Bacterial lipopolysaccharides (LPS), also referred to as endotoxins, are major outer surface membrane components present on almost all Gram-negative bacteria and are major determinants of sepsis-related clinical complications including septic shock. LPS acts as a strong stimulator of innate or natural immunity in a wide variety of eukaryotic species ranging from insects to humans including specific effects on the adaptive immune system. However, following immune stimulation, lipopolysaccharide can induce tolerance which is an essential immune-homeostatic response that prevents overactivation of the inflammatory response.
Bacterial lipopolysaccharides (LPS), also referred to as endotoxins, are major outer surface membrane components present on almost all Gram-negative bacteria and are major determinants of sepsis-related clinical complications including septic shock. LPS acts as a strong stimulator of innate or natural immunity in a wide variety of eukaryotic species ranging from insects to humans including specific effects on the adaptive immune system.
- immunological tolerance
- LPS
- cholera toxin B
- Indoleamine 2,3 dioxygenase
1. Introduction
2. Function of LPS
2.1. Virulence and Toxicity
Lipid A, which is the toxic component of LPS, and polysaccharide side chains, which are considered the non-toxic but immunogenic part of LPS, act as virulence determinants in Gram-negative bacteria [46,47,48][46][47][48]. O antigens have adhesive properties, phagocyte resistance, antigen protection, and antigen mutation properties [47,49][47][49]. Lipid A acts as an immunostimulant that induces biological responses to a specific organism [50,51,52][50][51][52].2.2. Biological Activity of Lipopolysaccharide
An animal’s biological immune responses can be analyzed using various parameters, such as an injection of live or killed Gram-negative cells or purified LPS in laboratory animals, which causes a broad spectrum of pathophysiological responses, such as fever, changes in blood counts, disseminated intravascular coagulation white blood cells, hypotension, and shock resulting in death. Injecting very small doses of endotoxin can cause death in most mammals. The sequence of events follows a regular pattern: (1) latency period; (2) physiological stress (diarrhea, exhaustion, shock); and (3) death. The rate at which death occurs depends on the dose of the endotoxin, the route of administration of the toxin, and the animal species.3. Lipopolysaccharide Signaling and Immune Activation Mechanisms in Higher Organisms
3.1. Lipopolysaccharide Detoxification Mechanisms in Higher Animals
