Marine natural products are potent and promising sources of drugs among other natural products of plant, animal, and microbial origin. Marine drugs are classified into six categories, where the basis of classification is nonuniform but maintains the flow of context within the category. Most of the drugs are categorized on the basis of the complexity of structures such as “spongonucleosides”, “antibody-drug conjugates”, and “peptides or proteins used as drugs or used in drug preparations”, but some are categorized on the basis of their mechanism of action, such as “microtubule inhibitors” and “deoxyribonucleic acid (DNA) alkylating agents”, or their natural source of abundance, such as “fish oil and its components as drugs”.
- marine natural products
- marine drugs
- microtubule inhibitors
- drug conjugated with an antibody
- peptides or proteins
- fish oil
1. Nucleoside Analogs
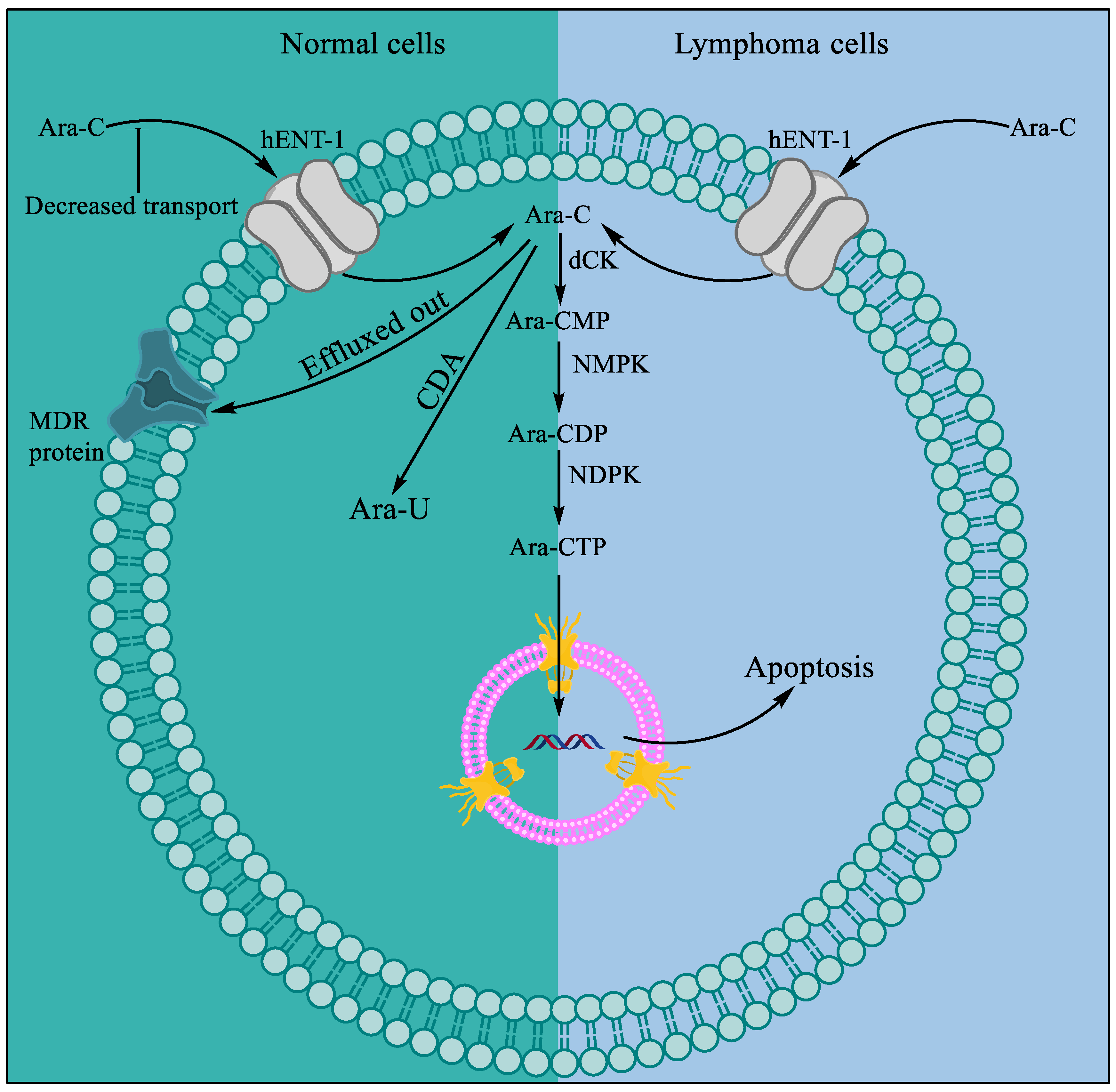
1.1. Cytarabine
1.2. Vidarabine
1.3. Fludarabine
1.4. Nelarabine
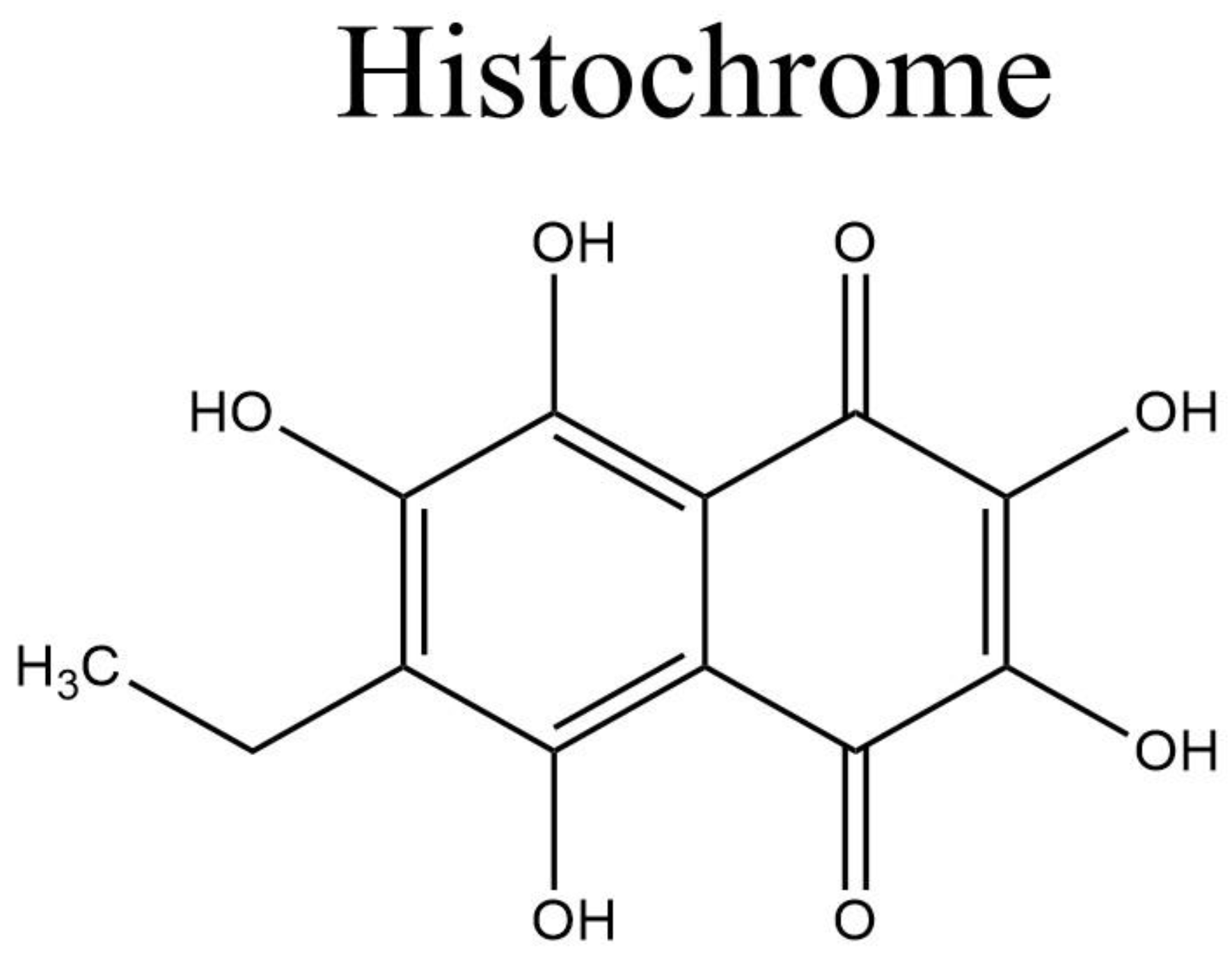
1.5. Histochrome®: Sodium Salt of Echinochrome A—A Common Sea Urchin Pigment
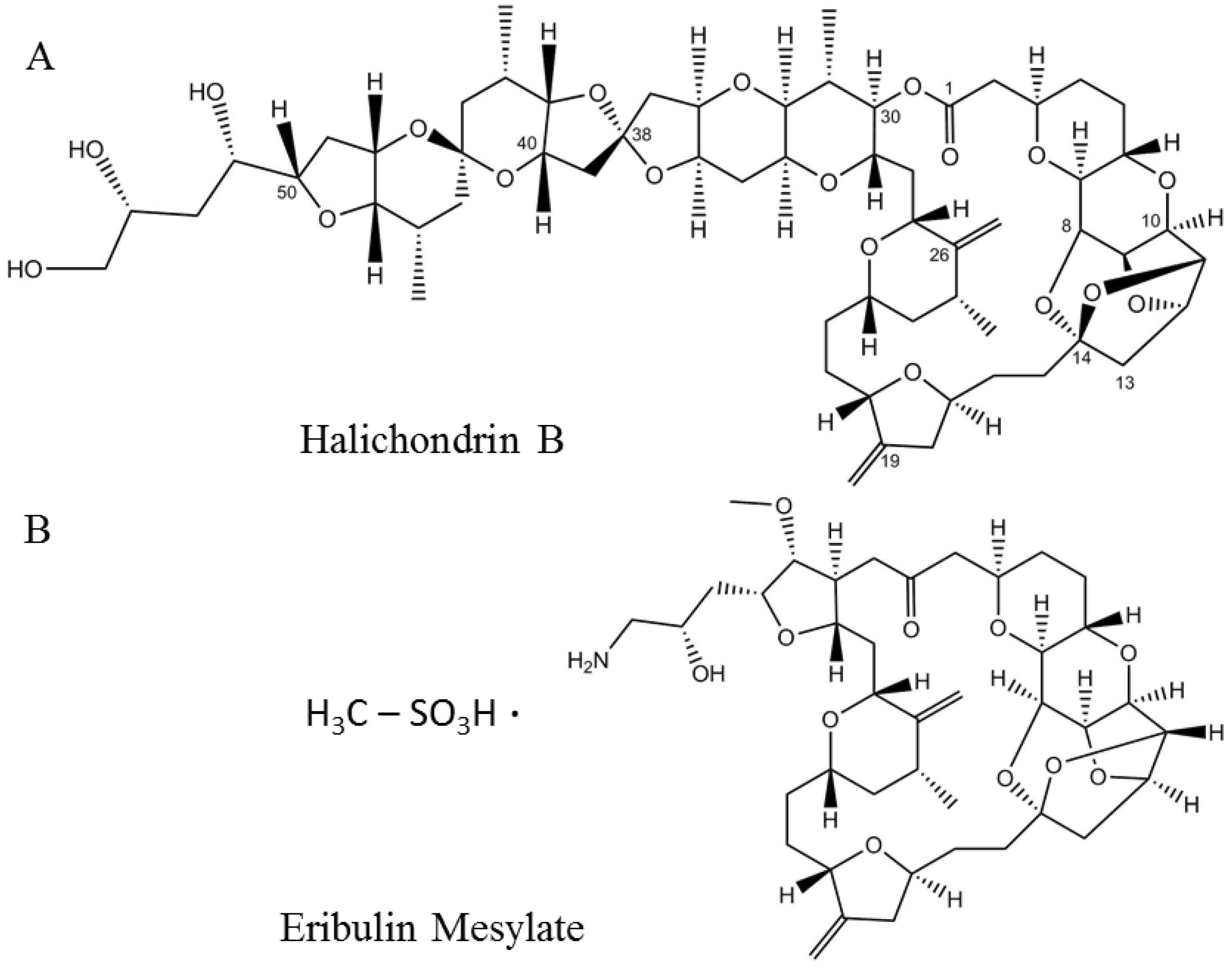
2. Microtubule Inhibitors
3. DNA Alkyating Agents
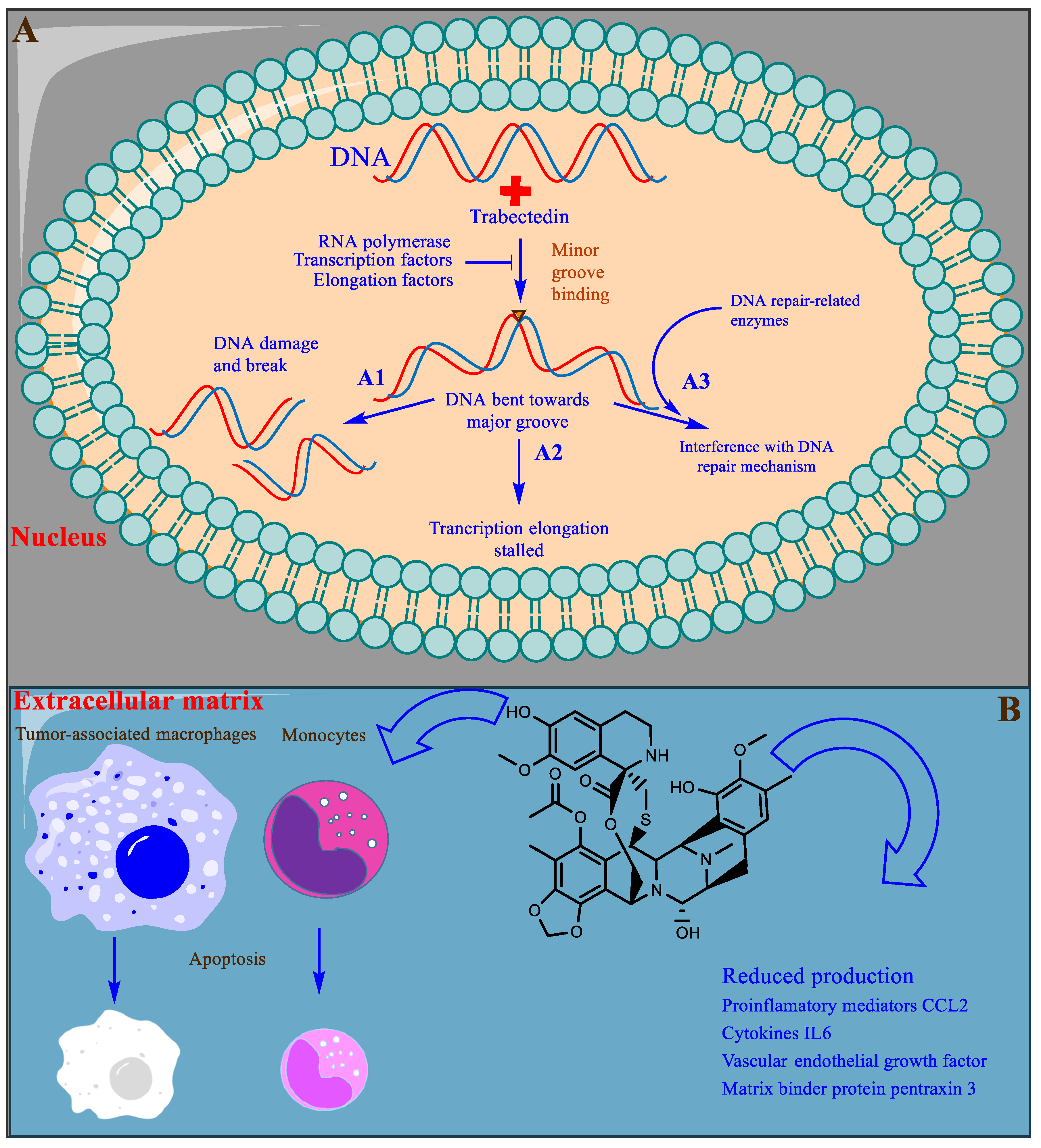
3.1. Trabectedin
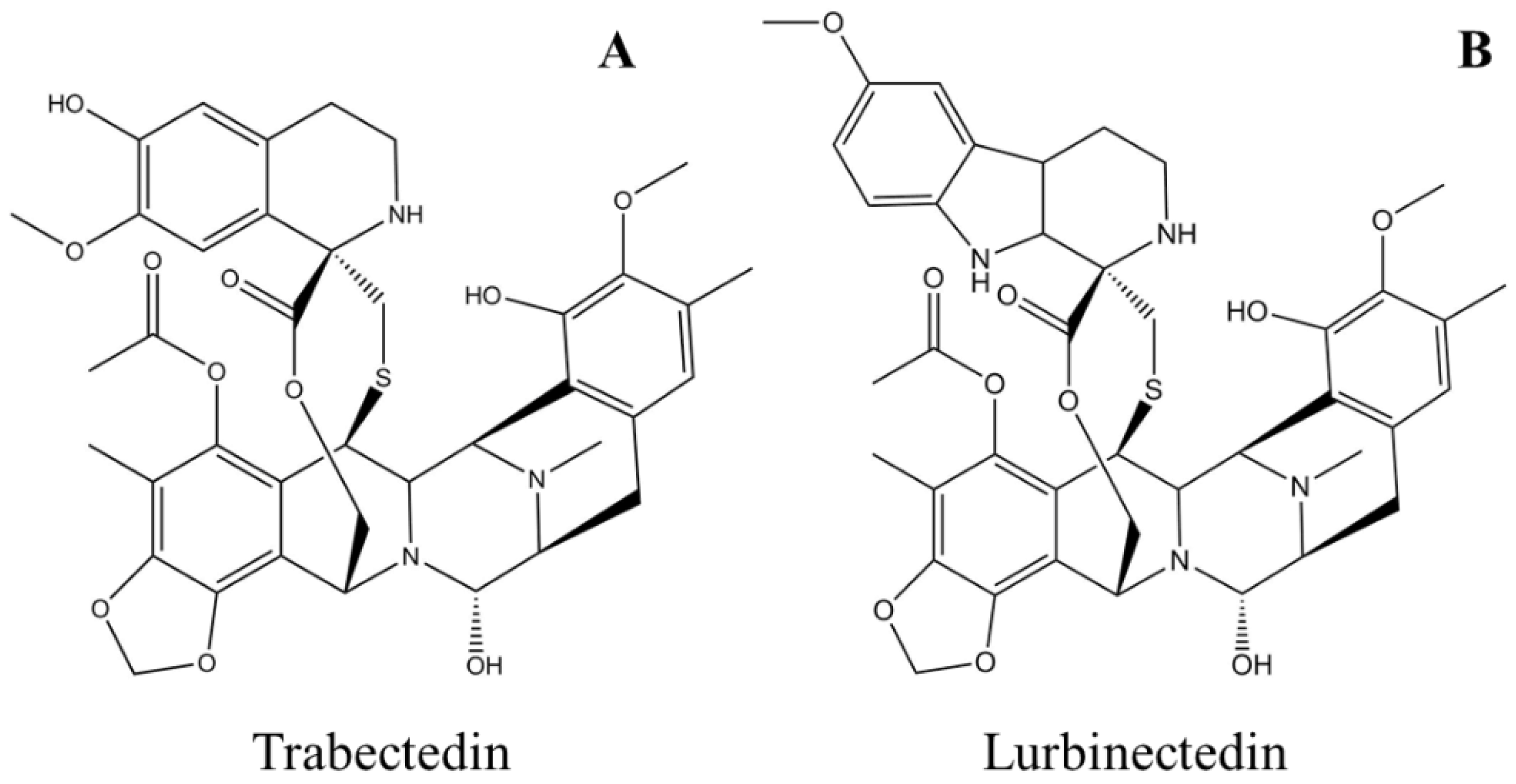
3.2. Lurbinectedin
4. Antibody-Drug Conjugates
