Antigorite is a Mg-rich 1:1 trioctahedral-structured layered silicate mineral of the serpentine group. Antigorite with layered structure can be used as a lubricant and friction reducing material to repair the friction pair of iron agent on line.
- Antigorite
- Tribological performance
- Lubricity
- Anti-wear
1. Introduction
1. Introduction
Antigorite is a Mg-rich 1:1 trioctahedral-structured layered silicate mineral of the serpentine group.
Layered minerals are known as laminar structure minerals or layer lattice minerals, have layer crystal structure in which the atoms within a layer are held together by a chemical bond stronger than the bond between the layers. This provides an isotropic shear property with preferred easy shear parallel to the basal planes of the crystallites, and result in the ability of basal planes to slide easily over one another
[1]
. This may be the reason that layered minerals can provide low friction coefficients and be used as solid lubricants
.
The earlier researches on frictional behavior of antigorite were conducted by geologists and geophysicists, with the intent of seeking information about the strength and sliding stability of natural faults containing antigorite
. In the recent decades, there has been considerable interest in using antigorite to reduce friction and wear of machinery and equipment. Significant progress has been achieved in the research and development of antigorite’s application in the lubrication of machine components. The research results indicated that antigorite micro–nano powder (AMNP) may significantly reduce friction coefficient and wear of friction pairs, and be one kind of excellent anti-frictional and lubrication materials
[12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27]
.
2. Mineralogy and Powder Characteristics of Antigorite
The theoretical chemical composition of antigorite is SiO
2
43.37%, MgO 43.63%, H
2
O 13.00%, with an ideal formula of Mg
6
Si
4
O
10
(OH)
8
. They can exhibit the substitution of Si
4+
cations by Al
3+
, Fe
3+
, or Cr
3+
, and Mg
2+
by Fe
2+
, Mn
2+
, Ca
2+
, or Ni
2+
. The actual composition of naturally occurring antigorites exhibits variation in the SiO
2
, MgO and H
2
O, and minor proportion of Al
2
O
3
, FeO, Fe
2
O
3
, MnO, CaO, Cr
2
O
3
and NiO are also present
.
Antigorite belongs to the group of trioctahedral 1:1 layered silicates, consisting of one tetrahedral (T) and one octahedral (O) sheet. The T sheet is formed by the two-dimensional polymerization of Si-centered tetrahedra sharing three out of four oxygen atoms with other tetrahedra. The unshared oxygen atoms are bonded to Mg atoms that jointly with OH groups form the octahedral sheets (O sheet). The T- and O- sheets are variably arranged and stacked one above another, and the thickness of the T-sheet is thinner than that of the O- sheet, resulting is a subtle dimensional misfit between the two. This misfit can be reduced by substitutions in the tetrahedron, which in turn reduces interlayer strain and consequently enhances the mineral’s stability
. The layers in the structure are linked by hydrogen bonds that form by pairing oxygen on the basal tetrahedral surface of one layer with an OH-group on the upper octahedral surface of the layer below. These bonds are generally long and weak but can be modified by the degree of substitution
[33]
. This crystal structure gives rise to typically platy or lamellar along (001) and perfect cleavages on {001} of antigorite.
Mohs hardness of antigorite is between 2.5 to 3.5, the Vickers microhardness are within a range from 196.3 to 204.9
[4]
. Its theoretical and measured densities are 2.61 g/cm
3
and about 2.65 g/cm
3
, respectively
[34]
.
