Gut microbiota can have opposing functions from pro-tumorigenic to anti-tumorigenic effects. Increasing preclinical and clinical evidence suggests that the intestinal microbiota affects cancer patients’ response to immune checkpoint inhibitors (ICIs) immunotherapy, such as anti-programmed cell death protein 1 (PD-1) and its ligand (PD-L1) and anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4). Microbiota-induced inflammation possibly contributes to tumor growth and cancer development. Microbiota-derived metabolites can also be converted to carcinogenic agents related to genetic mutations and DNA damage in organs such as the colon. However, other attributes of microbiota, such as greater diversity and specific bacterial species and their metabolites, are linked to better clinical outcomes and potentially improved anti-tumor immunity. In addition, the intratumoral microbial composition strongly affects T-cell-mediated cytotoxicity and anti-tumor immune surveillance, adding more complexity to the cancer-microbiome-immune axis.
- immunotherapy
- immune checkpoint inhibitors
- microbiota
- cancer
1. Introduction
2. Gut Microbiota and Anti-Tumor Immunity
Innate and adaptive immune responses are vital components of the anti-tumor immunity against cancer. Diverse immune agents mediate tumor immune surveillance, but T-cell-mediated cytotoxicity is the principal mechanism of anti-tumor immunity. T-cells are central in anti-tumor response due to their ability to recognize specific peptides through the interaction of MHC-TCR, their cytotoxic effects, and their ability to exhibit immunological memory. Mainly, CD4+ and CD8+ T-cells are responsible for preventing tumor growth and cancer development. Therefore, ICIs mainly rely on T-cells for their efficacy. Notably, the gut microbiota is a tumor-extrinsic factor that can modulate anti-tumor defense mechanisms and impact the efficacy of cancer immunotherapy with ICIs (Figure 31).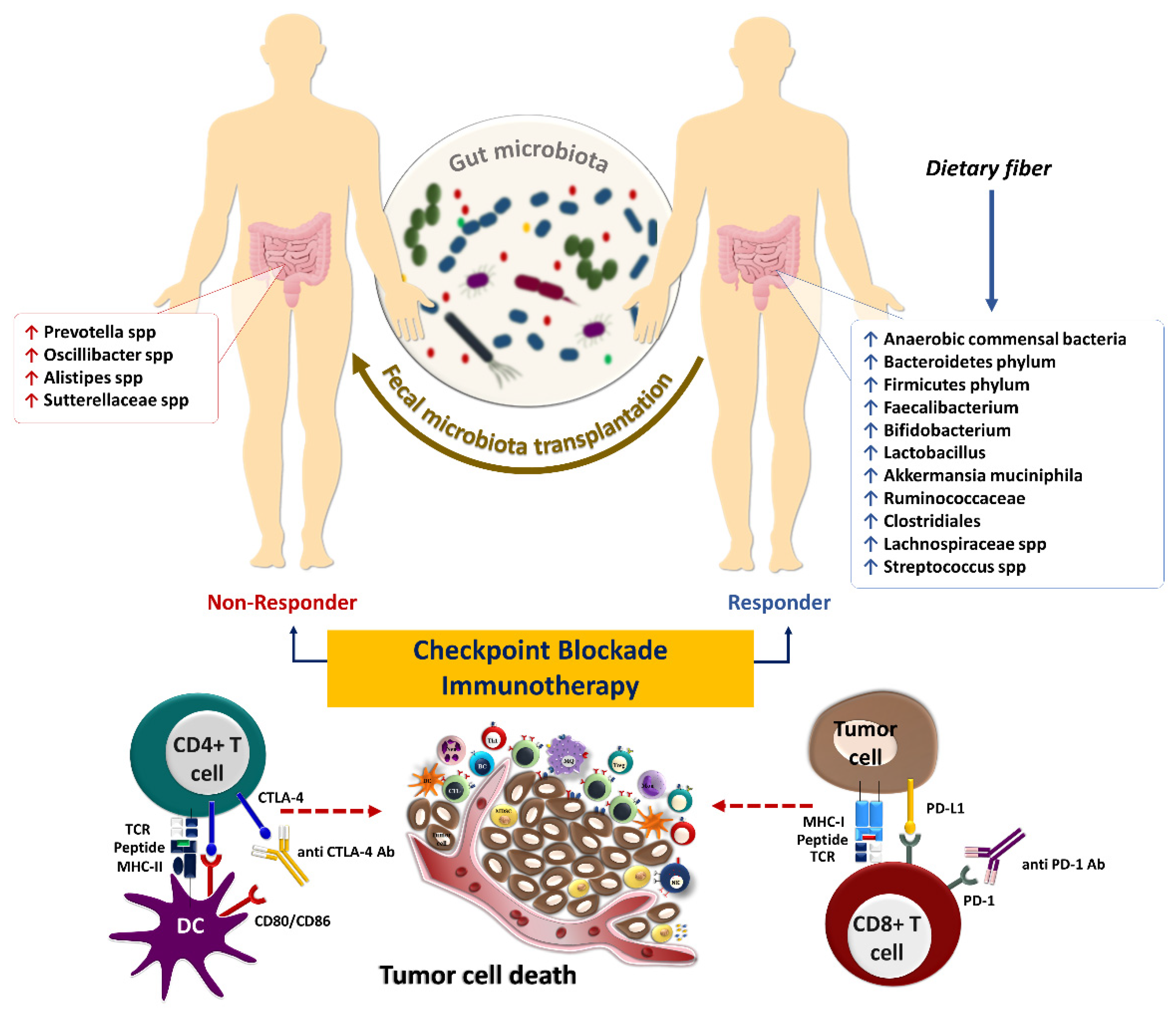
3. Gut Microbiota and Response to Systemic Therapy, including Immunotherapy
The interaction of immune checkpoint molecules such as PD-1 and its ligand, PD-L1, suppresses T-cell function and infiltration to the TME. The PD-1/PD-L1 interaction contributes to immune tolerance and, ultimately, immune escape of tumor cells [97][28]. The interaction of PD-1, mainly expressed on T-cells in the late phase of their activation, with its ligand PD-L1, expressed on tumor cells or other cells in the TME, inhibits the activation and effector functions of tumor-specific T-cells [98][29]. In contrast, CTLA-4 is expressed in the early phase of T-cell activation and competes with CD28 expressed on the surface of activated T-cells, with higher affinity, in binding to CD80 and CD86 on antigen-presenting cells preventing proper co-stimulatory signals for T-cell activation. Furthermore, Tregs constitutively express CTLA-4, allowing them to inhibit the activation of conventional T-cells [99][30]. Hence, ICIs, including anti-PD-1 (nivolumab and pembrolizumab), anti-PD-L1 (durvalumab, avelumab, and atezolizumab), and anti-CTLA-4 (ipilimumab and tremelimumab) antibodies have been developed for cancer immunotherapy [100][31]. Most patients remain unresponsive to ICIs despite the ability of these drugs to reinvigorate tumor-reactive T-cells in clinical settings [101][32]. Therefore, primary resistance to