The practice of community water fluoridation used prophylactically against dental caries increased concern of adverse fluoride effects. Millions of people living in endemic fluorosis areas suffer from various pathological disturbances. Authors assessed the publications on fluoride toxicity until June 2020. Authors present evidence that fluoride is an enzymatic poison, inducing oxidative stress, hormonal disruptions, and neurotoxicity. Fluoride in synergy with aluminum acts as a false signal in G protein cascades of hormonal and neuronal regulations in much lower concentrations than fluoride acting alone. Our review shows the impact of fluoride on human health. We suggest focusing the research on fluoride toxicity to the underlying integrative networks. Ignorance of the pluripotent toxic effects of fluoride might contribute to unexpected epidemics in the future.
- autism spectrum disorders
- aluminofluoride complexes
- enzymes
- fluoride toxicity
- G proteins
- IQ deficits
- magnesium
- neurotoxicity
- phosphate
1. Recommended Levels
For optimal dental health, the World Health Organization recommends a level of fluoride from 0.5 to 1.0 mg/L (milligrams per liter), depending on climate.[6] Fluorosis becomes possible above this recommended dosage. As of 2015, the United States Health and Human Services Department recommends a maximum of 0.7 milligrams of fluoride per liter of water – updating and replacing the previous recommended range of 0.7 to 1.2 milligrams issued in 1962. The new recommended level is intended to reduce the occurrence of dental fluorosis while maintaining water fluoridation.[7]
2. Toxicity
Chronic
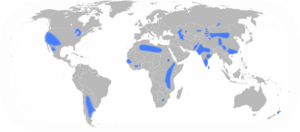
In India an estimated 60 million people have been poisoned by well water contaminated by excessive fluoride, which is dissolved from the granite rocks. The effects are particularly evident in the bone deformities of children. Similar or larger problems are anticipated in other countries including China, Uzbekistan, and Ethiopia.[5]
Acute
Historically, most cases of acute fluoride toxicity have followed accidental ingestion of sodium fluoride based insecticides or rodenticides.[9] Currently, in advanced countries, most cases of fluoride exposure are due to the ingestion of dental fluoride products.[10] Other sources include glass-etching or chrome-cleaning agents like ammonium bifluoride or hydrofluoric acid,[11][12] industrial exposure to fluxes used to promote the flow of a molten metal on a solid surface, volcanic ejecta (for example, in cattle grazing after an 1845–1846 eruption of Hekla and the 1783–1784 flood basalt eruption of Laki), and metal cleaners. Malfunction of water fluoridation equipment has happened several times, including a notable incident in Alaska.[4]
3. Occurrence
Organofluorine compounds
Twenty percent of modern pharmaceuticals contain fluorine.[13] These organofluorine compounds are not sources of fluoride poisoning. The carbon–fluorine bond is too strong to release fluoride.[14]
Fluoride in toothpaste
Children may experience gastrointestinal distress upon ingesting excessive amounts of flavored toothpaste. Between 1990 and 1994, over 628 people, mostly children, were treated after ingesting too much fluoride-containing toothpaste. "While the outcomes were generally not serious," gastrointestinal symptoms appear to be the most common problem reported.[15] However given the low concentration of fluoride present in dental products, this is potentially due to consumption of other major components.