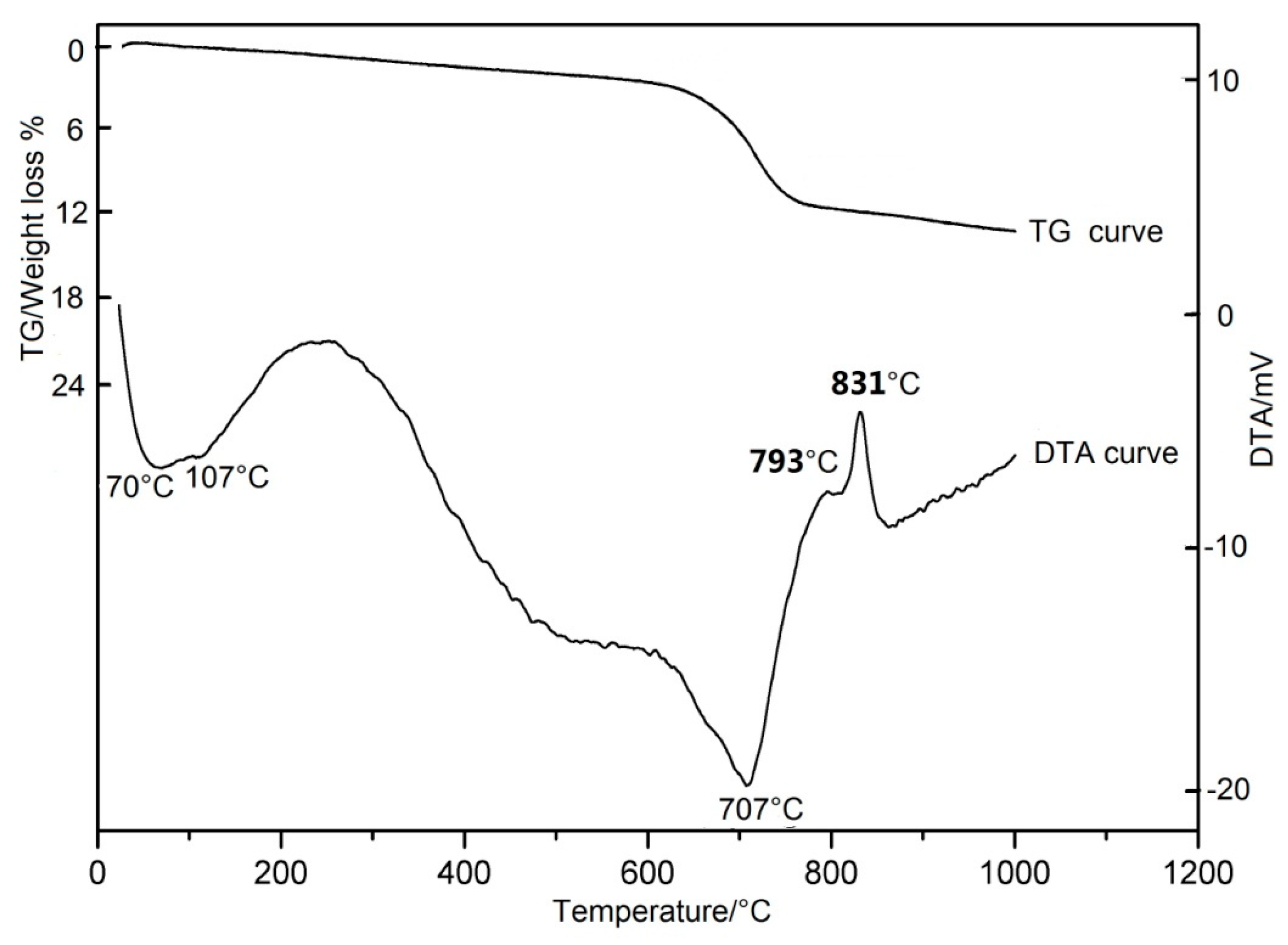
shows a typical DTA/TG thermogram (Differential Thermal Analysis/Thermogravimetric Analysis) of the antigorite powders. The DTA curve shows two endothermic peaks and two exothermic peaks. The first broad endothermic peak in the 70–107 °C is due to the loss of adsorbed water, the second intense endothermic peak in the 620–707 °C may be attributed to the loss of structural water of antigorite. The first apparent exothermic peak at 793 °C partially overlaps the 831 °C sharp endothermic peak (the second), which indicates the formation of the new minerals. The former corresponds to forsterite formation, the latter corresponds to the formation of enstatite + forsterite
. The TG curve shows outstanding weight losses during 620–707 °C, which corresponds to the intense endothermic peak in the DTA curves. Apparent activation energy of the reaction in the temperature range 612–708 °C was 255 kJ/mol for antigorite
[37]
.
Figure 1.
DTA/TG thermogram of the antigorite
[4]
.