The defense against infection in vertebrates is mediated by two interdependent arms of the immune system, known as innate and adaptive portions of the immune system. The innate immune system, consisting of antigen presenting cells, recognizes a diverse array of non-self-antigens and if overwhelmed, can signal and activate the adaptive immune system through well-established signaling pathways to stimulate an array of T-cells and B-cells to overcome the pathogen [53]. As LPS can have significant adverse effects on animals and humans, a process to detoxify LPS has been developed [54]. The detoxification mechanism of LPS occurs through enzymatic degradation or through complement-mediated detoxification, which leads to the breakdown of LPS.3.2. Host-Microbe Interactions (Lipopolysaccharide Activity) in Invertebrates—Insects
The innate immune system of insects plays an important role in the development of immunity [55]. In recent years, arthropods and insects have become the most useful models for describing the molecular regulation of the innate immune response [56]. Insects have highly effective defense mechanisms against invasive microorganisms, which include Gram-negative and Gram-positive molecules, LPS, and peptidoglycan [55,56,57][55][56][57]. These insect defense mechanisms include cellular and humoral responses. Cellular responses include phagocytosis and/or encapsulation of large parasites by bacterial nodules and blood cells [58]. In addition, the humoral response uses various antimicrobial peptides which are synthesized in the adipose body and some hemocytes after induction by septic lesions and which are then secreted into the hemolymph [59,60,61][59][60][61]. The insect defense system against LPS pathogens results in a transient increase in antimicrobial activity in the acellular hemolymph, including phagocytosis and encapsulation of invaders by blood cells and subsequent production of antimicrobial proteins (mainly in the insect’s adipose tissue) [62]. Strong immunoreactivity was found in the interaction between Galleria mellonella (large wax moth) and LPS. The high tolerance of LPS to insects can be explained by an extremely effective detoxification mechanism involving the binding of LPS to hemolymph lipophorins [63]. This observation suggests that LPS has the potential to induce immune activation. Activation of the proteolytic cascade and coagulation cascade using LPS triggers the limulus hemocyte to act as a signaling mechanism [64,65,66][64][65][66]. In addition, a blood cell membrane receptor for LPS has been isolated from Bombyx mori silkworm that can transmit an activation signal for the synthesis of the antibacterial peptide cecropin B [67,68,69][67][68][69].3.3. Expression of Genes and Signaling Action Induced by Lipopolysaccharide in Vertebrates and Invertebrates
It is a general phenomenon that antibacterial protein gene expression culminates a few hours after bacterial infection and decreases over time in vertebrates. This reduction in antibacterial protein gene expression has been shown to correlate with LPS deprivation [70,71][70][71]. TLRs, a class of pattern recognition receptors (PRRs) found in vertebrates, play an important role not only in initiating innate immunity, but also in activating adaptive immunity (Figure 1).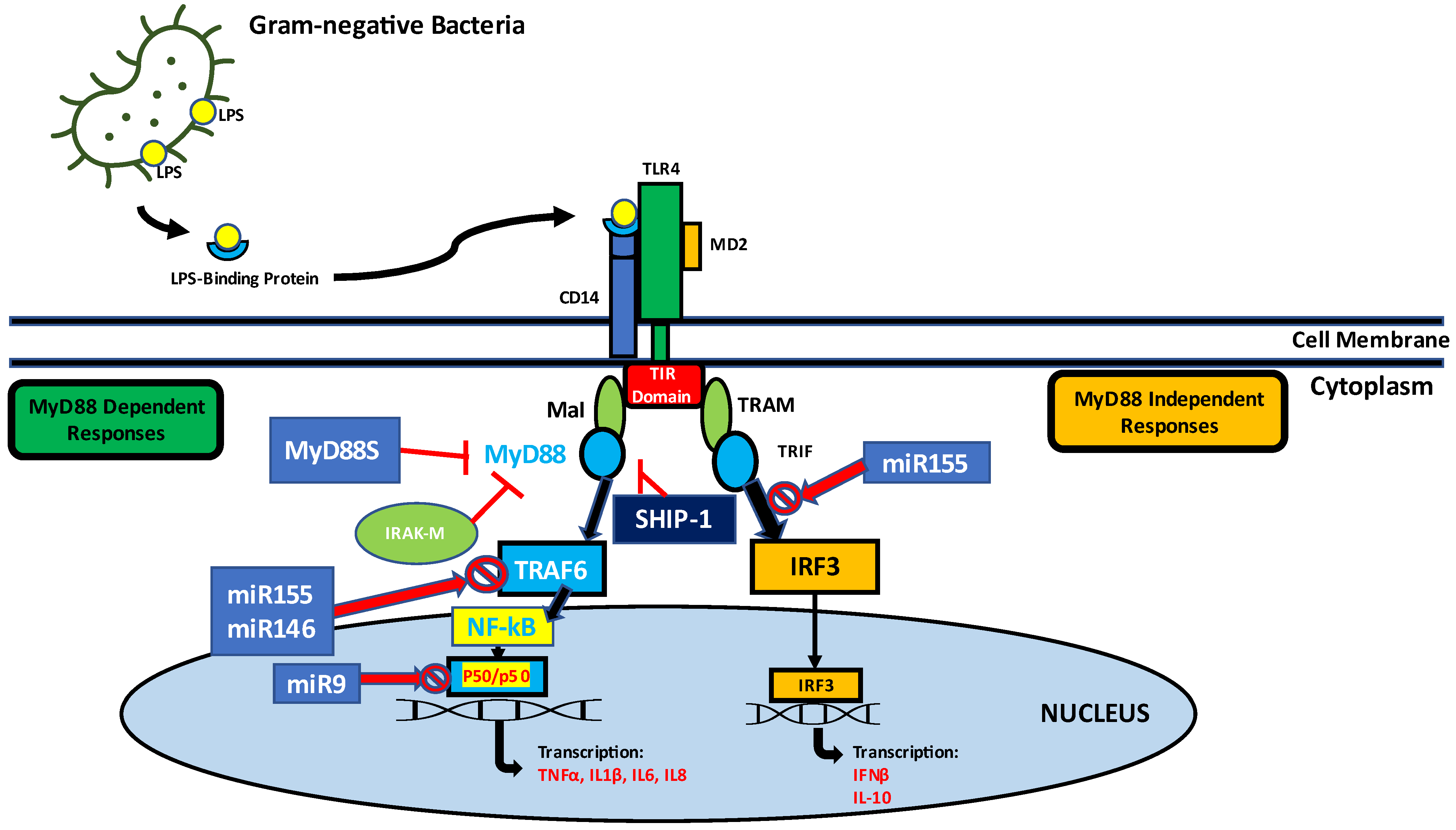
References
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8.