ICIs is an immense clinical problem that requires novel combination treatment strategies to improve the efficacy of these drugs. Intratumoral microbes possibly mediate resistance to immunotherapy with ICIs and other forms of systemic therapy such as chemotherapy. In some cases, bacteria are found in patients’ tumors and genetically engineered mouse models of pancreatic cancer that are associated with a more immunosuppressive TME [102][33]. It can be postulated that targeting intratumoral microbes with antibiotics could modulate chemotherapy resistance due to the direct tumor-supportive roles of intratumoral bacteria, such as bacterial-induced autophagy in tumor cells [103][34]. Moreover, intratumoral bacteria possibly diminish the efficacy of systemic cancer therapy via metabolizing the chemotherapeutic drug to its inactive form [104][35]. Therefore, lower chemotherapy drug concentrations can be achieved due to the presence of bacteria in human tumors than in other organs [105][36]. Some intratumoral bacteria can negatively impact anti-tumor immunity. In contrast, others can potentially prevent cancer progression by providing bacterial antigens in the tumor that can mimic neoantigens and activate anti-tumor immunity. For example, RNA sequencing and immunopeptidomics analysis have recently identified 248 and 35 unique HLA-I and HLA-II peptides derived from 41 intratumoral bacterial species from 17 metastatic melanoma tumors. Microbial neoantigens in melanoma tumors are processed, presented, and recognized by T-cells [105][36]. These findings confirmed that cancerous tissue could present bacterial neoantigens to tumor-infiltrating T-cells and reinforce immune TME. Furthermore, the presence of microbes in the tumor can potentially improve dendritic cell maturation. Indeed, dendritic cells stimulated with live or heat-killed commensal bacteria can express co-stimulation/maturation markers and produce pro-inflammatory cytokine/chemokine, such as IL-1β and TNF-α [106][37]. Also, combining anti-PD-1 immunotherapy with bacterial therapy using Clostridiales strains cleared almost all tumor cells and reduced the volume and weight of melanoma tumors [107][38]. Therefore, clinical studies based on bacteria-based therapies in the form of complete or partial consortia are warranted to sensitize tumors to ICI therapy. The resemblance of tumor-associated antigens and microbiota-derived epitopes potentially supports anti-tumor immunity. However, the intestinal microbiota has a dual effect on immunotherapy by enhancing or diminishing anti-tumor immune responses [108][39]. For instance, bacterial species including Faecalibacterium, Bifidobacterium, Lactobacillus, Akkermansia muciniphila, and Ruminococcaceae spp. play a considerable role in anti-tumor immune surveillance as well as the response to ICIs therapy [109,110][40][41]. Several seminal studies have already shown that intestinal microbiota composition is perturbed during cancer progression [111,112,113][42][43][44]. Microbiome sequencing and immune profiling of 233 patients with metastatic melanoma who received anti-PD-1 immunotherapy have shown that those with a more diverse gut microbiome had a higher ORR and improved survival outcomes. These findings indicate that a reduction in microbial diversity known as dysbiosis can result in poor response to ICIs [114][45]. Analysis of fecal microbiome