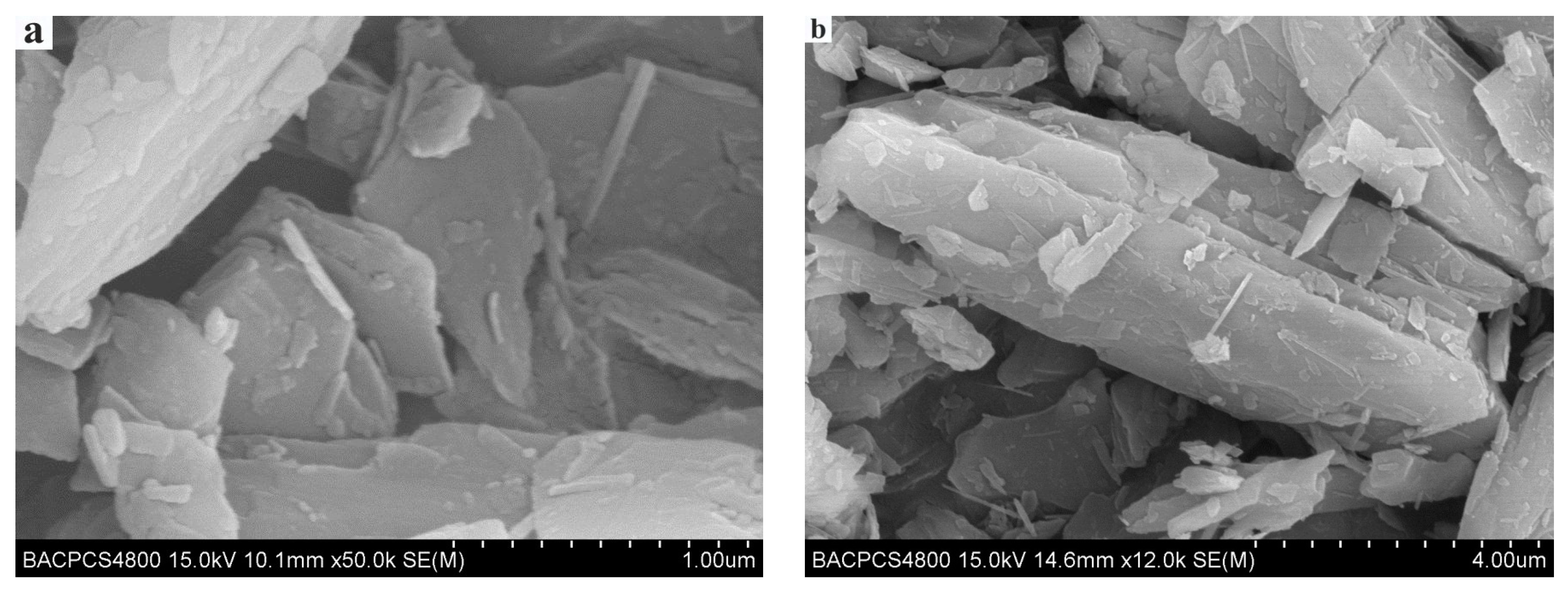
Natural antigorite is commonly bladed or fibrous. The antigorite as a lubrication material is usually bladed or lamellar (
), and processed into micro-nano scale powders by powder processing equipment
.
Figure 2.
Bladed and lamellar antigorite
[4]
. (
a
,
b
) are different particles in the same sample.
Antigorite showed a positive zeta potential over a wide pH range with a pH value of close to 10 at its isoelectric point
[38]
, which is readily wetted by water and is said to be hydrophilic
. In order to improve the compatibility of AMNP and organic lubrication medium, the surface modifications of AMNP have been investigated
. The formation of organically modified antigorite is carried out by applying the surfactants as boric acid ester, sorbitan monostearate (Span60), polysorbate 60 (Tween60), oleic acid, polyvinyl pyrrolidone (PVP), silane coupling agent (HK560), sodium dodecyl benzene sulfonate, trolamine, stearic acid, sodium stearate
. The studies investigating the surface modifications of AMNP have shown that modified powders present superior dispersion and suspension stability in the nonpolar solvent. It was found that the modifiers of oleic acid or Span60 combined with borate at 1:1 (mass ratio) had the best modification effect on AMNP
[44]
.