- Alexander, T.E.; Smith, I.M.; Lipsky, Z.W.; Lozeau, L.D.; Camesano, T.A. Role of lipopolysaccharides and lipoteichoic acids on C-Chrysophsin-1 interactions with model Gram-positive and Gram-negative bacterial membranes. Biointerphases 2020, 15, 031007.
- Brandenburg, K.; Schromm, A.B.; Weindl, G.; Heinbockel, L.; Correa, W.; Mauss, K.; de Tejada, G.M.; Garidel, P. An update on endotoxin neutralization strategies in Gram-negative bacterial infections. Expert Rev. Anti-Infect. Ther. 2020, 19, 495–517.
- Giordano, N.P.; Cian, M.B.; Dalebroux, Z.D. Outer Membrane Lipid Secretion and the Innate Immune Response to Gram-Negative Bacteria. Infect. Immun. 2020, 88, e00920-19.
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.-Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088.
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995.
- Rosadini, C.V.; Kagan, J.C. Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 2016, 44, 14–19.
- Tan, Y.; Kagan, J.C. A Cross-Disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol. Cell 2014, 54, 212–223.
- Kent, L.W.; Rahemtulla, F.; Hockett, R.D.; Gilleland, R.C.; Michalek, S.M. Effect of lipopolysaccharide and inflammatory cytokines on Interleukin-6 production by healthy human gingival fibroblasts. Infect. Immun. 1998, 66, 608–614.
- Choi, J.; Moon, S.; Bae, H.; Kim, Y.-W.; Lee, D.; Kim, S.; Seo, Y.; Wang, H.S.; Choi, Y.W.; Lee, M.W.; et al. Alnus Sibirica Extracts Suppress the Expression of Inflammatory Cytokines Induced by Lipopolysaccharides, Tumor Necrosis Factor-α, and Interferon-γ in Human Dermal Fibroblasts. Molecules 2019, 24, 2883.
- Bonham, K.; Orzalli, M.H.; Hayashi, K.; Wolf, A.I.; Glanemann, C.; Weninger, W.; Iwasaki, A.; Knipe, D.M.; Kagan, J.C. A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell 2014, 156, 705–716.
- Lai, J.; Ge, M.; Shen, S.; Yang, L.; Jin, T.; Cao, D.; Xu, H.; Zheng, X.; Qiu, S.; Wang, K.; et al. Activation of NFKB-JMJD3 signaling promotes bladder fibrosis via boosting bladder smooth muscle cell proliferation and collagen accumulation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 2403–2410.
- Min, Y.; Kim, M.-J.; Lee, S.; Chun, E.; Lee, K.-Y. Inhibition of TRAF6 ubiquitin-ligase activity by PRDX1 leads to inhibition of NFKB activation and autophagy activation. Autophagy 2018, 14, 1347–1358.
- Wang, P.; Zhou, S.; Ge, Y.; Lu, M.; Liu, Z.; Gong, R. Valproate hampers podocyte acquisition of immune phenotypes via intercepting the GSK3β facilitated NFkB activation. Oncotarget 2017, 8, 88332–88344.
- Van Acker, T.; Eyckerman, S.; Walle, L.V.; Gerlo, S.; Goethals, M.; Lamkanfi, M.; Bovijn, C.; Tavernier, J.; Peelman, F. The small GTPase Arf6 is essential for the Tram/Trif pathway in TLR4 signaling. J. Biol. Chem. 2014, 289, 1364–1376.
