), which modifies the Schottky barrier and therefore the probability of a hole (h+) traveling from the metal contact into the CNT valence band [10].3. Approaches to and Progress in Improving the Sensing Performance
3.1. Pure SWCNT-Based Sensors Produced by Tuning the Structure
A pure SWCNT-based sensor is able to detect target gases at trace concentrations, and the sensing performance varies with the source of the SWCNTs [56][41] even with same the pre-treatment. The quantity and morphology of SWCNTs play an important role in determining the gas sensing performance [32[30][42],57], with parameters such as tube density (from individual to networks), an isolated or bundled state, type and concentration of defects, and amounts of metallic or semiconducting tubes need to be taken into consideration.3.2. Functionalization of SWCNTs
As described above, much progress has been made in SWCNT-based gas sensors by controlling the network structure, defects, and conductivity type of SWCNTs. However, the inertness of sp2 carbon makes a pure SWCNT-based sensor have a low sensitivity for analytes such as H2, CH4, and CO2 [6,93][6][43]. Furthermore, it is difficult to selectively detect a target gas in a gas mixture using pure SWCNT sensors. In order to improve the sensing performance, receptors that selectively recognize, interact or react with the target gas are commonly anchored on the surface or ends of the SWCNTs. Various methods have been proposed to modify SWCNTs, which can be classified as covalent and noncovalent functionalization [94][44]. If the receptor reacts to form a covalent bond with the SWCNTs it’s called covalent functionalization. Covalent functionalization is strong and stable but lowers the intrinsic electronic properties of SWCNTs [32][30]. Therefore, the degree of functionalization must be carefully controlled to achieve an optimum result. Noncovalent functionalization mainly involves the absorption of molecules containing receptors which are attractive because they produce less perturbation to the intrinsic properties of SWCNTs. The drawback of noncovalent functionalization is that it is not stable, which limits the working conditions of the sensor.4. Conclusions
SWCNTs are one of the most promising materials for fabricating flexible gas sensors due to their unique geometries and extraordinary intrinsic properties, as well as their ability to be tailored to detect target gas molecules. SWCNT-based gas sensors have shown an excellent sensing performance including a fast response time and an extremely low LOD at room temperature. Considering the cost and efficiency, SWCNT networks comprised of isolated s-SWCNTs with an appropriate degree of functionalization could be a promising candidate sensing material. Combining the design of sensor structure and configuration with SWCNT flexibility and transparency, SWCNT-based gas sensors may have great promise for use in the IoT, wearable devices and aerospace applications.References
- Korotcenkov, G. Handbook of Gas Sensor Materials; Springer: New York, NY, USA, 2013; Volume 1, pp. 1–454.
- Duk-Dong, L.; Dae-Sik, L. Environmental gas sensors. IEEE Sens. J. 2001, 1, 214–224.
- Wang, Y.; Yeow, J.T.W. A Review of Carbon Nanotubes-Based Gas Sensors. J. Sens. 2009, 2009, 493904.
- Kohl, D. Function and applications of gas sensors. J. Phys. D Appl. Phys. 2001, 34, R125–R149.
- Yang, S.; Jiang, C.; Wei, S.-h. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4, 021304.
- Meyyappan, M. Carbon Nanotube-Based Chemical Sensors. Small 2016, 12, 2118–2129.
- Ryabtsev, S.V.; Shaposhnick, A.V.; Lukin, A.N.; Domashevskaya, E.P. Application of semiconductor gas sensors for medical diagnostics. Sens. Actuators B 1999, 59, 26–29.
- James, D.; Scott, S.M.; Ali, Z.; O’Hare, W.T. Chemical Sensors for Electronic Nose Systems. Microchim. Acta 2005, 149, 1–17.
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis—Review. J. Mater. Chem. 2011, 21, 15872–15884.
- Kauffman, D.R.; Star, A. Carbon Nanotube Gas and Vapor Sensors. Angew. Chem. Int. Ed. 2008, 47, 6550–6570.
- Bai, Y.; Zhang, R.; Ye, X.; Zhu, Z.; Xie, H.; Shen, B.; Cai, D.; Liu, B.; Zhang, C.; Jia, Z.; et al. Carbon nanotube bundles with tensile strength over 80 GPa. Nat. Nanotechnol. 2018, 13, 589–595.
- Wu, Y.; Zhao, X.; Shang, Y.; Chang, S.; Dai, L.; Cao, A. Application-Driven Carbon Nanotube Functional Materials. ACS Nano 2021, 15, 7946–7974.
- Dürkop, T.; Getty, S.A.; Cobas, E.; Fuhrer, M.S. Extraordinary Mobility in Semiconducting Carbon Nanotubes. Nano Lett. 2004, 4, 35–39.
- Javey, A.; Guo, J.; Wang, Q.; Lundstrom, M.; Dai, H. Ballistic carbon nanotube field-effect transistors. Nature 2003, 424, 654–657.
- Zhou, T.; Zhang, T. Recent Progress of Nanostructured Sensing Materials from 0D to 3D: Overview of Structure–Property-Application Relationship for Gas Sensors. Small Methods 2021, 5, 2100515.
- Mittal, M.; Kumar, A. Carbon nanotube (CNT) gas sensors for emissions from fossil fuel burning. Sens. Actuators B 2014, 203, 349–362.
- Agarwal, P.B.; Alam, B.; Sharma, D.S.; Sharma, S.; Mandal, S.; Agarwal, A. Flexible NO2 gas sensor based on single-walled carbon nanotubes on polytetrafluoroethylene substrates. Flexible Printed Electron. 2018, 3, 035001.