signatures of 94 melanoma patients who received anti-PD-1 immunotherapy showed that Ruminococcus (Mediterraneibacter) torques, Blautia producta, Blautia wexlerae, Blautia hansenii, Eubacterium rectale, Ruminococcus (Mediterraneibacter) gnavus, and Anaerostipes hadrus are increased in non-progressors. In comparison, the stool samples of progressors are enriched with Prevotella spp., Oscillibacter spp., Alistipes spp., and Sutterellaceae spp. Moreover, transcriptomic analyses of fecal samples of those patients have identified a remarkable upregulation of superoxide dismutase (SOD2), pro-inflammatory cytokines such as IL-1β and CXCL8, transcription factors NFKBIZ, NFKBIA, TNFAIP3, and LITAF in progressors. Fecal samples of progressor also had an abundance of inflammatory cells, including dendritic cells, monocytes, macrophages, and neutrophils [115][46]. Furthermore, shotgun metagenomic sequencing of fecal samples from 165 non-resectable advanced (stage III or IV) cutaneous melanoma patients prior to immunotherapy with ICIs including nivolumab, pembrolizumab or ipilimumab, or a combination of ipilimumab and nivolumab revealed a significant association between the composition of the gut microbiome especially the special panel of Bifidobacterium pseudocatenulatum, Roseburia spp. and Akkermansia muciniphila with ORR and PFS [116][47]. The diversity of the gut microbiome composition is also correlated with the survival rates in response to chemoradiation in patients with cervical cancer [117][48]. In a prospective observational study on microbial composition in patients with metastatic renal cell carcinoma (mRCC) who had received nivolumab or nivolumab plus ipilimumab, it was demonstrated that the high diversity of gut microbiome profiles is strongly linked to the benefits in treatment outcomes [118][49]. In a randomized clinical trial of bacterial therapy combined with dual immunotherapy, fecal metagenomic sequencing of 30 mRCC patients with histology of clear cell and/or sarcomatoid and intermediate- or poor-risk disease demonstrated that ORR and PFS were significantly longer in patients who received nivolumab plus ipilimumab with CBM588 (a butyrate-producing strain of Clostridium butyricum) [119][50]. In addition, Jin Y et al. showed that in thirty-seven patients with metastatic, advanced stage IIIb/IV or recurrent NSCLC who were recruited from two clinical trials, CheckMate 078 [NCT02613507] and CheckMate 870 [NCT03195491] and treated with nivolumab there is a strong association between intestinal microbiome diversity and the responses to anti–PD-1 immunotherapy [90][21]. The studies mentioned above indicate that gut microbiome diversity is associated with better outcomes. However, prospective studies that would couple microbiome profiling in cancer patients receiving systemic or localized therapy with their clinical outcome can further determine whether diversity can be used as a predictive biomarker of response to systemic or localized therapy in various cancers. Furthermore, microbiome-modifying strategies, such as complete consortia FMT from healthy donors that can potentially increase gut microbiome diversity, can be used as supportive therapy for cancer patients receiving systemic or localized treatment. A multicenter and retrospective study conducted by Takada K et al. has demonstrated that probiotics are linked to beneficial