- Wang, Y.; Yang, Y.; Liu, X.; Wang, N.; Cao, H.; Lu, Y.; Zhou, H.; Zheng, J. Inhibition of clathrin/dynamin-dependent internalization interferes with LPS-mediated TRAM–TRIF-dependent signaling pathway. Cell. Immunol. 2012, 274, 121–129.
- Zhang, S.; Yuquan, W.; Guo, Q.; Li, R.; Li, G.-B.; Tan, S.; Li, X.; Wei, Y.; Wu, M. Annexin A2 binds to endosomes and negatively regulates TLR4-triggered inflammatory responses via the TRAM-TRIF pathway. Sci. Rep. 2015, 5, 15859.
- Hilliard, A.; Mendonca, P.; Soliman, K.F. Involvement of NFƙB and MAPK signaling pathways in the preventive effects of Ganoderma lucidum on the inflammation of BV-2 microglial cells induced by LPS. J. Neuroimmunol. 2020, 345, 577269.
- Warren, H.S.; Fitting, C.; Hoff, E.; Adib-Conquy, M.; Beasley-Topliffe, L.; Tesini, B.; Liang, X.; Valentine, C.; Hellman, J.; Hayden, D.; et al. Resilience to bacterial infection: Difference between species could be due to proteins in serum. J. Infect. Dis. 2010, 201, 223–232.
- Reid, R.R.; Prodeus, A.P.; Khan, W.; Hsu, T.; Rosen, F.S.; Carroll, M.C. Endotoxin shock in antibody-deficient mice: Unraveling the role of natural antibody and complement in the clearance of lipopolysaccharide. J. Immunol. 1997, 159, 970–975.
- Boes, M.; Prodeus, A.P.; Schmidt, T.; Carroll, M.C.; Chen, J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 1998, 188, 2381–2386.
- Zhou, F.; Zhang, G.-X.; Rostami, A. LPS-treated bone marrow-derived dendritic cells induce immune tolerance through modulating differentiation of CD4+ regulatory T cell subpopulations mediated by 3G11 and CD127. Immunol. Res. 2016, 65, 630–638.
- Hayashi, T.; Gray, C.S.; Chan, M.; Tawatao, R.I.; Ronacher, L.; McGargill, M.A.; Datta, S.K.; Carson, D.A.; Corr, M. Prevention of autoimmune disease by induction of tolerance to Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 2009, 106, 2764–2769.
- Mbongue, J.; Nicholas, D.; Firek, A.; Langridge, W. The role of dendritic cells in tissue-specific autoimmunity. J. Immunol. Res. 2014, 2014.
- Joffre, O.; Nolte, M.A.; Spörri, R.; Sousa, C.R.E. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 2009, 227, 234–247.
- Kumar, H.; Kawai, T.; Akira, S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625.
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426.
- Galkina, E.; Ley, K. Immune and Inflammatory Mechanisms of Atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197.
- Oyoshi, M.K.; He, R.; Kumar, L.; Yoon, J.; Geha, R.S. Cellular and molecular mechanisms in atopic dermatitis. Adv. Immunol. 2009, 102, 135–226.
- Said, E.A.; Dupuy, F.P.; Trautmann, L.; Zhang, Y.; Shi, Y.; El-Far, M.; Hill, B.J.; Noto, A.; Ancuta, P.; Peretz, Y.; et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 2010, 16, 452–459.
- Agarwal, S.; Piesco, N.; Johns, L.; Riccelli, A. Differential Expression of IL-1β, TNF-α, IL-6, and IL-8 in Human Monocytes in Response to Lipopolysaccharides from Different Microbes. J. Dent. Res. 1995, 74, 1057–1065.
- Bryn, T.; Yaqub, S.; Mahic, M.; Henjum, K.; Aandahl, E.M.; Taskén, K. LPS-activated monocytes suppress T-cell immune responses and induce FOXP3+ T cells through a COX-2-PGE2-dependent mechanism. Int. Immunol. 2008, 20, 235–245.
- Adib-Conquy, M.; Adrie, C.; Moine, P.; Asehnoune, K.; Fitting, C.; Pinsky, M.R.; Dhainaut, J.F.; Cavaillon, J.M. NF-kappaB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am. J. Respir. Crit. Care Med. 2000, 162, 1877–1883.