3. Conclusions
Lubricity and anti-wear of antigorite may be related to its lamellar morphology, medium hardness, abundant hydroxyl water and surface unsaturated charge, strong ion exchange capacity, easy to slide between layers and high temperature phase transition. Under the action of friction kinetic energy and heat energy, the bladed AMNP forms a friction reaction layer having low roughness and high hardness on the friction pair surface by abrasive polishing and ion exchange, which effectively reduces the friction resistance and wear of the friction pairs.
As a lubricant additive, AMNP should have a bladed morphology with a blade diameter less than 2 microns, and should be subject to surface modification of organic matter.
The AMNP can be mixed with different types of lubricating oil to form a suspended solution, or evenly mixed with base grease to make a complex grease, or mixed with matrix materials (such as PTFE and Al alloys) to make solid composite materials. AMNP used as a lubricant additive for PTFE can significantly reduce the wear of PTFE, which can extend the service life of the PTFE friction seal components.
As already noted, the tribological properties of calcined AMNP under 300 °C are better than that of the uncalcined AMNP. The AMNP used as a lubricating material should not be calcined at temperatures >600 °C.
The application of AMNP in industrial equipment could reduce friction wear and improve cylinder pressure; reduce the consumption of engine fuel and lubricating oil, as well as the temperature of lubricating medium and the emission of pollution, obviously prolong the service life of the friction parts of the equipment, extend the overhaul period of the equipment and improve the service efficiency.
Antigorite as a lubricant has not yet formed a set of standards and specifications, which is a technical obstacle that restricts the large-scale application of AMNP in industry.
In published papers, some authors indiscriminately refer to “antigorite” as “serpentine”. In fact, the “serpentine” is a group of minerals that contains lizardite, antigorite and chrysotile
[46]
. Although the three minerals share the same composition (Mg
6
Si
4
O
10
(OH)
8
), they have different structures. Lizardite shows an ideal layer topology, whereas antigorite is a modulated layer and chrysotile is a bent layer. Of the three minerals, antigorite is currently the only additive lubricant, while fibrous chrysotile is not used because of its potential environmental hazards. The authors suggest that “antigorite” rather than “serpentine” should be used as a lubricant.
Some papers refer to antigorite as a “self-repairing” material based on its anti-wear properties
. The word “self-repairing” means that the object itself has a self-repairing function. In fact, antigorite was not repaired during the friction process, but the friction pair using AMNP was repaired. So, “self-repairing” may be an inappropriate term to describe the effects and functions of antigorite, and so should be abandoned.
References
- Allam, I.M. Solid lubricants temperatures for applications at elevated—A review. J. Mater. Sci. 1991, 26, 3977–3984.
- Bai, Z.M.; Wang, Z.Y.; Zhang, T.G.; Fu, F.; Yang, N. Synthesis and characterization of Co-Al-CO3 layered double metal hydroxides and assessment of their friction performances. Appl. Clay Sci. 2012, 59–60, 36–41.
- Bai, Z.M.; Wang, Z.Y.; Zhang, T.G.; Fu, F.; Yang, N. Characterization and friction performances of Co-Al-layered double-metal hydroxides synthesized in the presence of dodecylsulfate. Appl. Clay Sci. 2013, 75–76, 22–27.
- Bai, Z.M.; Yang, N.; Guo, M.; Li, S. Antigorite: Mineralogical characterization and friction performances. Tribol. Int. 2016, 101, 115–121.
- Raleigh, C.B.; Paterson, M.S. Experimental deformation of serpentinite and its tectonic implications. J. Geophys. Res. 1965, 70, 3965–3985.
- Dengo, C.A.; Logan, J.M. Implications of the mechanical and frictional behavior of serpentinite to seismogenic faulting. J. Geophys. Res. 1981, 86, 10771–10782.
- Reinen, L.A.; Weeks, J.D.; Tullis, T.E. The frictional behavior of serpentinite: Implications for aseismic creep on shallow crustal faults. Geophys. Res. Lett. 1991, 18, 1921–1924.
- Reinen, L.A.; Weeks, J.D.; Tullis, T.E. The frictional behavior of lizardite and antigorite serpentinites: Experiments, constitutive models, and Implications for natural faults. Pure Appl. Geophys. 1994, 143, 317–358.