4.1. Brentuximab Vedotin
4.2. Polatuzumab Vedotin
4.3. Enfortumab Vedotin
4.4. Belantamab Mafodotin
5. Peptides or Proteins Used as Drugs or in Drug Preparations
5.1. Plitidepsin
5.2. Ziconotide
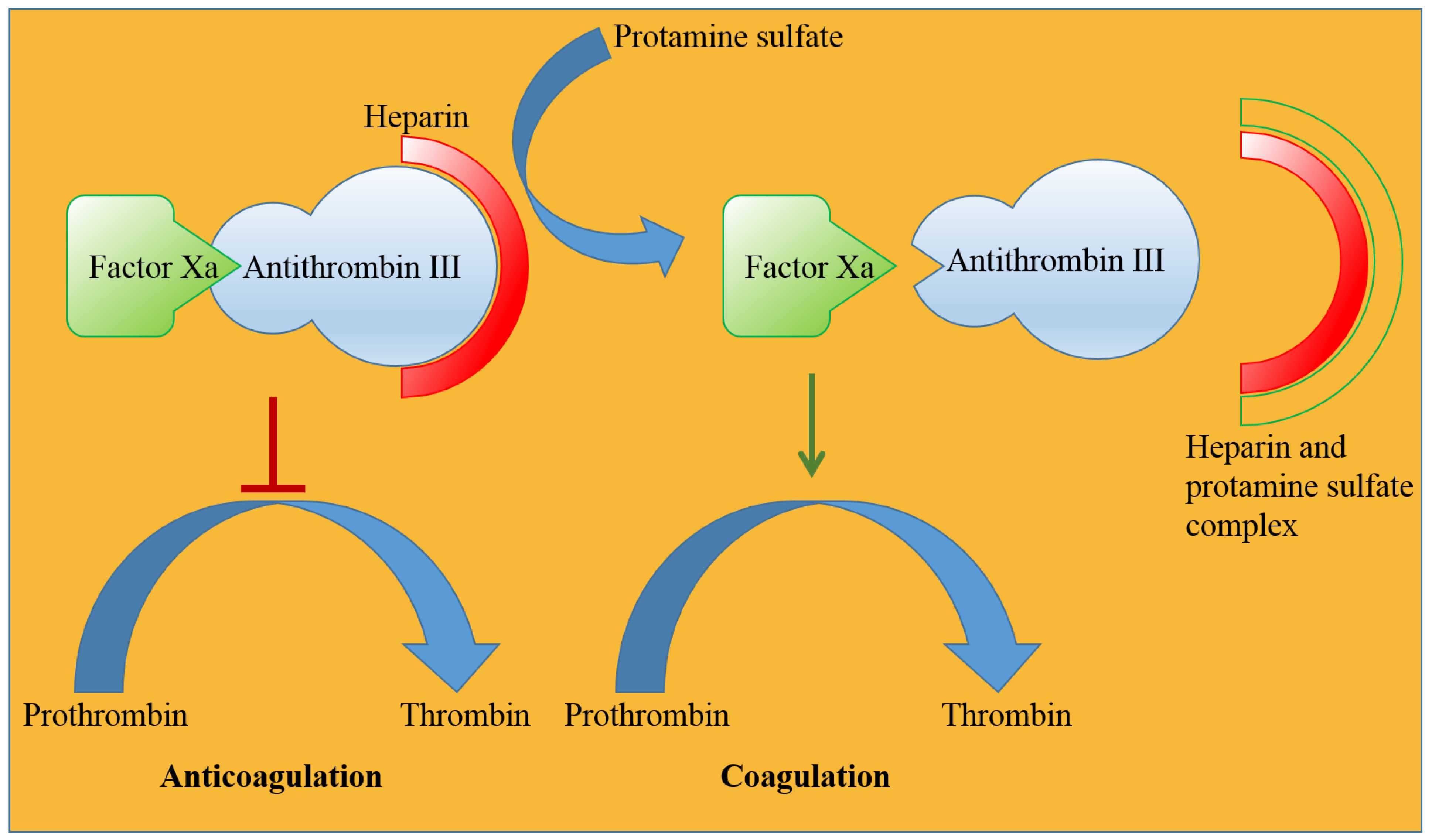
5.3. Protamine Sulfate
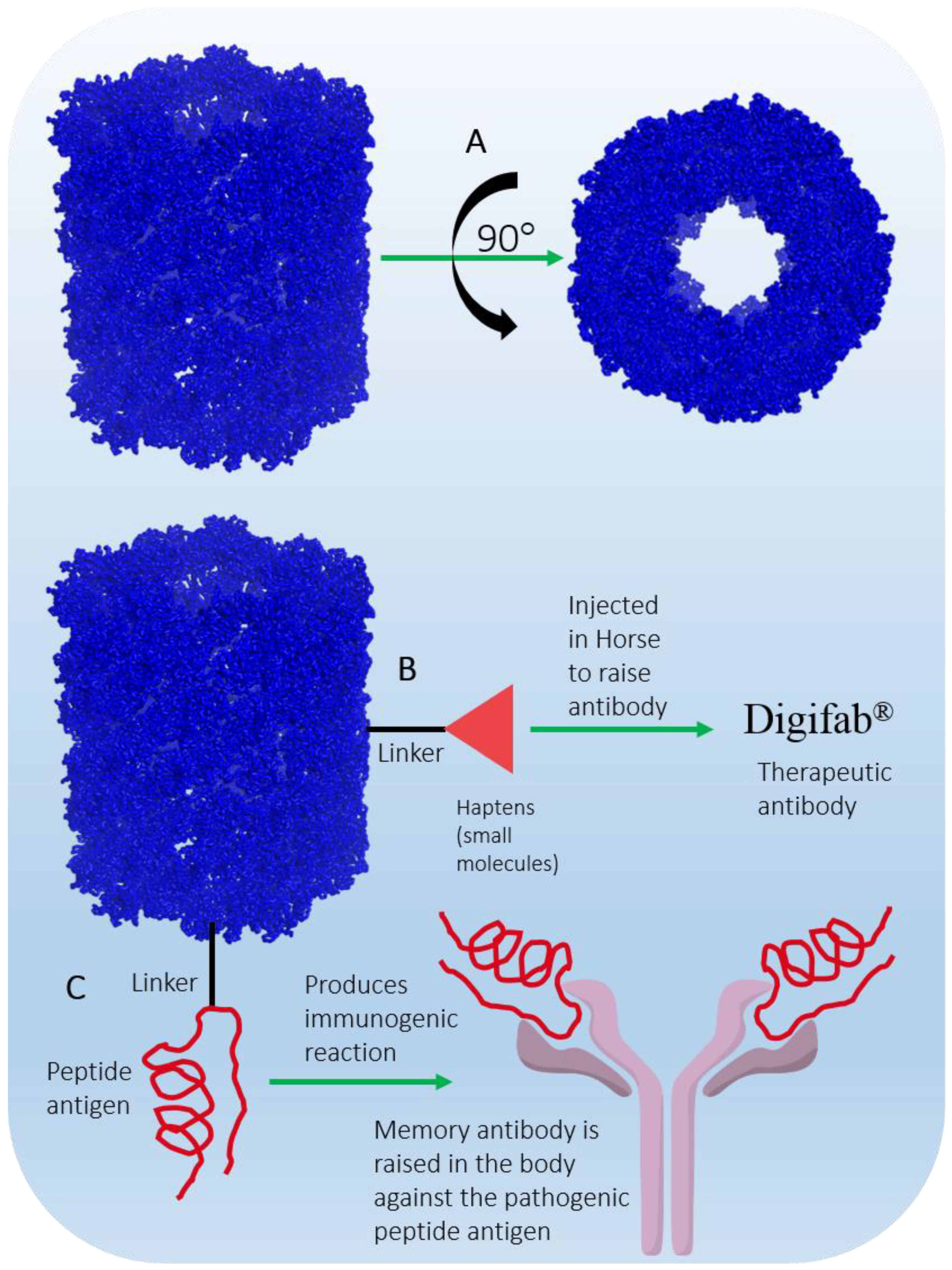
5.4. Keyhole Limpet Hemocyanin (KLH)
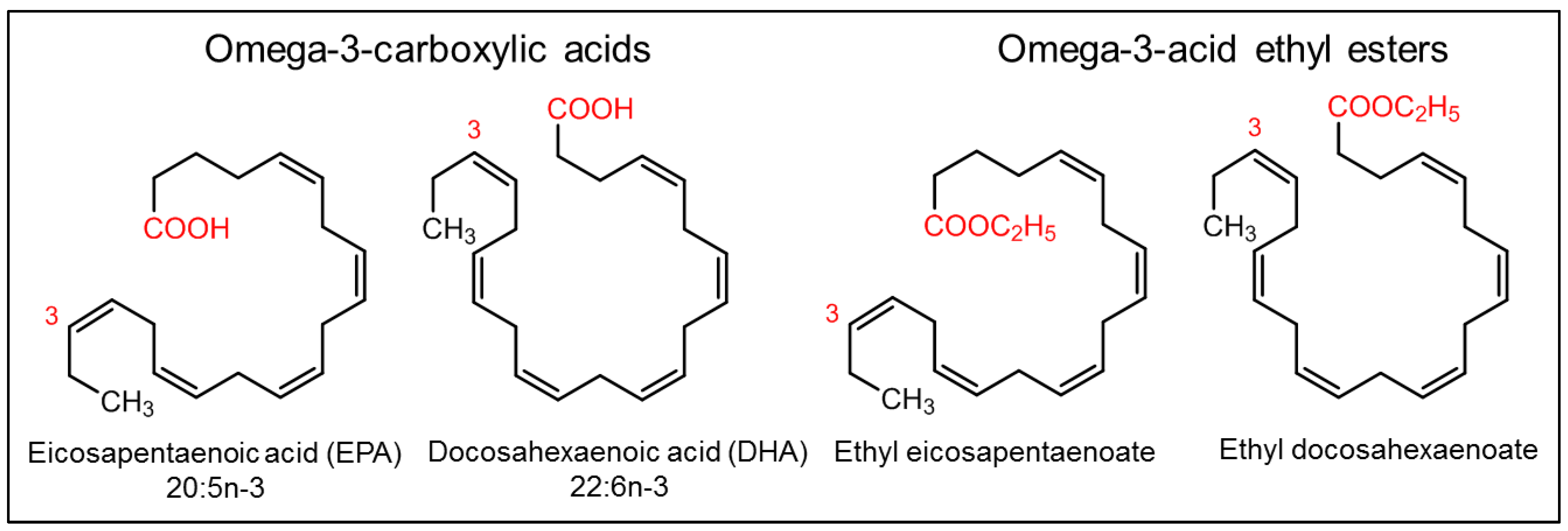
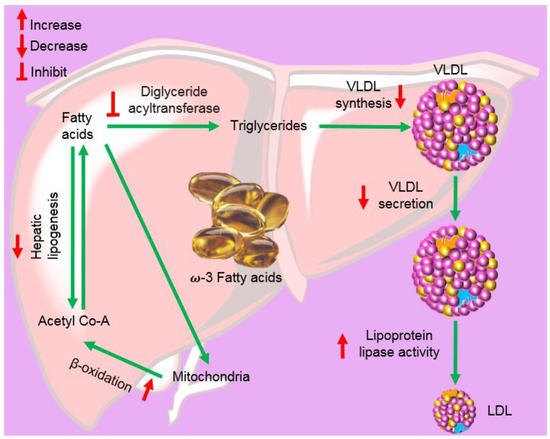
6. Fish Oil and Its Components
References
- Kremer, W.B. Drugs five years later: Cytarabine. Ann. Intern. Med. 1975, 82, 684–688.
- Drugs. Cytarabine. Available online: https://www.drugs.com/monograph/cytarabine.html (accessed on 30 June 2022).