Fluoride in drinking water
Around one-third of the world's population drinks water from groundwater resources. Of this, about 10 percent, approximately 300 million people, obtains water from groundwater resources that are heavily contaminated with arsenic or fluoride.[16] These trace elements derive mainly from leaching of minerals.[17] Maps are available of locations of potential problematic wells via the Groundwater Assessment Platform (GAP).[18]
4. Effects
Excess fluoride consumption has been studied as a factor in the following:
Brain
Some research has suggested that high levels of fluoride exposure may adversely affect neurodevelopment in children, but the evidence is of insufficient quality to allow any firm conclusions to be drawn.[19]
Bones
Whilst fluoridated water is associated with decreased levels of fractures in a population,[20] toxic levels of fluoride have been associated with a weakening of bones and an increase in hip and wrist fractures. The U.S. National Research Council concludes that fractures with fluoride levels 1–4 mg/L, suggesting a dose-response relationship, but states that there is "suggestive but inadequate for drawing firm conclusions about the risk or safety of exposures at [2 mg/L]".[21]:170 Consumption of fluoride at levels beyond those used in fluoridated water for a long period of time causes skeletal fluorosis. In some areas, particularly the Asian subcontinent, skeletal fluorosis is endemic. It is known to cause irritable-bowel symptoms and joint pain. Early stages are not clinically obvious, and may be misdiagnosed as (seronegative) rheumatoid arthritis or ankylosing spondylitis.[22]
Kidney
Fluoride induced nephrotoxicity is kidney injury due to toxic levels of serum fluoride, commonly due to release of fluoride from fluorine-containing drugs, such as methoxyflurane.[23][24][25] Within the recommended dose, no effects are expected, but chronic ingestion in excess of 12 mg/day are expected to cause adverse effects, and an intake that high is possible when fluoride levels are around 4 mg/L.[21]:281 Those with impaired kidney function are more susceptible to adverse effects.[21]:292 The kidney injury is characterised by failure to concentrate urine, leading to polyuria, and subsequent dehydration with hypernatremia and hyperosmolarity. Inorganic fluoride inhibits adenylate cyclase activity required for antidiuretic hormone effect on the distal convoluted tubule of the kidney. Fluoride also stimulates intrarenal vasodilation, leading to increased medullary blood flow, which interferes with the counter current mechanism in the kidney required for concentration of urine. Fluoride induced nephrotoxicity is dose dependent, typically requiring serum fluoride levels exceeding 50 micromoles per liter (about 1 ppm) to cause clinically significant renal dysfunction,[26] which is likely when the dose of methoxyflurane exceeds 2.5 MAC hours.[27][28] (Note: "MAC hour" is the multiple of the minimum alveolar concentration (MAC) of the anesthetic used times the number of hours the drug is administered, a measure of the dosage of inhaled anesthetics.) Elimination of fluoride depends on glomerular filtration rate. Thus, patients with chronic kidney disease will maintain serum fluoride for longer period of time, leading to increased risk of fluoride induced nephrotoxicity.
Teeth
The only generally accepted adverse effect of fluoride at levels used for water fluoridation is dental fluorosis, which can alter the appearance of children's teeth during tooth development; this is mostly mild and usually only an aesthetic concern. Compared to unfluoridated water, fluoridation to 1 mg/L is estimated to cause fluorosis in one of every 6 people (range 4–21), and to cause fluorosis of aesthetic concern in one of every 22 people (range 13.6–∞).[20]
Thyroid
Fluoride's suppressive effect on the thyroid is more severe when iodine is deficient, and fluoride is associated with lower levels of iodine.[clarification needed][29] Thyroid effects in humans were associated with fluoride levels 0.05–0.13 mg/kg/day when iodine intake was adequate and 0.01–0.03 mg/kg/day when iodine intake was inadequate.[21]:263 Its mechanisms and effects on the endocrine system remain unclear.[21]:266 Testing on mice shows that the medication gamma-Aminobutyric acid (GABA) can be used to treat fluoride toxicity of the thyroid and return normal function.[30]
Effects on aquatic organisms
Fluoride accumulates in the bone tissues of fish and in the exoskeleton of aquatic invertebrates. The mechanism of fluoride toxicity in aquatic organisms is believed to involve the action of fluoride ions as enzymatic poisons. In soft waters with low ionic content, invertebrates and fishes may develop adverse effects from fluoride concentration as low as 0.5 mg/L. Negative effects are less in hard waters and seawaters, as the bioavailability of fluoride ions is reduced with increasing water hardness[31] Seawater contains fluoride at a concentration of 1.3 mg/L.[32]
5. Mechanism
Like most soluble materials, fluoride compounds are readily absorbed by the stomach and intestines, and excreted through the urine. Urine tests have been used to ascertain rates of excretion in order to set upper limits in exposure to fluoride compounds and associated detrimental health effects.[33] Ingested fluoride initially acts locally on the intestinal mucosa, where it forms hydrofluoric acid in the stomach.
1. Introduction
More than 500 million people live in endemic fluorosis areas with an elevated level of fluoride in drinking water and biosphere with public health problems[1][2]. The fluorosis symptoms span from mild effects on teeth enamel, headaches, dizziness, loss of appetite, to severe pathological disturbances. These include dental and skeletal fluorosis, hypothyroidism, sleep disorders, inflammations, IQ deficits, and suspected autism[3][4][5][6].