clinical outcomes in patients with advanced or recurrent NSCLC treated with anti-PD-1 monotherapy [120][51]. However, another study has reported paradoxical results in melanoma patients who took probiotic supplements while receiving immunotherapy and had a worse survival outcome. The obtained results are in line with the outcome of probiotic-treated mice, which have a lower frequency of IFN-γ-producing CD8+ T-cells in TME and impaired anti-tumor response compared to the controls [121][52]. A cohort study of 338 patients with NSCLC has demonstrated that intestinal Akkermansia muciniphila is significantly accompanied by clinical benefits with increased response rates and OS following PD-1 blockade [122][53]. Notably, the enhancement of bacterial compositions directly contributes to the efficacy of ICIs and improves clinical outcomes in cancer patients [123][54]. Modifying the gut microbiota in immunotherapy-refractory melanoma patients sensitized their tumors to anti-PD1 rechallenge [114][45]. FMT from patients with metastatic melanoma who had previously been treated with anti-PD-1 monotherapy and achieved complete response for at least one year to immunotherapy-refractory melanoma patients re-sensitized 30% of the treated patients to anti-PD1 treatment. FMT from patient donors modulated the immune cell infiltration and gene expression profiles in the TME [22][55]. Additionally, in another prospective study, FMT from long-term responder melanoma patients to anti-PD-1-refractory patients sensitized patients to anti-PD-1 rechallenge, further establishing the role of the gut microbiome in modulating response to immunotherapy [124][56]. In responding patients, the gut microbiota significantly shifted toward donor microbiota after FMT, and responders had decreased IL-8, IL-18, and CCL2 levels in their serum post-FMT [124][56]. Furthermore, FMT from long-term survivor patients with advanced PDAC by oral gavage to a mouse model of pancreatic cancer previously treated with antibiotics demonstrated active modification of the tumor microbiota with enriched Clostridiales, which inhibited tumor growth in an IFN-γ-producing CD8+ T-cell-dependent manner. In contrast, FMT from PDAC short term-survivors to mice resulted in an increased CD4+ Foxp3+ Tregs and MDSC infiltration [125][57]. It has been reported that intestinal microbiota composition influences the immunological complications of ICIs in solid tumors. For instance, Dubin K et al. have reported that microbiome composition in patients with metastatic melanoma who received ipilimumab treatment is significantly correlated with the development of immune-mediated colitis [126][58]. McCulloch JA et al. have reported that the abundance of Lachnospiraceae spp. and Streptococcus spp. are linked to the favorable clinical response and immune-related adverse events, respectively, upon anti-PD-1 treatment of melanoma patients [115][46]. It has also been demonstrated that increased bacterial species of Bacteroidetes phylum in the gut is significantly associated with resistance to the development of checkpoint-blockade-induced colitis [126][58]. Identifying the individual microbial species responsible for immunomodulation could open a new horizon toward personalized medicine in cancer immunotherapy. Hence, the microbiome has been identified as a robust predictive biomarker in response to immunotherapy, mainly