- Heuser, M.; Ofran, Y.; Boissel, N.; Mauri, S.B.; Craddock, C.; Janssen, J.; Wierzbowska, A.; Buske, C. Acute myeloid leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 697–712.
- Chu, M.Y.; Fischer, G. A proposed mechanism of action of 1-β-d-arabinofuranosyl-cytosine as an inhibitor of the growth of leukemic cells. Biochem. Pharmacol. 1962, 11, 423–430.
- Furth, J.; Cohen, S.S. Inhibition of mammalian DNA polymerase by the 5’-triphosphate of 1-β-D-arabinofuranosylcytosine and the 5’-triphosphate of 9-β-D-arabinofuranosyladenine. Cancer Res. 1968, 28, 2061–2067.
- Graham, F.; Whitmore, G. The effect of 1-β-D-arabinofuranosylcytosine on growth, viability, and DNA synthesis of mouse L-cells. Cancer Res. 1970, 30, 2627–2635.
- Plunkett, W.; Gandhi, V. Evolution of the arabinosides and the pharmacology of fludarabine. Drugs 1994, 47, 30–38.
- Chu, M.-Y. Incorporation of arabinosyl cytosine into 2–7S ribonucleic acid and cell death. Biochem. Pharmacol. 1971, 20, 2057–2063.
- Creasey, W.A.; Papac, R.J.; Markiw, M.E.; Calabresi, P.; Welch, A.D. Biochemical and pharmacological studies with 1-β-d-arabinofuranosylcytosine in man. Biochem. Pharmacol. 1966, 15, 1417–1428.
- Benedict, W.F.; Harris, N.; Karon, M. Kinetics of 1-β-D-arabinofuranosylcytosine-induced chromosome breaks. Cancer Res. 1970, 30, 2477–2483.
- Kihlman, B.; Nichols, W.W.; Levan, A. The effect of deoxyadenosine and cytosine arabinoside on the chromosomes of human leukocytes in vitro. Hereditas 1963, 50, 139–143.
- Camiener, G.W.; Smith, C.G. Studies of the enzymatic deamination of cytosine arabinoside—I: Enzyme distribution and species specificity. Biochem. Pharmacol. 1965, 14, 1405–1416.
- Mulligan, L.; Mellett, L. Comparative Metabolism of Cytosine Arabinoside and Inhibition of Deamination by Tetrahydrouridine. Pharmacologist 1968, 10, 167.
- Glantz, M.J.; LaFollette, S.; Jaeckle, K.A.; Shapiro, W.; Swinnen, L.; Rozental, J.R.; Phuphanich, S.; Rogers, L.R.; Gutheil, J.C.; Batchelor, T. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J. Clin. Oncol. 1999, 17, 3110–3116.
- Zimm, S.; Collins, J.M.; Miser, J.; Chatterji, D.; Poplack, D.G. Cytosine arabinoside cerebrospinal fluid kinetics. Clin. Pharmacol. Ther. 1984, 35, 826–830.
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J. Clin. Oncol. 2018, 36, 2684–2692.
- Lim, W.-S.; Tardi, P.G.; Dos Santos, N.; Xie, X.; Fan, M.; Liboiron, B.D.; Huang, X.; Harasym, T.O.; Bermudes, D.; Mayer, L.D. Leukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine: Daunorubicin formulation, in bone marrow xenografts. Leuk. Res. 2010, 34, 1214–1223.
- Rein, L.A.; Rizzieri, D.A. Clinical potential of elacytarabine in patients with acute myeloid leukemia. Ther. Adv. Hematol. 2014, 5, 211–220.
- Baker, W.J.; Royer, G.L., Jr.; Weiss, R.B. Cytarabine and neurologic toxicity. J. Clin. Oncol. 1991, 9, 679–693.
- Tilly, H.; Castaigne, S.; Bordessoule, D.; Casassus, P.; Le Prise, P.; Tertian, G.; Desablens, B.; Henry-Amar, M.; Degos, L. Low-dose cytarabine versus intensive chemotherapy in the treatment of acute nonlymphocytic leukemia in the elderly. J. Clin. Oncol. 1990, 8, 272–279.
- Privat de Garilhe, M. Effect de deux nucleosides de l’arabinose sur la multiplication des virus de l’herpes et de la vaccine en culture cellulaire. CR Acad. Sci. 1964, 259, 2725–2728.
- Sidwell, R.W.; Arnett, G.; Dixon, G.J. Effect of selected biologically active compounds on in vitro cytomegalovirus (CMV) infections. In Proceedings of the 7th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 28–29 October 1967.
- Miller, F.; Dixon, G.; Ehrlich, J.; Sloan, B.J.; McLean, I. Antiviral activity of 9 beta D arabinofuranosyladenîne. I. Cell culture studies. Antimicrob. Agents Chemother. 1969, 8, 136–147.
- Lee, W.W.; Benitez, A.; Goodman, L.; Baker, B. Potential anticancer agents. 1 xl. synthesis of the β-anomer of 9-(d-arabinofuranosyl)-adenine. J. Am. Chem. Soc. 1960, 82, 2648–2649.
- Buchanan, R.A.; Hess, F. Vidarabine (Vira-A®): Pharmacology and clinical experience. Pharmacol. Ther. 1980, 8, 143–171.
- Mayer, A.M.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265.
- De Clercq, E.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747.
- Plunkett, W.; Chubb, S.; Alexander, L.; Montgomery, J.A. Comparison of the toxicity and metabolism of 9-β-D-arabinofuranosyl-2-fluoroadenine and 9-β-D-arabinofuranosyladenine in human lymphoblastoid cells. Cancer Res. 1980, 40, 2349–2355.
- Plunkett, W.; Benjamin, R.S.; Keating, M.J.; Freireich, E.J. Modulation of 9-β-D-arabinofuranosyladenine 5’-triphosphate and deoxyadenosine triphosphate in leukemic cells by 2′-deoxycoformycin during therapy with 9-β-D-arabinofuranosyladenine. Cancer Res. 1982, 42, 2092–2096.
- Plunkett, W.; Feun, L.G.; Benjamin, R.S.; Keating, M.; Freireich, E.J. “Modulation of Vidarabine Metabolism by 2’-Deoxycoformycin for Therapy of Acute Leukemia” Conference on 2′-Deoxycoformycin--Current Status and Future Directions, US Department of Health and Human Services, Public Health Service; National Institutes of Health: Washington, DC, USA, 1984; Volume 2, pp. 23–27.
- Montgomery, J.A.; Hewson, K. Synthesis of potential anticancer agents. X. 2-fluoroadenosine1. J. Am. Chem. Soc. 1957, 79, 4559.
- Rodriguez, G. Fludarabine phosphate. Investig. New Drugs 1994, 12, 75–92.
- Keating, M.; Kantarjian, H.; Talpaz, M.; Redman, J.; Koller, C.; Barlogie, B.; Velasquez, W.; Plunkett, W.; Freireich, E.J.; McCredie, K. Fludarabine: A new agent with major activity against chronic lymphocytic leukemia. Blood 1989, 74, 19–25.
- Rai, K.R.; Peterson, B.L.; Appelbaum, F.R.; Kolitz, J.; Elias, L.; Shepherd, L.; Hines, J.; Threatte, G.A.; Larson, R.A.; Cheson, B.D. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N. Engl. J. Med. 2000, 343, 1750–1757.
- McLaughlin, P.; Hagemeister, F.B.; Romaguera, J.E.; Sarris, A.H.; Pate, O.; Younes, A.; Swan, F.; Keating, M.; Cabanillas, F. Fludarabine, mitoxantrone, and dexamethasone: An effective new regimen for indolent lymphoma. J. Clin. Oncol. 1996, 14, 1262–1268.
- Thompson, P.A.; Tam, C.S.; O’Brien, S.M.; Wierda, W.G.; Stingo, F.; Plunkett, W.; Smith, S.C.; Kantarjian, H.M.; Freireich, E.J.; Keating, M.J. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood 2016, 127, 303–309.
- Carpenter, J., Jr.; Vogel, C.; Wang, G.; Raney, M. Phase II evaluation of fludarabine in patients with metastatic breast cancer: A southeastern cancer study group trial. Cancer Treat. Rep. 1986, 70, 1235–1236.
- Taylor, S.; Eyre, H. Randomized phase II trials of acivicin (AT-125, NSC 163501) and fludarabine (2-fluoro-ara-AMP, NSC 312887) (2FLAMP) in recurrent malignant gliomas: A SWOG study. Proc. Annu. Meet Am. Soc. Clin. Oncol. 1987, 6, 71.
- Taylor, S.A.; Crowley, J.; Vogel, F.S.; Townsend, J.J.; Eyre, H.J.; Jaeckle, K.A.; Hynes, H.E.; Guy, J.T. Phase II evaluation of fludarabine phosphate in patients with central nervous system tumors. Investig. New Drugs 1991, 9, 195–197.
- Weiss, G.; Metch, B.; Von Hoff, D.; Taylor, S.; Saiers, J. Phase II trial of fludarabine phosphate in patients with head and neck cancer: A southwest oncology group study. Cancer Treat. Rep. 1987, 71, 1313–1314.
- Casper, E.S.; Mittelman, A.; Kelson, D.; Young, C.W. Phase I clinical trial of fludarabine phosphate (F-ara-AMP). Cancer Chemother. Pharmacol. 1985, 15, 233–235.
- Hutton, J.J.; Von Hoff, D.D.; Kuhn, J.; Phillips, J.; Hersh, M.; Clark, G. Phase I clinical investigation of 9-β-d-arabinofuranosyl-2-fluoroadenine 5’-monophosphate (NSC 312887), a new purine antimetabolite. Cancer Res. 1984, 44, 4183–4186.
- Cheson, B.D.; Bennett, J.M.; Rai, K.R.; Grever, M.R.; Kay, N.E.; Schiffer, C.A.; Oken, M.M.; Keating, M.J.; Boldt, D.H.; Kempin, S.J.; et al. Guidelines for clinical protocols for chronic lymphocytic leukemia: Recommendations of the National Cancer Institute-sponsored working group. Am. J. Hematol. 1988, 29, 152–163.
- Hiddemann, W.; Rottmann, R.; Wörmann, B.; Thiel, A.; Essink, M.; Ottensmeier, C.; Freund, M.; Büchner, T.; van de Loo, J. Treatment of advanced chronic lymphocytic leukemia by fludarabine. Ann. Hematol. 1991, 63, 1–4.
- Hurst, P.G.; Habib, M.P.; Garewal, H.; Bluestein, M.; Paquin, M.; Greenberg, B.R. Pulmonary toxicity associated with fludarabine monophosphate. Investig. New Drugs 1987, 5, 207–210.
- Chun, H.; Leyland-Jones, B.; Caryk, S.; Hoth, D. Central nervous system toxicity of fludarabine phosphate. Cancer Treat. Rep. 1986, 70, 1225–1228.