In 1931, H. Trendley Dean, head of the Dental Hygiene Unit at the National Institute of Health (NIH), began investigating the epidemiology of fluorosis in the USA. Dean and his staff determined the fluoride levels in drinking water causing fluorosis. They discovered that fluoride levels of up to one milligram fluoride per liter of drinking water (one ppm) did not cause dental fluorosis in most people. Dean’s reports formed the foundation of the concept that the ingestion of fluoride will augment the teeth enamel and make it less susceptible to dental caries[7]. Dean suggested adding fluoride to water sources deficient in fluoride to bring its concentration up to the optimal value. After trials in the USA, the World Health Organization (WHO) recommended community water fluoridation (CWF), and many countries implemented this program[8][9][10]. The CWF recommendation has been followed for over 70 years in developed countries, such as the USA, Canada, Australia, New Zealand, Ireland, and some parts of the United Kingdom (UK)[9].
CWF started an era of increased income from fluoride as never before in human history. Recently, 370 million people from 27 countries have a supply of fluoridated drinking water[9]. The number of fluoride sources has further expanded since fluoride is used for the improvement of food, beverages, hygiene, and medical products including fluorinated drugs.
The fluorine atom does not exist in nature in a free and unmixed state. The mineral fluorite (CaF2) is the main source of fluorine for commercial use. Fluorine readily reacts with other elements to form fluoride compounds, within which it always adopts an oxidation state of −1. Inorganic compounds are formed with hydrogen, metals, and non-metals. Therefore, fluoride refers to the negatively charged ion of the fluorine atom. In general, soil fluoride is not available to plants. There are exceptions such as tea plants that are natural accumulators of fluoride from fertilizers[1][2][4]. Fruits such as blueberry and grape concentrate fluoride from soil and it might appear in commercial juices. Fluorine excess had been found to be detrimental also to plants. Injury to plants is common all around industries making aluminum, fertilizers, or glass. Special plants might produce the toxic organofluorine compound fluoroacetate, which is used as a defense against grazing by herbivores.
Conflicting views occur in debates addressing whether fluorine is an essential element for humans. The researchers of the Panel of the European Food Safety Authority (EFSA) state that fluoride has no known essential function in human physiology and development[11]. On the other hand, the WHO and the Centers for Disease Control and Prevention (CDC) consider fluoride to be an important dietary element for humans because of “resistance to dental caries is a physiologically important function"[12].
2. The Implication of Fluoride Toxicity on Human Health
Early reports about fluoride toxicity in humans appeared in the journal Physiological Reviews already in 1933. Aluminum smelter workers and persons living near the factory where fluoride was in high concentration in the atmosphere suffered with psychiatric and neurological disturbances. Endemic fluorosis is caused by persistent fluoride exposure through ingestion or inhalation, and most commonly, as a result of high fluoride levels in drinking water and beverages[13][14]. The WHO’s drinking water quality Guideline Value for fluoride is 1.5 mg/L [14].
Dental fluorosis results after excess fluoride ingestion, most commonly in drinking water, during tooth formation. For example, dental fluorosis appears in 43–63% of schoolchildren in endemic areas of China with total fluoride intake 2.7–19.8 mg/day[5]. The rate of dental fluorosis also increases in countries with CWF. In the 2010–2012 survey, dental fluorosis was reported among adolescents aged 12–15 years in the USA with a surprisingly high rate of 65%[15]. Dental fluorosis might be used as a sensitive indicator of excessive fluoride exposure[16].
Skeletal fluorosis impacts millions of people in regions with high natural levels of fluoride, like India, China, Pakistan, Iran, and the Gulf region, to name a few [13]. The U.S. Environmental Protection Agency (USEPA) sets a maximum contaminant level of 4.0 mg/L to protect against skeletal fluorosis. WHO indicates a clear risk of skeletal fluorosis for a total intake of 14 mg fluoride per day. Nevertheless, the recent findings revealed that consumption of fluoride at even 10 times lower concentrations of 1.5 mg/L caused its high incidence in India[17]. The chemical analyses show that 80% of water sources in rural areas exceed the WHO fluoride permissible limits and residents are affected by skeletal fluorosis.
Waldbott et al. examined about 500 people affected by chronic fluoride intake from CWF[18]. These authors observed chronic fatigue, headaches, loss of the ability to concentrate, depression, gastrointestinal symptoms, and deterioration of muscular coordination.