- Moore, D.E.; Lockner, D.A.; Summers, R.; Ma, S.L.; Byerlee, J.D. Strength of chrysotile- serpentinite gouge under hydrothermal conditions: Can it explain a weak San Andreas fault? Geology 1996, 24, 1041–1044.
- Morrow, C.A.; Moore, D.E.; Lockner, D.A. The effect of mineral bond strength and adsorbed water on fault gouge frictional strength. Geophys. Res. Lett. 2000, 27, 815–818.
- Jung, H.; Fei, Y.W.; Silver, P.G.; Green, H.W. Frictional sliding in serpentine at very high pressure. Earth Planet. Sci. Lett. 2009, 277, 273–279.
- Yang, H.; Li, S.H.; Jin, Y.S. Study on tribological behavior of Mg6Si4O10(OH)8 additive package with steel tribo-pair. Tribology 2005, 25, 308–311.
- Tupotilov, N.N.; Ostrikov, V.V.; Kornev, A.Y. Finely disperse minerals as antiwear additives for lube oils. Chem. Technol. Fuels Oils 2008, 44, 29–33.
- Lyubimova, D.N.; Dolgopolova, K.N.; Kozakovb, A.T.; Nikolskiib, A.V. Improvement of performance of lubricating materials with additives of clayey minerals. J. Frict. Wear 2011, 32, 442–451.
- Pogodaev, L.I.; Buyanovskii, I.A.; Kryukov, E.Y.; Kuz’min, V.N.; Usachev, V.V. The mechanism of interaction between natural laminar hydrosilicates and friction surfaces. J. Mach. Manuf. Reliab. 2009, 38, 476–484.
- Dolgopolov, K.N.; Lyubaimov, D.N.; Chigarenkoc GGPonomarenko, A.G.; Chigarenko, G.G.; Boiko, M.V. The structure of lubricating layers appearing during friction in the presence of additives of mineral friction modifiers. J. Frict. Wear 2009, 30, 377–380.
- Dolgopolov, K.N.; Lyubaimov, D.N.; Kozakovb, A.T.; Nikol’skii, A.V.; Glazunova, E.A. Tribochemical aspects of interactions between high-dispersed serpentine particles and metal friction surface. J. Frict. Wear 2012, 33, 108–114.
- Zhang, B.; Xu, B.S.; Xu, Y.; Wang, X.L.; Zhang, B.S. Effect of magnesium silicate hydroxide on the friction behavior of ductile cast iron pair and the self-repairing performance. J. Chin. Ceram. Soc. 2009, 37, 492–495.
- Zhang, B.S.; Xu, B.S.; Xu, Y.; Gao, F.; Shi, P.J.; Wu, Y.X. Cu nanoparticles effect on the tribological properties of hydrosilicate powders as lubricant additive for steel–steel contacts. Tribol. Int. 2011, 44, 878–886.
- Jin, Y.S. The effect of internal oxidation from serpentine on generating reconditioning layer on worn ferrous metal surfaces. Chin. Surf. Eng. 2010, 23, 45–50.
- Yu, H.L.; Xu, Y.; Shi, P.J.; Wang, H.M.; Zhao, Y.; Xu, B.S.; Bai, Z.M. Tribological behaviors of surface-coated serpentine ultrafine powders as lubricant additive. Tribol. Int. 2010, 43, 667–675.
- Yu, H.L.; Xu, Y.; Shi, P.J.; Wang, H.M.; Zhang, W.; Xu, B.S. Effect of thermal activation on the tribological behaviours of serpentine ultrafine powders as an additive in liquid paraffin. Tribol. Int. 2011, 44, 1736–1741.
- Qi, X.W.; Jia, Z.N.; Yang, Y.L.; Fan, B.L. Characterization and auto-restoration mechanism of nanoscale serpentine powder as lubricating oil additive under high temperature. Tribol. Int. 2011, 44, 805–810.
- Zhao, F.Y.; Bai, Z.M.; Zhao, D.; Yan, C.M. Synthesis and tribological properties of serpentine/La composite powders. J. Chin. Ceram. Soc. 2012, 40, 126–130.
- Zhao, F.Y.; Bai, Z.M. Tribological properties of serpentine, La (OH)3 and their composite particles as lubricant additives. Wear 2012, 288, 72–77.