- Warrell, R.P., Jr.; Berman, E. Phase I and II study of fludarabine phosphate in leukemia: Therapeutic efficacy with delayed central nervous system toxicity. J. Clin. Oncol. 1986, 4, 74–79.
- Jensen, K.; L’Aurelle, A.J.; Jacobson, P.A.; Kachler, S.; Kirstein, M.N.; Lamba, J.; Klotz, K.-N. Cytotoxic purine nucleoside analogues bind to A1, A2A, and A3 adenosine receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 519–525.
- Ribeiro, J.; Sebastiao, A.; de Mendonça, A. Adenosine receptors in the nervous system: Pathophysiological implications. Prog. Neurobiol. 2002, 68, 377–392.
- Helman, D.L., Jr.; Byrd, J.C.; Ales, N.C.; Shorr, A.F. Fludarabine-related pulmonary toxicity: A distinct clinical entity in chronic lymphoproliferative syndromes. Chest 2002, 122, 785–790.
- Frederiksen, S. Specificity of adenosine deaminase toward adenosine and 2’-deoxyadenosine analogues. Arch. Biochem. Biophys. 1966, 113, 383–388.
- Avramis, V.I.; Champagne, J.; Sato, J.; Krailo, M.; Ettinger, L.J.; Poplack, D.G.; Finklestein, J.; Reaman, G.; Hammond, G.D.; Holcenberg, J.S. Pharmacology of fludarabine phosphate after a phase I/II trial by a loading bolus and continuous infusion in pediatric patients. Cancer Res. 1990, 50, 7226–7231.
- Malspeis, L.; Grever, M.R.; Staubus, A.; Young, D. Pharmacokinetics of 2-F-ara-A (9-beta-D-arabinofuranosyl-2-fluoroadenine) in cancer patients during the phase I clinical investigation of fludarabine phosphate. Semin. Oncol. 1990, 17, 18–32.
- Plunkett, W.; Huang, P.; Gandhi, V. Metabolism and action of fludarabine phosphate. Semin. Oncol. 1990, 17, 3–17.
- Plunkett, W.; Saunders, P.P. Metabolism and action of purine nucleoside analogs. Pharmacol. Ther. 1991, 49, 239–268.
- Danhauser, L.; Plunkett, W.; Keating, M.; Cabanillas, F. 9-β-D-Arabinofuranosyl-2-fluoroadenine 5’-monophosphate pharmacokinetics in plasma and tumor cells of patients with relapsed leukemia and lymphoma. Cancer Chemother. Pharmacol. 1986, 18, 145–152.
- Huang, P.; Chubb, S.; Plunkett, W. Termination of DNA synthesis by 9-beta-D-arabinofuranosyl-2-fluoroadenine. a mechanism for cytotoxicity. J. Biol. Chem. 1990, 265, 16617–16625.
- Spriggs, D.; Robbins, G.; Mitchell, T.; Kufe, D. Incorporation of 9-β-D-arabinofuranosyl-2-fluoroadenine into HL-60 cellular RNA and DNA. Biochem. Pharmacol. 1986, 35, 247–252.
- Krenitsky, T.A.; Koszalka, G.W.; Tuttle, J.V.; Rideout, J.L.; Elion, G.B. An enzymic synthesis of purine D-arabinonucleosides. Carbohydr. Res. 1981, 97, 139–146.
- Lambe, C.U.; Averett, D.R.; Paff, M.T.; Reardon, J.E.; Wilson, J.G.; Krenitsky, T.A. 2-Amino-6-methoxypurine arabinoside: An agent for T-cell malignancies. Cancer Res. 1995, 55, 3352–3356.
- Gandhi, V.; Keating, M.J.; Bate, G.; Kirkpatrick, P. Nelarabine. Nat. Rev. Drug Discov. 2006, 5, 17–18.
- Mitchell, B.S.; Mejias, E.; Daddona, P.E.; Kelley, W.N. Purinogenic immunodeficiency diseases: Selective toxicity of deoxyribonucleosides for T cells. Proc. Natl. Acad. Sci. USA 1978, 75, 5011–5014.
- Rodriguez, C.O., Jr.; Stellrecht, C.M.; Gandhi, V. Mechanisms for T-cell selective cytotoxicity of arabinosylguanine. Blood 2003, 102, 1842–1848.
- DeAngelo, D.J.; Yu, D.; Johnson, J.L.; Coutre, S.E.; Stone, R.M.; Stopeck, A.T.; Gockerman, J.P.; Mitchell, B.S.; Appelbaum, F.R.; Larson, R.A. Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and leukemia group B study 19801. Blood 2007, 109, 5136–5142.
- Gandhi, V.; Tam, C.; O’Brien, S.; Jewell, R.C.; Rodriguez, C.O., Jr.; Lerner, S.; Plunkett, W.; Keating, M.J. Phase I trial of nelarabine in indolent leukemias. J. Clin. Oncol. 2008, 26, 1098–1105.
- Mishchenko, N.P.; Fedoreev, S.A.; Bagirova, V.L. Histochrome: A new original domestic drug. Pharm. Chem. J. 2003, 37, 48–52.
- Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine polyhydroxynaphthoquinone, echinochrome a: Prevention of atherosclerotic inflammation and probable molecular targets. J. Clin. Med. 2020, 9, 1494.
- Park, J.H.; Lee, N.K.; Lim, H.J.; Mazumder, S.; Kumar Rethineswaran, V.; Kim, Y.J.; Jang, W.B.; Ji, S.T.; Kang, S.; Kim, D.Y.; et al. Therapeutic cell protective role of histochrome under oxidative stress in human cardiac progenitor cells. Mar. Drugs 2019, 17, 368.
- Hirata, Y.; Uemura, D. Halichondrins-antitumor polyether macrolides from a marine sponge. Pure Appl. Chem. 1986, 58, 701–710.
- Bauer, A. Story of eribulin mesylate: Development of the longest drug synthesis. In Synthesis of Heterocycles in Contemporary Medicinal Chemistry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 209–270.
- Seshadri, P.; Deb, B.; Kumar, P. Multifarious targets beyond microtubules-role of eribulin in cancer therapy. Front. Biosci. 2021, 13, 157–172.
- USFDA. Highlights of Prescribing Information: HALAVEN™. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/201532lbl.pdf (accessed on 30 June 2022).
- Eisai (Global) Eisai Announces Canadian Approval of Its Anticancer Agent Halaven™. Available online: https://www.eisai.com/news/news201179.html (accessed on 30 June 2022).
- USFDA. Highlights of Prescribing Information: HALAVEN™. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/201532s015lbl.pdf (accessed on 30 June 2022).
- Polastro, L.; Aftimos, P.G.; Awada, A. Eribulin mesylate in the management of metastatic breast cancer and other solid cancers: A drug review. Expert Rev. Anticancer. Ther. 2014, 14, 649–665.
- Towle, M.J.; Salvato, K.A.; Budrow, J.; Wels, B.F.; Kuznetsov, G.; Aalfs, K.K.; Welsh, S.; Zheng, W.; Seletsky, B.M.; Palme, M.H.; et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001, 61, 1013–1021.
- Ganjoo, K.N.; Patel, S.R. Trabectedin: An anticancer drug from the sea. Expert Opin. Pharmacother. 2009, 10, 2735–2743.
- Guan, Y.; Sakai, R.; Rinehart, K.; Wang, A. Molecular and crystal structures of ecteinascidins: Potent antitumor compounds from the caribbean tunicate ecteinascidia turbinata. J. Biomol. Struct. Dyn. 1993, 10, 793–818.
- Corey, E.J.; Gin, D.Y.; Kania, R.S. Enantioselective total synthesis of ecteinascidin 743. J. Am. Chem. Soc. 1996, 118, 9202–9203.
- He, W.; Zhang, Z.; Ma, D. A scalable total synthesis of the antitumor agents ET-743 and lurbinectedin. Angew. Chem. Int. Ed. 2019, 58, 3972–3975.
- EMA. Public Summary of Opinion on Orphan Designation: Trabectedin for the Treatment of Ovarian Cancer. Available online: https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/03/171-public-summary-positive-opinion-orphan-designation-trabectedin-treatment-ovarian-cancer_en.pdf (accessed on 30 June 2022).
- EMA. Yondelis. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/yondelis (accessed on 30 June 2022).
- De Sanctis, R.; Marrari, A.; Santoro, A. Trabectedin for the treatment of soft tissue sarcomas. Expert Opin. Pharmacother. 2016, 17, 1569–1577.
- EMA. Committee for Medicinal Products for Human Use Post-Authorisation Summary of Positive Opinion for Yondelis. Available online: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-yondelis-24-september-2009_en.pdf (accessed on 30 June 2022).
- Zijoo, R.; von Mehren, M. Efficacy of trabectedin for the treatment of liposarcoma. Expert Opin. Pharmacother. 2016, 17, 1953–1962.
- USFDA. Highlights of Prescribing Information: YONDELIS™. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207953s000lbl.pdf (accessed on 30 June 2022).
- Gordon, E.M.; Sankhala, K.K.; Chawla, N.; Chawla, S.P. Trabectedin for soft tissue sarcoma: Current status and future perspectives. Adv. Ther. 2016, 33, 1055–1071.
- Grosso, F.; D’Ambrosio, L.; Zucchetti, M.; Ibrahim, T.; Tamberi, S.; Matteo, C.; Rulli, E.; Comandini, D.; Palmerini, E.; Baldi, G.G.; et al. Pharmacokinetics, safety, and activity of trabectedin as first-line treatment in elderly patients who are affected by advanced sarcoma and are unfit to receive standard chemotherapy: A phase 2 study (TR1US study) from the Italian sarcoma group. Cancer 2020, 126, 4726–4734.
- Beumer, J.H.; Rademaker-Lakhai, J.M.; Rosing, H.; Hillebrand, M.J.; Bosch, T.M.; Lopez-Lazaro, L.; Schellens, J.H.; Beijnen, J.H. Metabolism of trabectedin (ET-743, Yondelis) in patients with advanced cancer. Cancer Chemother. Pharmacol. 2007, 59, 825–837.
- Gago, F.; Hurley, L.H. Devising a structural basis for the potent cytotoxic effects of Ecteinascidin 743. In Small Molecule DNA and RNA Binders: From Synthesis to Nulceic Acid; The Royal Society of Chemistry: London, UK, 2002; pp. 643–675.
- Jimeno, J.; Maki, R.G.; Casali, P.; Faircloth, G.; Martinez, N.; Nieto, A.; Cañigueral, S.; Rinehart, K. Therapeutic impact of ET-743 (yondelis; trabectidin), a new marine-derived compound, in sarcoma. Curr. Opin. Orthop. 2003, 14, 419–428.