- Yoon, B.; Choi, S.-J.; Swager, T.M.; Walsh, G.F. Flexible Chemiresistive Cyclohexanone Sensors Based on Single-Walled Carbon Nanotube–Polymer Composites. ACS Sens. 2021, 6, 3056–3062.
- Bezdek, M.J.; Luo, S.X.L.; Liu, R.Y.; He, Q.; Swager, T.M. Trace Hydrogen Sulfide Sensing Inspired by Polyoxometalate-Mediated Aerobic Oxidation. ACS Cent. Sci. 2021, 7, 1572–1580.
- Guo, S.Y.; Hou, P.X.; Wang, H.X.; Shi, C.; Fang, H.T.; Liu, C. Transparent and flexible hydrogen sensor based on semiconducting single-wall carbon nanotube networks. Carbon 2019, 151, 156–159.
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371.
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon nanotubes as optical biomedical sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950.
- Kim, J.-H.; Heller, D.A.; Jin, H.; Barone, P.W.; Song, C.; Zhang, J.; Trudel, L.J.; Wogan, G.N.; Tannenbaum, S.R.; Strano, M.S. The rational design of nitric oxide selectivity in single-walled carbon nanotube near-infrared fluorescence sensors for biological detection. Nat. Chem. 2009, 1, 473–481.
- Hu, Y.; Ma, X.; Zhang, Y.; Che, Y.; Zhao, J. Detection of Amines with Fluorescent Nanotubes: Applications in the Assessment of Meat Spoilage. ACS Sens. 2016, 1, 22–25.
- Snow, E.S.; Perkins, F.K.; Houser, E.J.; Badescu, S.C.; Reinecke, T.L. Chemical detection with a single-walled carbon nanotube capacitor. Science 2005, 307, 1942–1945.
- Sivaramakrishnan, S.; Rajamani, R.; Smith, C.S.; McGee, K.A.; Mann, K.R.; Yamashita, N. Carbon nanotube-coated surface acoustic wave sensor for carbon dioxide sensing. Sens. Actuators B Chem. 2008, 132, 296–304.
- Dai, J.; Ogbeide, O.; Macadam, N.; Sun, Q.; Yu, W.; Li, Y.; Su, B.-L.; Hasan, T.; Huang, X.; Huang, W. Printed gas sensors. Chem. Soc. Rev. 2020, 49, 1756–1789.
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583.
- Pandhi, T.; Chandnani, A.; Subbaraman, H.; Estrada, D. A Review of Inkjet Printed Graphene and Carbon Nanotubes Based Gas Sensors. Sensors 2020, 20, 5642.
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663.
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-Transduced Chemical Sensors Based on Two-Dimensional Nanomaterials. Chem. Rev. 2019, 119, 478–598.
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171.
- Guo, L.; Yang, Z.; Li, Y.; Zu, B.; Dou, X. Sensitive, real-time and anti-interfering detection of nitro-explosive vapors realized by ZnO/rGO core/shell micro-Schottky junction. Sens. Actuators B 2017, 239, 286–294.
- Fennell, J.F.; Liu, S.F.; Azzarelli, J.M.; Weis, J.G.; Rochat, S.; Mirica, K.A.; Ravnsbæk, J.B.; Swager, T.M. Nanowire Chemical/Biological Sensors: Status and a Roadmap for the Future. Angew. Chem. Int. Ed. 2016, 55, 1266–1281.
- Savagatrup, S.; Schroeder, V.; He, X.; Lin, S.; He, M.; Yassine, O.; Salama, K.N.; Zhang, X.-X.; Swager, T.M. Bio-Inspired Carbon Monoxide Sensors with Voltage-Activated Sensitivity. Angew. Chem. Int. Ed. 2017, 56, 14066–14070.
- Bezdek, M.J.; Luo, S.-X.L.; Ku, K.H.; Swager, T.M. A chemiresistive methane sensor. Proc. Natl. Acad. Sci. USA 2021, 118, e2022515118.
- Liu, C.; Hu, J.; Wu, G.; Cao, J.; Zhang, Z.; Zhang, Y. Carbon Nanotube-Based Field-Effect Transistor-Type Sensor with a Sensing Gate for Ppb-Level Formaldehyde Detection. ACS Appl. Mater. Interfaces 2021, 13, 56309–56319.
- Xiao, M.; Liang, S.; Han, J.; Zhong, D.; Liu, J.; Zhang, Z.; Peng, L. Batch Fabrication of Ultrasensitive Carbon Nanotube Hydrogen Sensors with Sub-ppm Detection Limit. ACS Sens. 2018, 3, 749–756.
- Niu, L.; Luo, Y.; Li, Z. A highly selective chemical gas sensor based on functionalization of multi-walled carbon nanotubes with poly(ethylene glycol). Sens. Actuators B 2007, 126, 361–367.
- Boyd, A.; Dube, I.; Fedorov, G.; Paranjape, M.; Barbara, P. Gas sensing mechanism of carbon nanotubes: From single tubes to high-density networks. Carbon 2014, 69, 417–423.
- Ghaddab, B.; Sanchez, J.B.; Mavon, C.; Paillet, M.; Parret, R.; Zahab, A.A.; Bantignies, J.L.; Flaud, V.; Beche, E.; Berger, F. Detection of O3 and NH3 using hybrid tin dioxide/carbon nanotubes sensors: Influence of materials and processing on sensor’s sensitivity. Sens. Actuators B 2012, 170, 67–74.
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752.
- Umadevi, D.; Sastry, G.N. Feasibility of carbon nanomaterials as gas sensors: A computational study. Curr. Sci. 2014, 106, 1224–1234.
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732.