the blockade of immune checkpoints. Intestinal Bifidobacterium pseudolongum-driven inosine metabolites promote Th1 differentiation and activation, shaping a robust immune response following anti-CTLA-4 and anti-PD-L1 immunotherapy in preclinical models of melanoma and CRC [127][59]. A preclinical study on mouse models of CRC has indicated that oral administration of Clostridiales strains actively leads to the intratumoral infiltration and activation of CD8+ T-cells. It has also been reported that the Enterococcus species secrete SagA enzyme leading to degradation of the bacterial cell wall, the release of muramyl peptide fragments, and activation of the NOD2 signaling pathway, which in turn improves the response to anti–PD-L1 immunotherapy in the mouse models of melanoma and colon cancer [128][60]. Based on these findings, it could be proposed that treatment with some bacterial strains as a limited consortium of probiotic therapy may improve the efficacy of anti-PD-1/PD-L1 therapy.References
- Lewandowska, A.M.; Rudzki, M.; Rudzki, S.; Lewandowski, T.; Laskowska, B. Environmental risk factors for cancer—Review paper. Ann. Agric. Environ. Med. 2019, 26, 1–7.
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011, 29, 235–271.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46.
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198.
- Boland, P.; Pavlick, A.C.; Weber, J.; Sandigursky, S. Immunotherapy to treat malignancy in patients with pre-existing autoimmunity. J. Immunother. Cancer 2020, 8, 1–9.
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506.
- Barbosa, A.M.; Gomes-Gonçalves, A.; Castro, A.G.; Torrado, E. Immune System Efficiency in Cancer and the Microbiota Influence. Pathobiology 2021, 88, 170–186.
- Wu, J.; Wang, S.; Zheng, B.; Qiu, X.; Wang, H.; Chen, L. Modulation of Gut Microbiota to Enhance Effect of Checkpoint Inhibitor Immunotherapy. Front. Immunol. 2021, 12, 669150.
- Szczyrek, M.; Bitkowska, P.; Chunowski, P.; Czuchryta, P.; Krawczyk, P.; Milanowski, J. Diet, Microbiome, and Cancer Immunotherapy-A Comprehensive Review. Nutrients 2021, 13, 2217.
- Vieira, A.T.; Galvão, I.; Amaral, F.A.; Teixeira, M.M.; Nicoli, J.R.; Martins, F.S. Oral treatment with Bifidobacterium longum 51A reduced inflammation in a murine experimental model of gout. Benef. Microb. 2015, 6, 799–806.
- Vieira, A.T.; Rocha, V.M.; Tavares, L.; Garcia, C.C.; Teixeira, M.M.; Oliveira, S.C.; Cassali, G.D.; Gamba, C.; Martins, F.S.; Nicoli, J.R. Control of Klebsiella pneumoniae pulmonary infection and immunomodulation by oral treatment with the commensal probiotic Bifidobacterium longum 5(1A). Microb. Infect. 2016, 18, 180–189.
- Lakritz, J.R.; Poutahidis, T.; Levkovich, T.; Varian, B.J.; Ibrahim, Y.M.; Chatzigiagkos, A.; Mirabal, S.; Alm, E.J.; Erdman, S.E. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int. J. Cancer 2014, 135, 529–540.
- Liu, C.Y.; Xu, J.Y.; Shi, X.Y.; Huang, W.; Ruan, T.Y.; Xie, P.; Ding, J.L. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab. Investig. 2013, 93, 844–854.
- Kim, Y.G.; Udayanga, K.G.; Totsuka, N.; Weinberg, J.B.; Núñez, G.; Shibuya, A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE₂. Cell Host Microbe 2014, 15, 95–102.
- Li, R.; Zhou, R.; Wang, H.; Li, W.; Pan, M.; Yao, X.; Zhan, W.; Yang, S.; Xu, L.; Ding, Y.; et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019, 26, 2447–2463.