- Pommier, Y.; Kohlhagen, G.; Bailly, C.; Waring, M.; Mazumder, A.; Kohn, K.W. DNA sequence-and structure-selective alkylation of guanine N2 in the DNA minor groove by ecteinascidin 743, a potent antitumor compound from the Caribbean tunicate Ecteinascidia turbinata. Biochemistry 1996, 35, 13303–13309.
- Erba, E.; Bergamaschi, D.; Bassano, L.; Damia, G.; Ronzoni, S.; Faircloth, G.; d’Incalci, M. Ecteinascidin-743 (ET-743), a natural marine compound, with a unique mechanism of action. Eur. J. Cancer 2001, 37, 97–105.
- Takebayashi, Y.; Pourquier, P.; Zimonjic, D.B.; Nakayama, K.; Emmert, S.; Ueda, T.; Urasaki, Y.; Kanzaki, A.; Akiyama, S.-I.; Popescu, N.; et al. Antiproliferative activity of ecteinascidin 743 is dependent upon transcription-coupled nucleotide-excision repair. Nat. Med. 2001, 7, 961–966.
- Soares, D.G.; Escargueil, A.E.; Poindessous, V.; Sarasin, A.; de Gramont, A.; Bonatto, D.; Henriques, J.A.P.; Larsen, A.K. Replication and homologous recombination repair regulate DNA double-strand break formation by the antitumor alkylator ecteinascidin 743. Proc. Natl. Acad. Sci. USA 2007, 104, 13062–13067.
- Liguori, M.; Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages as incessant builders and destroyers of the cancer stroma. Cancers 2011, 3, 3740–3761.
- Germano, G.; Frapolli, R.; Belgiovine, C.; Anselmo, A.; Pesce, S.; Liguori, M.; Erba, E.; Uboldi, S.; Zucchetti, M.; Pasqualini, F. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013, 23, 249–262.
- D’incalci, M.; Badri, N.; Galmarini, C.; Allavena, P. Trabectedin, a drug acting on both cancer cells and the tumour microenvironment. Br. J. Cancer 2014, 111, 646–650.
- Leal, J.; Martínez-Díez, M.; García-Hernández, V.; Moneo, V.; Domingo, A.; Bueren-Calabuig, J.; Negri, A.; Gago, F.; Guillén-Navarro, M.; Avilés, P. PM01183, a new DNA minor groove covalent binder with potent in vitro and in vivo anti-tumour activity. Br. J. Pharmacol. 2010, 161, 1099–1110.
- Newswire, P.R. Jazz Pharmaceuticals Announces U.S. FDA Accelerated Approval of zepzelca™ (lurbinectedin) for the Treatment of Metastatic Small Cell Lung Cancer. Available online: https://www.prnewswire.com/news-releases/jazz-pharmaceuticals-announces-us-fda-accelerated-approval-of-zepzelca-lurbinectedin-for-the-treatment-of-metastatic-small-cell-lung-cancer-301077082.html (accessed on 30 June 2022).
- Baena, J.; Modrego, A.; Zeaiter, A.; Kahatt, C.; Alfaro, V.; Jimenez-Aguilar, E.; Mazarico, J.M.; Paz-Ares, L. Lurbinectedin in the treatment of relapsed small cell lung cancer. Future Oncol. 2021, 17, 2279–2289.
- USFDA. Highlights of Prescribing Information: ZEPZELCA™. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213702s000lbl.pdf (accessed on 30 June 2022).
- Trigo, J.; Subbiah, V.; Besse, B.; Moreno, V.; López, R.; Sala, M.A.; Peters, S.; Ponce, S.; Fernández, C.; Alfaro, V.; et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: A single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020, 21, 645–654.
- Fernandez-Teruel, C.; Gonzalez, I.; Trocóniz, I.F.; Lubomirov, R.; Soto, A.; Fudio, S. Population-pharmacokinetic and covariate analysis of lurbinectedin (PM01183), a new RNA polymerase II Inhibitor, in pooled phase I/II trials in patients with cancer. Clin. Pharmacokinet. 2019, 58, 363–374.
- Elez, M.E.; Tabernero, J.; Geary, D.; Macarulla, T.; Kang, S.P.; Kahatt, C.; Pita, A.S.-M.; Teruel, C.F.; Siguero, M.; Cullell-Young, M. First-in-human phase I study of Lurbinectedin (PM01183) in patients with advanced solid tumors. Clin. Cancer Res. 2014, 20, 2205–2214.
- Moneo, V.; Martínez, P.; de Castro, B.; Cascajares, S.; Avila, S.; Garcia-Fernandez, L.F.; Galmarini, C.M. Abstract A174: Comparison of the antitumor activity of trabectedin, lurbinectedin, zalypsis and PM00128 in a panel of human cells deficient in transcription/NER repair factors. Am. Assoc. Cancer Res. 2017, 5, 628–638.
- Nuñez, G.S.; Robles CM, G.; Giraudon, C.; Martínez-Leal, J.F.; Compe, E.; Coin, F.; Aviles, P.; Galmarini, C.M.; Egly, J.-M. Lurbinectedin specifically triggers the degradation of phosphorylated RNA polymerase II and the formation of DNA breaks in cancer cells. Mol. Cancer Ther. 2016, 15, 2399–2412.
- Pernice, T.; Bishop, A.G.; Guillen, M.J.; Cuevas, C.; Aviles, P. Development of a liquid chromatography/tandem mass spectrometry assay for the quantification of PM01183 (lurbinectedin), a novel antineoplastic agent, in mouse, rat, dog, Cynomolgus monkey and mini-pig plasma. J. Pharm. Biomed. Anal. 2016, 123, 37–41.
- Romano, M.; Frapolli, R.; Zangarini, M.; Bello, E.; Porcu, L.; Galmarini, C.M.; García-Fernández, L.F.; Cuevas, C.; Allavena, P.; Erba, E. Comparison of in vitro and in vivo biological effects of trabectedin, lurbinectedin (PM01183) and zalypsis®(PM00104). Int. J. Cancer 2013, 133, 2024–2033.
- Belgiovine, C.; Bello, E.; Liguori, M.; Craparotta, I.; Mannarino, L.; Paracchini, L.; Beltrame, L.; Marchini, S.; Galmarini, C.M.; Mantovani, A.; et al. Lurbinectedin reduces tumour-associated macrophages and the inflammatory tumour microenvironment in preclinical models. Br. J. Cancer 2017, 117, 628–638.
- Taniguchi, H.; Sen, T.; Rudin, C.M. Targeted therapies and biomarkers in small cell lung cancer. Front. Oncol. 2020, 10, 741.
- USFDA. Highlights of Prescribing Information: ADCETRIS™. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125388_S056S078lbl.pdf (accessed on 30 June 2022).
- Horie, R.; Watanabe, T. Seminars in Immunology. In CD30: Expression and Function in Health and Disease; Elsevier: Amsterdam, The Netherlands, 1998; pp. 457–470.
- Van der Weyden, C.; Pileri, S.; Feldman, A.; Whisstock, J.; Prince, H. Understanding CD30 biology and therapeutic targeting: A historical perspective providing insight into future directions. Blood Cancer J. 2017, 7, e603.
- Moskowitz, C.H.; Nademanee, A.; Masszi, T.; Agura, E.; Holowiecki, J.; Abidi, M.H.; Chen, A.I.; Stiff, P.; Gianni, A.M.; Carella, A. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 385, 1853–1862.
- Hansen, H.P.; Trad, A.; Dams, M.; Zigrino, P.; Moss, M.; Tator, M.; Schön, G.; Grenzi, P.C.; Bachurski, D.; Aquino, B. CD30 on extracellular vesicles from malignant Hodgkin cells supports damaging of CD30 ligand-expressing bystander cells with brentuximab-vedotin, in vitro. Oncotarget 2016, 7, 30523.
- Amaya, M.; Jimeno, A.; Kamdar, M. Polatuzumab vedotin to treat relapsed or refractory diffuse large B-cell lymphoma, in combination with bendamustine plus rituximab. Drugs Today 2020, 56, 287–294.
- Malecek, M.-K.; Watkins, M.P.; Bartlett, N.L. Polatuzumab vedotin for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma. Expert Opin. Biol. Ther. 2021, 21, 831–839.
- Pfeifer, M.; Zheng, B.; Erdmann, T.; Koeppen, H.; McCord, R.; Grau, M.; Staiger, A.; Chai, A.; Sandmann, T.; Madle, H. Anti-CD22 and anti-CD79B antibody drug conjugates are active in different molecular diffuse large B-cell lymphoma subtypes. Leukemia 2015, 29, 1578–1586.
- International Non-Hodgkin’s Lymphoma Prognostic Factors Project, A predictive model for aggressive non-Hodgkin’s lymphoma. N. Engl. J. Med. 1993, 329, 987–994.
- USFDA. Clinical Review: Polatuzumab Vedotin™. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761121Orig1s000MedR.pdf (accessed on 30 June 2022).
- USFDA. Highlights of Prescribing Information: POLIVY™. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761121s000lbl.pdf (accessed on 30 June 2022).
- FDA. Padcev®. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-enfortumab-vedotin-ejfv-locally-advanced-or-metastatic-urothelial-cancer (accessed on 30 June 2022).
- FDA Approved Drug Products: Padcev (Enfortumab Vedotin-Ejfv) for IV Injection. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761137s000lbl.pdf (accessed on 30 June 2022).
- Hanna, K.S. Clinical overview of enfortumab vedotin in the management of locally advanced or metastatic urothelial carcinoma. Drugs 2020, 80, 1–7.
- McGregor, B.A.; Sonpavde, G. Enfortumab Vedotin, a fully human monoclonal antibody against Nectin 4 conjugated to monomethyl auristatin E for metastatic urothelial carcinoma. Expert Opin. Investig. Drugs 2019, 28, 821–826.
- FDA. Blenrep®. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-granted-accelerated-approval-belantamab-mafodotin-blmf-multiple-myeloma (accessed on 30 June 2022).
- FDA Approved Drug Products: Blenrep Belantaman Mafodotin-Blmf Intravenous Injection. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761158s006lbl.pdf (accessed on 30 June 2022).
- McMillan, A.; Warcel, D.; Popat, R. Antibody-drug conjugates for multiple myeloma. Expert Opin. Biol. Ther. 2021, 21, 889–901.
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V.; et al. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019, 9, 37.
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238.