- López-Collazo, E.; del Fresno, C. Pathophysiology of endotoxin tolerance: Mechanisms and clinical consequences. Crit. Care 2013, 17, 242.
- Randolph, G.J.; Jakubzick, C.; Qu, C. Antigen presentation by monocytes and monocyte-derived cells. Curr. Opin. Immunol. 2008, 20, 52–60.
- Morin, J.; Faideau, B.; Gagnerault, M.; Lepault, F.; Boitard, C.; Boudaly, S. Passive transfer of flt-3L-derived dendritic cells delays diabetes development in NOD mice and associates with early production of interleukin (IL)-4 and IL-10 in the spleen of recipient mice. Clin. Exp. Immunol. 2003, 134, 388–395.
- Kim, N.-S.; Torrez, T.; Langridge, W. LPS enhances CTB-INSULIN induction of IDO1 and IL-10 synthesis in human dendritic cells. Cell. Immunol. 2019, 338, 32–42.
- Klaska, I.P.; Muckersie, E.; Martin-Granados, C.; Christofi, M.; Forrester, J.V. Lipopolysaccharide-primed heterotolerant dendritic cells suppress experimental autoimmune uveoretinitis by multiple mechanisms. Immunology 2016, 150, 364–377.
- Braun-Fahrländer, C.; Riedler, J.; Herz, U.; Eder, W.; Waser, M.; Grize, L.; Maisch, S.; Carr, D.; Gerlach, F.; Bufe, A.; et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 2002, 347, 869–877.
- Bashir, M.E.H.; Louie, S.; Shi, H.N.; Nagler-Anderson, C. Toll-Like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J. Immunol. 2004, 172, 6978–6987.
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 1551.
- Mbongue, J.C.; Nicholas, D.A.; Zhang, K.; Kim, N.S.; Hamilton, B.N.; Larios, M.; Zhang, G.; Umezawa, K.; Firek, A.F.; Langridge, W.H. Induction of indoleamine 2, 3-dioxygenase in human dendritic cells by a cholera toxin B subunit-proinsulin vaccine. PLoS ONE 2015, 10, e0118562.
- Kim, N.-S.; Mbongue, J.C.; Nicholas, D.A.; Esebanmen, G.E.; Unternaehrer, J.J.; Firek, A.F.; Langridge, W.H.R. Chimeric Vaccine Stimulation of Human Dendritic Cell Indoleamine 2, 3-Dioxygenase Occurs via the Non-Canonical NF-κB Pathway. PLoS ONE 2016, 11, e0147509.
- Zariri, A.; van der Ley, P. Biosynthetically engineered lipopolysaccharide as vaccine adjuvant. Expert Rev. Vaccines 2015, 14, 861–876.
- Chilton, P.M.; Hadel, D.M.; To, T.T.; Mitchell, T.C.; Darveau, R.P. Adjuvant activity of naturally occurring monophosphoryl lipopolysaccharide preparations from mucosa-associated bacteria. Infect. Immun. 2013, 81, 3317–3325.
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700.
- Gaspar, J.A.; Thomas, J.A.; Marolda, C.L.; Valvano, M.A. Surface expression of O-specific lipopolysaccharide in Escherichia coli requires the function of the TolA protein. Mol. Microbiol. 2000, 38, 262–275.
- Whitfield, C. Biosynthesis and Assembly of Capsular Polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006, 75, 39–68.
- Vinés, E.D.; Marolda, C.L.; Balachandran, A.; Valvano, M.A. Defective O-Antigen polymerization in tolA and pal mutants of Escherichia coli in response to Extracytoplasmic stress. J. Bacteriol. 2005, 187, 3359–3368.
- Hancock, R.E.; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000, 8, 402–410.
- Papo, N.; Shai, Y. A molecular mechanism for lipopolysaccharide protection of gram-negative bacteria from antimicrobial peptides. J. Biol. Chem. 2005, 280, 10378–10387.
- Surapaneni, K.M.; Vishnu Priya, V.; Mallika, J. Effect of pioglitazone, quercetin, and hydroxy citric acid on vascular endothelial growth factor messenger RNA (VEGF mRNA) expression in experimentally induced nonalcoholic steatohepatitis (NASH). Turk. J. Med. Sci. 2015, 45, 542–546.