- Pu, Y.; Ji, Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front. Immunol. 2022, 13, 874589.
- Hezaveh, K.; Shinde, R.S.; Klötgen, A.; Halaby, M.J.; Lamorte, S.; Ciudad, M.T.; Quevedo, R.; Neufeld, L.; Liu, Z.Q.; Jin, R.; et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 2022, 55, 324–340.e328.
- Thiele Orberg, E.; Fan, H.; Tam, A.J.; Dejea, C.M.; Destefano Shields, C.E.; Wu, S.; Chung, L.; Finard, B.B.; Wu, X.; Fathi, P.; et al. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. 2017, 10, 421–433.
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568.
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355.
- Jin, Y.; Dong, H.; Xia, L.; Yang, Y.; Zhu, Y.; Shen, Y.; Zheng, H.; Yao, C.; Wang, Y.; Lu, S. The Diversity of Gut Microbiome is Associated With Favorable Responses to Anti-Programmed Death 1 Immunotherapy in Chinese Patients With NSCLC. J. Thorac. Oncol. 2019, 14, 1378–1389.
- Lin, S.J.; Yan, D.C.; Lee, W.I.; Kuo, M.L.; Hsiao, H.S.; Lee, P.Y. Effect of azithromycin on natural killer cell function. Int. Immunopharmacol. 2012, 13, 8–14.
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108.
- Jiang, L.; Xiao, X.; Ren, J.; Tang, Y.; Weng, H.; Yang, Q.; Wu, M.; Tang, W. Proteomic analysis of bladder cancer indicates Prx-I as a key molecule in BI-TK/GCV treatment system. PLoS ONE 2014, 9, e98764.
- Yoon, Y.; Kim, G.; Jeon, B.N.; Fang, S.; Park, H. Bifidobacterium Strain-Specific Enhances the Efficacy of Cancer Therapeutics in Tumor-Bearing Mice. Cancers 2021, 13, 957.
- Demin, N.N.; Karmanova, I.G.; Rubinskaya, N.L.; Khomutetskaya, O.E. Comparative neurochemical and physiological characteristics of catalepsy-type rest and sleep. Neurosci. Behav. Physiol. 1978, 9, 98–102.
- Li, Y.; Tinoco, R.; Elmen, L.; Segota, I.; Xian, Y.; Fujita, Y.; Sahu, A.; Zarecki, R.; Marie, K.; Feng, Y.; et al. Gut microbiota dependent anti-tumor immunity restricts melanoma growth in Rnf5(-/-) mice. Nat. Commun. 2019, 10, 1492.
- Russell, B.L.; Sooklal, S.A.; Malindisa, S.T.; Daka, L.J.; Ntwasa, M. The Tumor Microenvironment Factors That Promote Resistance to Immune Checkpoint Blockade Therapy. Front. Oncol. 2021, 11, 641428.
- Ghosh, C.; Luong, G.; Sun, Y. A snapshot of the PD-1/PD-L1 pathway. J. Cancer 2021, 12, 2735–2746.
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106.
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of immune checkpoints-PD-1, PD-L1, CTLA-4-new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021, 40, 949–982.
- Dai, Z.; Zhang, J.; Wu, Q.; Fang, H.; Shi, C.; Li, Z.; Lin, C.; Tang, D.; Wang, D. Intestinal microbiota: A new force in cancer immunotherapy. Cell Commun. Signal. 2020, 18, 90.
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416.
- Chen, J.; Li, T.; Liang, J.; Huang, Q.; Huang, J.D.; Ke, Y.; Sun, H. Current status of intratumour microbiome in cancer and engineered exogenous microbiota as a promising therapeutic strategy. Biomed. Pharmacother. 2022, 145, 112443.
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160.
- Kalaora, S.; Lee, J.S.; Barnea, E.; Levy, R.; Greenberg, P.; Alon, M.; Yagel, G.; Bar Eli, G.; Oren, R.; Peri, A.; et al. Immunoproteasome expression is associated with better prognosis and response to checkpoint therapies in melanoma. Nat. Commun. 2020, 11, 896.