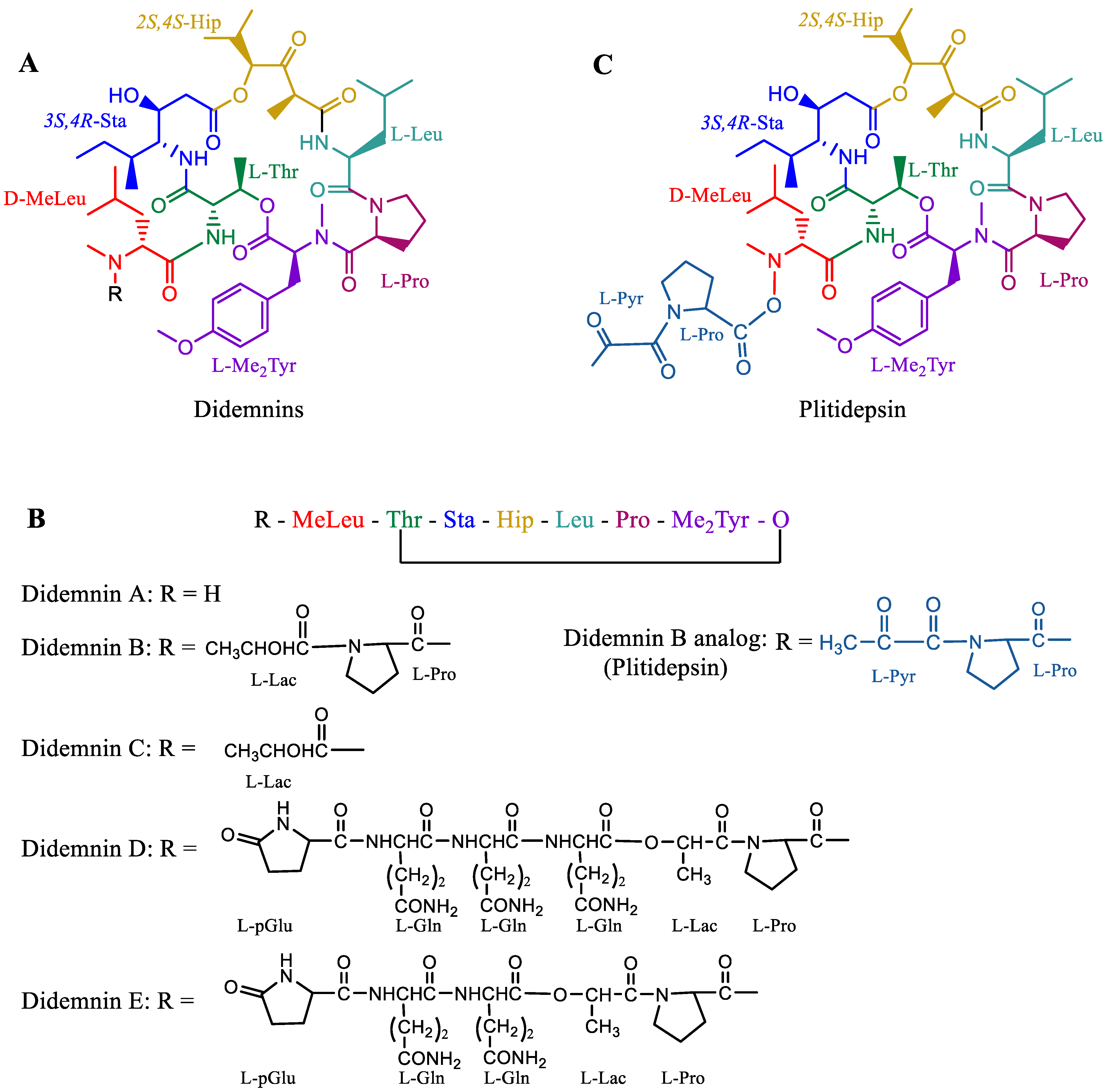
- Rinehart, K.L., Jr.; Gloer, J.B.; Cook, J.C., Jr.; Mizsak, S.A.; Scahill, T.A. Structures of the didemnins, antiviral and cytotoxic depsipeptides from a caribbean tunicate. J. Am. Chem. Soc. 1981, 103, 1857–1859.
- Rinehart, K.L.; Kishore, V.; Bible, K.C.; Sakai, R.; Sullins, D.W.; Li, K.-M. Didemnins and tunichlorin: Novel natural products from the marine tunicate trididemnum solidum. J. Nat. Pproducts 1988, 51, 1–21.
- TGA, A. Australian Public Assessment Report for Plitidepsin. Available online: https://www.tga.gov.au/sites/default/files/auspar-plitidepsin-190513.pdf (accessed on 30 June 2022).
- BioWorld. EU Court Sides with Pharmamar in Aplidin Approval Dispute. Available online: https://www.bioworld.com/articles/499498-eu-court-sides-with-pharmamar-in-aplidin-approval-dispute (accessed on 30 June 2022).
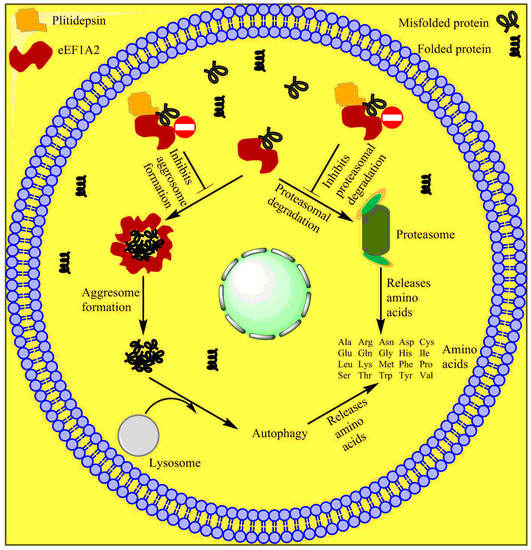
- White, K.M.; Rosales, R.; Yildiz, S.; Kehrer, T.; Miorin, L.; Moreno, E.; Jangra, S.; Uccellini, M.B.; Rathnasinghe, R.; Coughlan, L.; et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 2021, 371, 926–931.
- Gomez, J.; Extremera, S.; Nieto, A. Overall survival (OS) results of randomized phase III study (ADMYRE trial) of plitidepsin and dexamethasone (DXM) vs. DXM alone in patients with relapsed/refractory multiple myeloma (RRMM): Evaluation of the crossover impact. Am. Soc. Clin. Oncol. J. 2018, 36, 8018.
- Spicka, I.; Ocio, E.M.; Oakervee, H.E.; Greil, R.; Banh, R.H.; Huang, S.-Y.; D’Rozario, J.M.; Dimopoulos, M.A.; Martínez, S.; Extremera, S.; et al. Randomized phase III study (ADMYRE) of plitidepsin in combination with dexamethasone vs. dexamethasone alone in patients with relapsed/refractory multiple myeloma. Ann. Hematol. 2019, 98, 2139–2150.
- Ribrag, V.; Caballero, D.; Fermé, C.; Zucca, E.; Arranz, R.; Briones, J.; Gisselbrecht, C.; Salles, G.; Gianni, A.M.; Gomez, H. Multicenter phase II study of plitidepsin in patients with relapsed/refractory non-Hodgkin’s lymphoma. Haematologica 2013, 98, 357–363.
- Muñoz-Alonso, M.J.; González-Santiago, L.; Martínez, T.; Losada, A.; Galmarini, C.M.; Muñoz, A. The mechanism of action of plitidepsin. Curr. Opin. Investig. Drugs 2009, 10, 536–542.
- Alonso-Álvarez, S.; Pardal, E.; Sánchez-Nieto, D.; Navarro, M.; Caballero, M.D.; Mateos, M.V.; Martín, A. Plitidepsin: Design, development, and potential place in therapy. Drug Des. Dev. Ther. 2017, 11, 253–264.
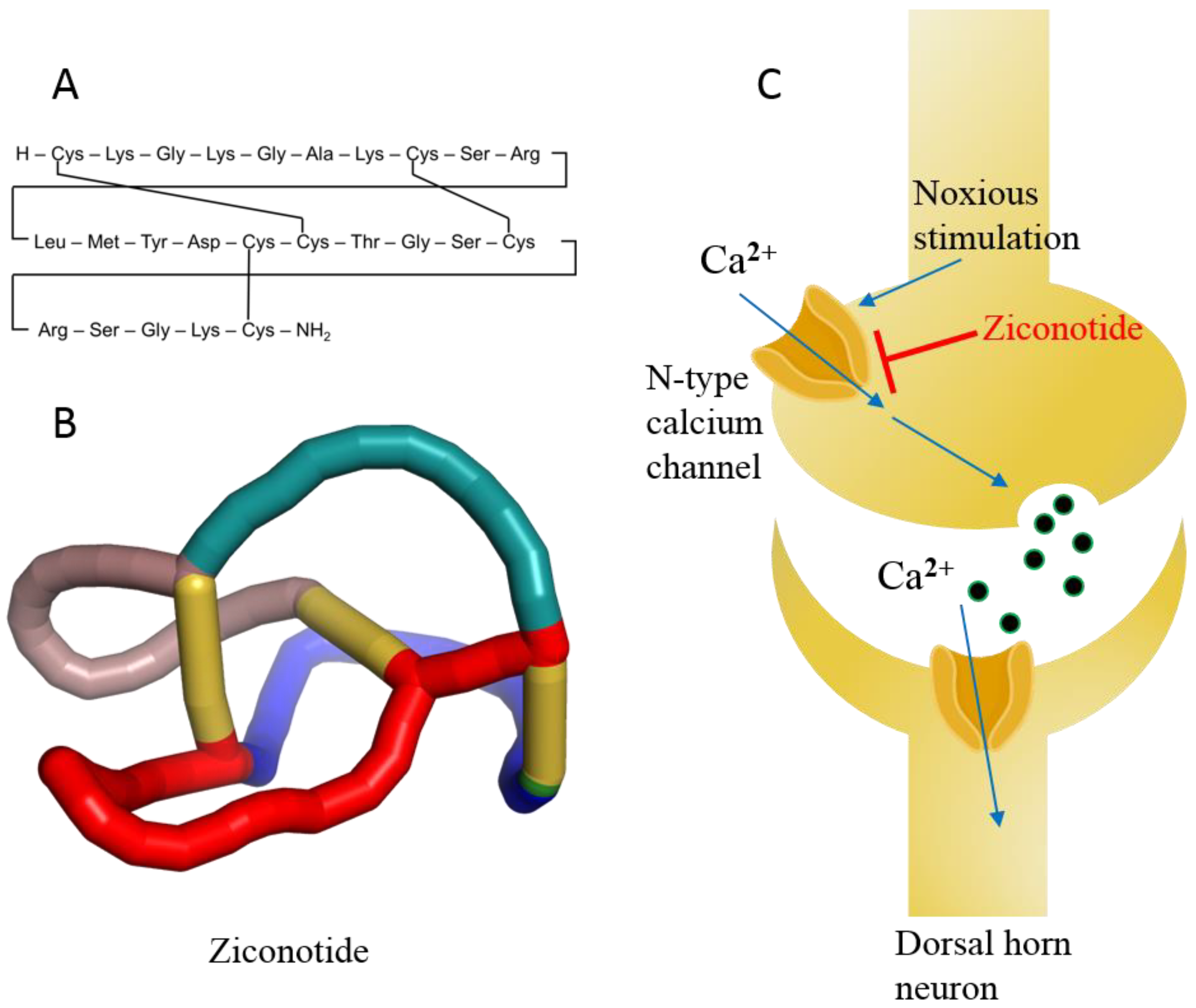
- Terlau, H.; Olivera, B.M. Conus venoms: A rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004, 84, 41–68.