- Nitkin, C.R.; Xia, S.; Menden, H.; Yu, W.; Xiong, M.; Heruth, D.P.; Ye, S.Q.; Sampath, V. FOSL1 is a novel mediator of endotoxin/lipopolysaccharide-induced pulmonary angiogenic signaling. Sci. Rep. 2020, 10, 1–14.
- Mamat, U.; Wilke, K.; Bramhill, D.; Schromm, A.B.; Lindner, B.; Kohl, T.A.; Corchero, J.L.; Villaverde, A.; Schaffer, L.; Head, S.R.; et al. Detoxifying Escherichia coli for endotoxin-free production of recombinant proteins. Microb. Cell Factories 2015, 14, 57.
- Hoffmann, J.A. The immune response of Drosophila. Nature 2003, 426, 33–38.
- Royet, J. Drosophila melanogaster innate immunity: An emerging role for peptidoglycan recognition proteins in bacteria detection. Experientia 2004, 61, 537–546.
- Hultmark, D. Immune reactions in Drosophila and other insects: A model for innate immunity. Trends Genet. 1993, 9, 178–183.
- Lackie, A. Immune mechanisms in insects. Parasitol. Today 1988, 4, 98–105.
- Cociancich, S.; Bulet, P.; Hetru, C.; Hoffmann, J. The inducible antibacterial peptides of insects. Parasitol. Today 1994, 10, 132–139.
- Cociancich, S.; Dupont, A.; Hegy, G.; Lanot, R.; Holder, F.; Hetru, C.; Hoffmann, J.A.; Bulet, P. Novel inducible antibacterial peptides from a hemipteran insect, the sap-sucking bug Pyrrhocoris apterus. Biochem. J. 1994, 300, 567–575.
- Koizumi, N.; Imamura, M.; Kadotani, T.; Yaoi, K.; Iwahana, H.; Sato, R. The lipopolysaccharide-binding protein participating in hemocyte nodule formation in the silkworm Bombyx mori is a novel member of the C-type lectin superfamily with two different tandem carbohydrate-recognition domains. FEBS Lett. 1999, 443, 139–143.
- Wittwer, D.; Weise, C.; Götz, P.; Wiesner, A. LPS (Lipopolysaccharide)-activated immune responses in a hemocyte cell line from Estigmene acraea (Lepidoptera). Dev. Comp. Immunol. 1997, 21, 323–336.
- Kato, Y.; Motoi, Y.; Taniai, K.; Kadono-Okuda, K.; Yamamoto, M.; Higashino, Y.; Shimabukuro, M.; Chowdhury, S.; Xu, J.; Sugiyama, M.; et al. Lipopolysaccharide-lipophorin complex formation in insect hemolymph: A common pathway of lipopolysaccharide detoxification both in insects and in mammals. Insect Biochem. Mol. Biol. 1994, 24, 547–555.
- Kawabata, S.-I.; Nagayama, R.; Hirata, M.; Shigenaga, T.; Agarwala, K.L.; Saito, T.; Cho, J.; Nakajima, H.; Takagi, T.; Iwanaga, S. Tachycitin, a Small Granular Component in Horseshoe Crab Hemocytes, Is an Antimicrobial Protein with Chitin-Binding Activity. J. Biochem. 1996, 120, 1253–1260.
- Kawabata, S.-I.; Saeki, K.; Iwanaga, S. Limulus kexin: A new type of Kex2-like endoprotease specifically expressed in hemocytes of the horseshoe crab. FEBS Lett. 1996, 386, 201–204.
- Kawabata, S.-I.; Tokunaga, F.; Kugi, Y.; Motoyama, S.; Miura, Y.; Hirata, M.; Iwanaga, S. Limulus factor D, a 43-kDa protein isolated from horseshoe crab hemocytes, is a serine protease homologue with antimicrobial activity. FEBS Lett. 1996, 398, 146–150.
- Xu, W.-H.; Sato, Y.; Ikeda, M.; Yamashita, O. Molecular characterization of the gene encoding the precursor protein of diapause hormone and pheromone biosynthesis activating neuropeptide (DH-PBAN) of the silkworm, Bombyx mori and its distribution in some insects. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1995, 1261, 83–89.