- Norton, J.E., Jr.; Kommineni, S.; Akrivoulis, P.; Gutierrez, D.A.; Hazuda, D.J.; Swaminathan, G. Primary Human Dendritic Cells and Whole-Blood Based Assays to Evaluate Immuno-Modulatory Properties of Heat-Killed Commensal Bacteria. Vaccines 2021, 9, 225.
- Montalban-Arques, A.; Katkeviciute, E.; Busenhart, P.; Bircher, A.; Wirbel, J.; Zeller, G.; Morsy, Y.; Borsig, L.; Glaus Garzon, J.F.; Müller, A.; et al. Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host Microbe 2021, 29, 1573–1588.e1577.
- Inamura, K. Roles of microbiota in response to cancer immunotherapy. Semin. Cancer Biol. 2020, 65, 164–175.
- Routy, B.; Gopalakrishnan, V.; Daillere, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396.
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97.
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 2012, 7, e39743.
- Wu, N.; Yang, X.; Zhang, R.; Li, J.; Xiao, X.; Hu, Y.; Chen, Y.; Yang, F.; Lu, N.; Wang, Z.; et al. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb. Ecol. 2013, 66, 462–470.
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280.
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103.
- McCulloch, J.A.; Davar, D.; Rodrigues, R.R.; Badger, J.H.; Fang, J.R.; Cole, A.M.; Balaji, A.K.; Vetizou, M.; Prescott, S.M.; Fernandes, M.R.; et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 2022, 28, 545–556.
- Lee, K.A.; Thomas, A.M.; Bolte, L.A.; Bjork, J.R.; de Ruijter, L.K.; Armanini, F.; Asnicar, F.; Blanco-Miguez, A.; Board, R.; Calbet-Llopart, N.; et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat. Med. 2022, 28, 535–544.
- Sims, T.T.; El Alam, M.B.; Karpinets, T.V.; Dorta-Estremera, S.; Hegde, V.L.; Nookala, S.; Yoshida-Court, K.; Wu, X.; Biegert, G.W.G.; Delgado Medrano, A.Y.; et al. Gut microbiome diversity is an independent predictor of survival in cervical cancer patients receiving chemoradiation. Commun. Biol. 2021, 4, 237.
- Salgia, N.J.; Bergerot, P.G.; Maia, M.C.; Dizman, N.; Hsu, J.; Gillece, J.D.; Folkerts, M.; Reining, L.; Trent, J.; Highlander, S.K.; et al. Stool Microbiome Profiling of Patients with Metastatic Renal Cell Carcinoma Receiving Anti-PD-1 Immune Checkpoint Inhibitors. Eur. Urol. 2020, 78, 498–502.
- Dizman, N.; Meza, L.; Bergerot, P.; Alcantara, M.; Dorff, T.; Lyou, Y.; Frankel, P.; Cui, Y.; Mira, V.; Llamas, M.; et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat. Med. 2022, 28, 704–712.
- Takada, K.; Shimokawa, M.; Takamori, S.; Shimamatsu, S.; Hirai, F.; Tagawa, T.; Okamoto, T.; Hamatake, M.; Tsuchiya-Kawano, Y.; Otsubo, K.; et al. Clinical impact of probiotics on the efficacy of anti-PD-1 monotherapy in patients with nonsmall cell lung cancer: A multicenter retrospective survival analysis study with inverse probability of treatment weighting. Int. J. Cancer 2021, 149, 473–482.
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640.
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 2022, 28, 315–324.
- Cheng, P.; Shen, P.; Shan, Y.; Yang, Y.; Deng, R.; Chen, W.; Lu, Y.; Wei, Z. Gut Microbiota-Mediated Modulation of Cancer Progression and Therapy Efficacy. Front. Cell Dev. Biol. 2021, 9, 626045.
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609.
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602.
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e712.
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391.
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489.
- Griffin, M.E.; Espinosa, J.; Becker, J.L.; Luo, J.D.; Carroll, T.S.; Jha, J.K.; Fanger, G.R.; Hang, H.C. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science 2021, 373, 1040–1046.