- Jin, A.-H.; Muttenthaler, M.; Dutertre, S.; Himaya, S.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Conotoxins: Chemistry and biology. Chem. Rev. 2019, 119, 11510–11549.
- Kaas, Q.; Westermann, J.-C.; Craik, D.J. Conopeptide characterization and classifications: An analysis using conoserver. Toxicon 2010, 55, 1491–1509.
- Kumar, P.S.; Kumar, D.S.; Umamaheswari, S. A perspective on toxicology of conus venom peptides. Asian Pac. J. Trop. Med. 2015, 8, 337–351.
- Bäckryd, E. Do the potential benefits outweigh the risks? An update on the use of ziconotide in clinical practice. Eur. J. Pain 2018, 22, 1193–1202.
- Bozorgi, H.; Motaghi, E.; Zamani, M.; Ghavimi, R. Neuronal calcium channels blocker, ziconotide (ɷ-conotoxin MVIIA), reverses morphine withdrawal-induced memory impairments via alteration in hippocampal NMDA receptor expression in rats. Toxin Rev. 2018, 39, 1–10.
- Wermeling, D.; Drass, M.; Ellis, D.; Mayo, M.; McGuire, D.; O’Connell, D.; Hale, V.; Chao, S. Pharmacokinetics and pharmacodynamics of intrathecal ziconotide in chronic pain patients. J. Clin. Pharmacol. 2003, 43, 624–636.
- Schmidtko, A.; Lötsch, J.; Freynhagen, R.; Geisslinger, G. Ziconotide for treatment of severe chronic pain. Lancet 2010, 375, 1569–1577.
- Smith, H.S.; Deer, T.R. Safety and efficacy of intrathecal ziconotide in the management of severe chronic pain. Ther. Clin. Risk Manag. 2009, 5, 521–534.
- Nair, A.; Poornachand, A.; Kodisharapu, P. Ziconotide: Indications, adverse effects, and limitations in managing refractory chronic pain. Indian J. Palliat. Care 2018, 24, 118–119.
- Deer, T.R.; Prager, J.; Levy, R.; Rathmell, J.; Buchser, E.; Burton, A.; Caraway, D.; Cousins, M.; de Andrés, J.; Diwan, S.; et al. Polyanalgesic consensus conference 2012: Recommendations for the management of pain by intrathecal (intraspinal) drug delivery: Report of an interdisciplinary expert panel. Neuromodulation 2012, 15, 436–466.
- Deer, T.; Hagedorn, J.M. How has ziconotide impacted non-cancer pain management? Expert Opin. Pharmacother. 2020, 21, 507–511.
- Matis, G.; de Negri, P.; Dupoiron, D.; Likar, R.; Zuidema, X.; Rasche, D. Intrathecal pain management with ziconotide: Time for consensus? Brain Behav. 2021, 11, e02055.
- Jorpes, E.; Thaning, T. Neutralisation of action of heparin by protamine. Lancet 1939, 234, 975–976.
- Sokolowska, E.; Kalaska, B.; Miklosz, J.; Mogielnicki, A. The toxicology of heparin reversal with protamine: Past, present and future. Expert Opin. Drug Metab. Toxicol. 2016, 12, 897–909.
- Drugs. Protamine Sulfate. Available online: https://www.drugs.com/international/protamine-sulfate.html (accessed on 30 June 2022).
- Lindblad, B. Protamine sulphate: A review of its effects: Hypersensitivity and toxicity. Eur. J. Vasc. Surg. 1989, 3, 195–201.
- Sorgi, F.; Bhattacharya, S.; Huang, L. Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther. 1997, 4, 961–968.
- DeLucia, A., 3rd; Wakefield, T.W.; Kadell, A.; Wrobleski, S.K.; VanDort, M.; Stanley, J.C. Tissue distribution, circulating half-life, and excretion of intravenously administered protamine sulfate. J. Am. Soc. Artif. Intern. Organs 1993, 39, M715–M718.
- Blajchman, M. An overview of the mechanism of action of antithrombin and its inherited deficiency states. Blood Coagul. Fibrinolysis 1994, 5, S5–S11.
- Hirsh, J.; Warkentin, T.E.; Dalen, J.E.; Deykin, D.; Poller, L. Heparin: Mechanism of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest 1995, 108, 258S–275S.
- Okajima, Y.; Kanayama, S.; Maeda, Y.; Urano, S.; Kitani, T.; Watada, M.; Nakagawa, M.; Ijichi, H. Studies on the neutralizing mechanism of antithrombin activity of heparin by protamine. Thromb. Res. 1981, 24, 21–29.
- Medpagetoday. FDA Clears Andexanet Alfa for Rapid NOAC Reversal. Available online: https://www.medpagetoday.com/cardiology/prevention/72701 (accessed on 30 June 2022).
- Klhsite. About KLH. Available online: https://www.klhsite.com/about-klh/ (accessed on 30 June 2022).
- DrugBank. Keyhole Limpet Hemocyanin. Available online: https://go.drugbank.com/drugs/DB05299 (accessed on 30 June 2022).
- Weigle, W.O. Immunochemical properties of hemocyanin. Immunochemistry 1964, 1, 295–302.
- Curtis, J.; Hersh, E.M.; Harris, J.; McBride, C.; Freireich, E.J. The human primary immune response to keyhole limpet haemocyanin: Interrelationships of delayed hypersensitivity, antibody response and in vitro blast transformation. Clin. Exp. Immunol. 1970, 6, 473–491.
- Curtis, J.E.; Hersh, E.M.; Butler, W.T.; Rossen, R.D. Antigen dose in the human immune response: Dose-response relationships in the human immune response to keyhole limpet hemocyanin. J. Lab. Clin. Med. 1971, 78, 61–69.
- Herscowitz, H.; Harold, W.; Stavitsky, A. Immunochemical and immunogenic properties of a purified keyhole limpet haemocyanin. Immunology 1972, 22, 51–61.
- Söhngen, S.M.; Stahlmann, A.; Harris, J.R.; Müller, S.A.; Engel, A.; Markl, J. Mass determination, subunit organization and control of oligomerization states of keyhole limpet hemocyanin (KLH). Eur. J. Biochem. 1997, 248, 602–614.
- Harris, J.; Markl, J. Keyhole limpet hemocyanin (KLH): A biomedical review. Micron 1999, 30, 597–623.
- Nseyo, U.O.; Lamm, D.L. Seminars in Surgical Oncology. In Immunotherapy of Bladder Cancer; Wiley Online Library: Hoboken, NJ, USA, 1997; pp. 342–349.
- Biosyn. IMMUCOTHEL™. Available online: https://biosyncorp.com/klh/ (accessed on 30 June 2022).
- Biosyn. VACMUNE™. Available online: https://biosyncorp.com/vacmuner/ (accessed on 30 June 2022).
- International, B. DigiFab. Available online: https://digifab.health/en-us (accessed on 30 June 2022).
- Swaminathan, A.; Lucas, R.M.; Dear, K.; McMichael, A.J. Keyhole limpet haemocyanin–a model antigen for human immunotoxicological studies. Br. J. Clin. Pharmacol. 2014, 78, 1135–1142.
- Shekelle, R.B.; Missell, L.; Paul, O.; Shryock, A.M.; Stamler, J.; Vollset, S.E.; Heuch, I.; Bjelke, E.; Curb, J.D.; Reed, D.M. Fish consumption and mortality from coronary heart disease. N. Engl. J. Med. 1985, 313, 820–824.
- Dolecek, T.; Granditis, G. Dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial (MRFIT). World Rev. Nutr. Diet. 1991, 66, 205–216.
- Kromhout, D.; Feskens, E.J.; Bowles, C.H. The protective effect of a small amount of fish on coronary heart disease mortality in an elderly population. Int. J. Epidemiol. 1995, 24, 340–345.
- Zhang, J.; Sasaki, S.; Amano, K.; Kesteloot, H. Fish consumption and mortality from all causes, ischemic heart disease, and stroke: An ecological study. Prev. Med. 1999, 28, 520–529.
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757.
- Kumar, N.G.; Contaifer, D.; Madurantakam, P.; Carbone, S.; Price, E.T.; van Tassell, B.; Brophy, D.F.; Wijesinghe, D.S. Dietary bioactive fatty acids as modulators of immune function: Implications on human health. Nutrients 2019, 11, 2974.
- Maki, K.C.; Johns, C.; Harris, W.S.; Puder, M.; Freedman, S.D.; Thorsteinsson, T.; Daak, A.; Rabinowicz, A.L.; Sancilio, F.D. Bioequivalence demonstration for Ω-3 acid ethyl Ester formulations: Rationale for modification of current guidance. Clin. Ther. 2017, 39, 652–658.
- Guthrie, G.; Burrin, D. Impact of parenteral lipid emulsion components on cholestatic liver disease in neonates. Nutrients 2021, 13, 508.
- Saremi, A.; Arora, R. The utility of omega-3 fatty acids in cardiovascular disease. Am. J. Ther. 2009, 16, 421–436.
- Ballantyne, C.M.; Braeckman, R.A.; Soni, P.N. Icosapent ethyl for the treatment of hypertriglyceridemia. Expert Opin. Pharmacother. 2013, 14, 1409–1416.
- Bazarbashi, N.; Miller, M. Icosapent ethyl: Drug profile and evidence of reduced residual cardiovascular risk in patients with statin-managed LDL-C cholesterol. Expert Rev. Cardiovasc. Ther. 2020, 18, 175–180.
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 1–12.
- Touloukian, R.J.; Seashore, J.H. Hepatic secretory obstruction with total parenteral nutrition in the infant. J. Pediatric Surg. 1975, 10, 353–360.
- Rangel, S.J.; Calkins, C.M.; Cowles, R.A.; Barnhart, D.C.; Huang, E.Y.; Abdullah, F.; Arca, M.J.; Teitelbaum, D.H. Parenteral nutrition–associated cholestasis: An American pediatric surgical association outcomes and clinical trials committee systematic review. J. Pediatric Surg. 2012, 47, 225–240.