3. The Economic Consequences of Fluoride Toxicity
It is difficult to precisely evaluate the economic consequences of endemic fluorosis. It is now known to be global in scope, occurring on all continents. Except for China, defluorination projects were closed in most countries due to a collapse in their cost.
Conversely, CWF is recognized as one of the most cost-effective, equitable, and safe measures to prevent cavities and improve oral health. Thousands of studies showed that CWF prevents cavities and saves money, both for families and the health care system. An economic review of multiple studies used by CDC found that savings for communities ranged from $1.10 to $135 for every $1 invested. Per capita annual costs of CWF range from $0.11 to $24.38, while per capita annual benefits range from $5.49 to $93.19[19]. Fluoride has modest benefit in terms of reduction of dental caries but also significant costs concerning dental and skeletal fluorosis, hypothyroidism, and mental and cognitive disturbances. Ingestion of fluoride constitutes an unacceptable risk with virtually no proven benefit. The possibility that chronic fluoride intake might evoke chronic diseases with high socioeconomic impact must be involved. Hirzy et al. calculated that the economic impact of IQ loss among USA children is the loss of tens of billions of dollars[4]. The available information supports a reasonable conclusion that economic losses associated with ASD may be also quite large. The annual societal costs for children with ASD were estimated between $11.5–60.9 billion in the USA in 2011. They included a variety of direct and indirect costs, from medical care to special education, and lost parental productivity [20]. Children and adolescents with ASD had average medical expenditures that exceeded those without ASD by $4110–6200 per year. According to estimates of Leigh and Du, annual costs due to ASD in the USA in 2015 were around $268 billion[21]. However, if the rate of increase in the ASD prevalence continues, Cakir et al. estimated in 2020, that costs could reach nearly $15 trillion by 2029 [22].
We suggest that the reduction of fluoride from the daily life of pregnant women as well as of children in infancy could be an efficient way to prevent fluoride developmental neurotoxicity[23].
4. Conclusions
Fluoride toxicity has been demonstrated in many studies. We present evidence that fluoride interferes with enzyme activities, induces oxidative stress, and causes hormonal disturbances, and neurotoxicity. The health impacts of increased fluoride exposition of millions of people in endemic fluorosis areas are of global public concern. At present, there is a divergence between the practice of CWF, which is regarded as valuable and safe for reducing dental caries, and current scientific evidence, which indicates that fluoride is a potent neurotoxin disturbing prenatal as well as postnatal brain development. The dose recommended for dental caries reduction is close to the dose causing pathological effects. Moreover, fluoride in synergy with trace amounts of Al3+ has the potential to affect all phosphoryl transfer reactions and most regulations of fundamental biological processes in much lower concentrations than fluoride alone.
The potential neurotoxicity associated with exposure to fluoride has generated controversy about CWF. Given the large number of studies showing cognitive deficits associated with elevated fluoride exposure under different settings, the general tendency of fluoride-associated neurotoxicity seems overwhelming. We concur with the conclusions of many authors over the world that fluoride neurotoxicity is a serious risk associated with elevated fluoride exposure, whether due to CWF, natural fluoride release from soil minerals, food supplements or tea consumption, especially when the exposure occurs during early brain development.
Fluoride is not an essential nutrient. No physiological function can be defined during the development and growth, for which it is required. Fluoride toxicity is a slow, hidden process. Evolving evidence should inspire scientists and health authorities to re-evaluate claims about the safety of fluoride, especially for the fetus and infant for whom it has no benefit at all.
Of all sources of fluoride, artificially fluoridated water is the most available practical source to eliminate fluoride intake to reduce its human hazards. Our review explains that fluoride could evoke unexpected epidemics in the future.