- Xu, W.-H.; Sato, Y.; Ikeda, M.; Yamashita, O. Stage-dependent and temperature-controlled expression of the gene encoding the precursor protein of diapause hormone and pheromone biosynthesis activating neuropeptide in the silkworm, bombyx mori. J. Biol. Chem. 1995, 270, 3804–3808.
- Sugiyama, M.; Kuniyoshi, H.; Kotani, E.; Taniai, K.; Kadono-Okuda, K.; Kato, Y.; Yamamoto, M.; Shimabukuro, M.; Chowdhury, S.; Xu, J.; et al. Characterization of a Bombyx mori cDNA encoding a novel member of the attacin family of insect antibacterial proteins. Insect Biochem. Mol. Biol. 1995, 25, 385–392.
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14.
- Kang, D.; Liu, G.; Lundström, A.; Gelius, E.; Steiner, H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc. Natl. Acad. Sci. USA 1998, 95, 10078–10082.
- Fabrick, J.; Baker, J.; Kanost, M. cDNA cloning, purification, properties, and function of a β-1,3-glucan recognition protein from a pyralid moth, Plodiainterpunctella. Insect Biochem. Mol. Biol. 2003, 33, 579–594.
- Ma, C.; Kanost, M. A β1,3-Glucan Recognition protein from an insect, manduca sexta, agglutinates microorganisms and activates the Phenoloxidase cascade. J. Biol. Chem. 2000, 275, 7505–7514.
- Ochiai, M.; Ashida, M. Purification of a beta-1,3-glucan recognition protein in the prophenoloxidase activating system from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 1988, 263, 12056–12062.
- Ashida, M.; Ochiai, M.; Niki, T. Immunolocalization of prophenoloxidase among hemocytes of the silkworm, Bombyx mori. Tissue Cell 1988, 20, 599–610.
- Dimopoulos, G.; Richman, A.; Müller, H.M.; Kafatos, F.C. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc. Natl. Acad. Sci. USA 1997, 94, 11508–11513.
- Dimopoulos, G.; Seeley, D.; Wolf, A.; Kafatos, F.C. Malaria infection of the mosquito Anopheles gambiae activates immune-responsive genes during critical transition stages of the parasite life cycle. EMBO J. 1998, 17, 6115–6123.
- Yeh, M.-S.; Lai, C.-Y.; Liu, C.-H.; Kuo, C.-M.; Cheng, W. A second proPO present in white shrimp Litopenaeus vannamei and expression of the proPOs during a Vibrio alginolyticus injection, molt stage, and oral sodium alginate ingestion. Fish Shellfish Immunol. 2009, 26, 49–55.
- Yeh, M.-S.; Liu, C.-H.; Hung, C.-W.; Cheng, W. cDNA cloning, identification, tissue localisation, and transcription profile of a transglutaminase from white shrimp, Litopenaeus vannamei, after infection by Vibrio alginolyticus. Fish Shellfish Immunol. 2009, 27, 748–756.
- Litman, G.W.; Rast, J.P.; Fugmann, S.D. The origins of vertebrate adaptive immunity. Nat. Rev. Immunol. 2010, 10, 543–553.
- Miller, J.S.; Nguyen, T.; Stanley-Samuelson, D.W. Eicosanoids mediate insect nodulation responses to bacterial infections. Proc. Natl. Acad. Sci. USA 1994, 91, 12418–12422.
- Jomori, T.; Natori, S. Function of the lipopolysaccharide-binding protein of Periplaneta americana as an opsonin. FEBS Lett 1992, 296, 283–286.
- Shigenaga, T.; Takayenoki, Y.; Kawasaki, S.; Seki, N.; Muta, T.; Toh, Y.; Ito, A.; Iwanaga, S. Separation of large and small granules from horseshoe crab (Tachypleus tridentatus) hemocytes and characterization of their components1. J. Biochem. 1993, 114, 307–316.
- Marmaras, V.J.; Charalambidis, N.D.; Zervas, C. Immune response in insects: The role of phenoloxidase in defense reactions in relation to melanization and sclerotization. Arch. Insect Biochem. Physiol. 1996, 31, 119–133.