- Zhao, F.Y.; Kasrai, M.; Sham, T.K.; Bai, Z.M. Characterization of tribofilms generated from serpentine and commercial oil using X-ray absorption spectroscopy. Tribol Lett. 2013, 50, 287–297.
- Zhao, F.Y.; Kasrai, M.; Sham, T.K.; Bai, Z.M.; Zhao, D. Characterization of tribofilms derived from zinc dialkyl dithiophosphate and serpentine by X-ray absorption spectroscopy. Tribol. Int. 2014, 73, 167–176.
- Caruso, L.J.; Chernosky, J.J.V. The stability of lizardite. Can. Miner. 1979, 17, 757–769.
- Fuchs, Y.; Linares, J.; Mellini, M. Mossbauer and infrared spectrometry of lizardite-1T from Monte Fico, Elba. Phys. Chem. Miner. 1998, 26, 111–115.
- Capitani, G.; Mellini, M. The modulated crystal structure of antigorite: The m = 17 polysome. Am. Mineral. 2004, 89, 147–158.
- Uehara, S.; Shirozu, H. Variations in chemical composition and structural properties of antigorites. J. Mineral. Petrol. Sci. 1985, 12, 299–318.
- Evans, B.W.; Dyar, M.D.; Kuehner, S.M. Implication of ferrous and ferric iron in antigorite. Am. Mineral. 2012, 97, 184–196.
- Mellini, M.; Zanazzi, P.F. Crystal structures of lizardite 1T and lizardite 2H1 from Coli, Italy. Am. Mineral. 1987, 72, 943–948.
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA; pp. 20151–21110. Available online: http://www.handbookofmineralogy.org/ (accessed on 22 August 2020).
- MacKenzie, K.J.D.; Meinhold, R.H. Thermal reactions of chrysotile revisited; a 29 Si and 25 Mg MAS NMR study. Am. Miner. 1994, 79, 43–50.
- Viti, C. Serpentine minerals discrimination by thermal analysis. Am. Miner. 2010, 95, 631–638.
- Gualtieri, A.F.; Giacobbe, C.; Viti, C. The dehydroxylation of serpentine group minerals. Am. Miner. 2012, 97, 666.
- Alvarez-Silva, M.; Uribe-Salas, A.; Watersa, K.E.; Finch, J.A. Zeta potential study of pentlandite in the presence of serpentine and dissolved mineral species. Miner. Eng. 2016, 85, 66–71.
- Manser, R.M. Handbook of Silicate Flotation; Warren Spring Laboratory: Stevenage, UK, 1975.
- Li, G.J.; Zhao, P.; Bai, Z.M. Surface characteristics of serpentine. J. Chin. Ceram. Soc. 2017, 45, 1204–1210.
- Feng, B.; Lu, Y.P.; Feng, Q.M. Mechanisms of surface charge development of serpentine mineral. Trans. Nonferrous Met. Soc. China 2013, 23, 1123–1128.
- Cao, J.; Zhang, Z.Z.; An, S.H.; Wang, C. Surface modification and tribology properties in bass oil of ultra-fine serpentine. J. Chin. Ceram. Soc. 2008, 36, 1210–1214.
- Cao, J.; Zhang, Z.Z.; Zhao, F.X.; Jiang, C.J.; Duan, Z.W. The Effect of different surfactants on the dispersion properties of serpentine in the alcohol solvent. Lubr. Eng. 2007, 32, 83–86.
- Li, G.J.; Bai, Z.M.; Zhao, P. Antifriction repair function of serpentine on Fe based metal friction pairs. J. Chin. Ceram. Soc. 2018, 46, 306–314.
- Li, G.J.; Bai, Z.M.; Huang, W.J.; Ju, Y. Research of serpentine powder surface modification. Bull. China Ceram. Soc. 2008, 6, 1091–1095.
- Veblen, D.R.; Wylie, A.G. Mineralogy of amphiboles and 1:1 layer silicates. In Reviews in Mineralogy and Geochemistry; Mineralogical Society of America: Washington, DC, USA, 1993; Volume 28, pp. 61–131.
- Xue, B.; Jing, P.X.; Ma, W.D. Tribological properties of NiAl Matrix composites filled with serpentine powders. J. Mater. Eng. Perform. 2017, 26, 5816–5824.