References
- Ghosh, A.; Mukherjee, K.; Ghosh, S.K.; Saha, B. Sources and toxicity of fluoride in the environment. Res. Chem. Intermed. 2013, 39, 2881–2915. [Google Scholar] [CrossRef]
- Vithanage, M.; Bhattacharya, P. Fluoride in the environment: Sources, distribution and defluoridation. Env. Chem. Lett. 2015, 13, 131–147. [Google Scholar] [CrossRef]
- Strunecká, A.; Patocka, J.; Blaylock, R.; Chinoy, N. Fluoride interactions: From molecules to disease. Curr. Signal. Transd. Ther. 2007, 2, 190–213. [Google Scholar] [CrossRef]
- Hirzy, J.; Connett, P.; Xiang, Q.; Spittle, B.; Kennedy, D. Developmental neurotoxicity of fluoride: A quantitative risk analysis toward establishing a safe dose for children. Fluoride 2016, 49, 379–400. [Google Scholar]
- Strunecká, A.; Strunecký, O.; Guan, Z. The resemblance of fluorosis pathology to that of autism spectrum disorder: A mini-review. Fluoride 2019, 52, 105–115. [Google Scholar]
- Saeed, M.; Malik, R.N.; Kamal, A. Fluorosis and cognitive development among children (6–14 years of age) in the endemic areas of the world: A review and critical analysis. Environ. Sci. Pollut. Res. Int. 2020, 27, 2566–2579. [Google Scholar] [CrossRef]
- Dean, H.T. Post-war implications of fluorine and dental health: Epidemiological aspects. J. Public Health 1944, 34, 133–143. [Google Scholar] [CrossRef]
- Arnold, F.A., Jr.; Dean, H.T.; Jay, P.; Knutson, J.W. Effect of fluoridated public water supplies on dental caries prevalence. Public Health Rep. 1956, 71, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Whelton, H.P.; Spencer, A.J.; Do, L.G.; Rugg-Gunn, A.J. Fluoride revolution and dental caries: Evolution of policies for global use. J. Dent. Res. 2019, 98, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Fawell, J.; Bailey, K.; Chilton, J.; Dahi, E.; Fewtrell, L.; Magara, Y. Fluoride in Drinking-Water; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies. Scientific opinion on dietary reference values for fluoride, 2013. EFSA J. 2013, 11, 3332. [Google Scholar]
- CDC. Basics of Oral Health. Available online: https://www.cdc.gov/oralhealth/basics/index.html (accessed on 1 September 2020).
- Waugh, D.T.; Godfrey, M.; Limeback, H.; Potter, W. Black tea source, production, and consumption: Assessment of health risks of fluoride intake in New Zealand. J. Environ. Public Health 2017, 2017, 5120504. [Google Scholar] [CrossRef] [PubMed]
- Hattab, F. An update on fluorides and fluorosis with reference to oral health status in the Gulf Region: Review. Asian. J. Dent. Sc. 2020, 3, 27–48. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 2nd Edition: Volume 1—Recommendations; World Health Organisation: Geneva, Switzerland, 1993. [Google Scholar]
- Neurath, C.; Limeback, H.; Osmunson, B.; Connett, M.; Kanter, V.; Wells, C.R. Dental fluorosis trends in US oral health surveys: 1986 to 2012. JDR Clin. Trans. Res. 2019, 4, 298–308. [Google Scholar] [CrossRef]
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health 2019, 18, 110. [Google Scholar] [CrossRef]
- Srivastava, S.; Flora, S.J.S. Fluoride in drinking water and skeletal fluorosis: A review of the global impact. Curr. Environ. Health Rep. 2020, 7, 140–146. [Google Scholar] [CrossRef]
- Waldbott, G.L.; Burgstahler, A.W.; McKinney, H.L. Fluoridation: The great dilemma. In Fluoridation: The Great Dilemma; Coronado Press: Lawrence, KS, USA, 1978; pp. 1–152. [Google Scholar]
- CDC. Cost Savings of Community Water Fluoridation. Available online: https://www.cdc.gov/fluoridation/basics/cost.htm (accessed on 1 September 2020).
- Leigh, J.P.; Du, J. Brief report: Forecasting the economic burden of autism in 2015 and 2025 in the United States. J. Autism. Dev. Disord. 2015, 45, 4135–4139. [Google Scholar] [CrossRef]
- Cakir, J.; Frye, R.E.; Walker, S.J. The lifetime social cost of autism: 1990–2029. RASD 2020, 72, 101502. [Google Scholar] [CrossRef]
- Strunecka, A.; Blaylock, R.L.; Strunecky, O. Fluoride, aluminum, and aluminofluoride complexes in pathogenesis of the autism spectrum disorders: A possible role of immunoexcitotoxicity. J. Appl. Biomed. 2016, 14, 171–176. [Google Scholar] [CrossRef]
