Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Ioannis Ntafoulis.
Glioblastoma (GBM) remains one of the most difficult tumors to treat. The one of major obstacle in GBM treatment is the blood–brain barrier (BBB), which prevents effective delivery of drugs to the central nervous system (CNS). Another key player impeding drug delivery into the CNS is the family of drug efflux pumps and more specifically the ATP-binding cassette (ABC) transporters.
- drug repurposing
- glioblastoma
- blood–brain barrier
- efflux pumps
1. Introduction
Glioblastoma (GBM) is the most aggressive form of diffuse gliomas and the most lethal among all types of brain tumors, comprising 12–15% of all adult intracranial tumors and 50–60% of astrocytic neoplasms [1]. According to the 2021 WHO classification of central nervous system (CNS) tumors, former (grade 4) GBM is now classified based on the presence or absence of mutations in the isocitrate dehydrogenase (IDH) gene: IDH wild-type (IDHwt) glioblastoma or IDH-mutant (IDHmut) grade 4 astrocytoma [2]. Molecularly, IDHwt glioblastomas are characterized by the presence of TERT promoter mutation, EGRF amplification, +7/−10 chromosome copy number changes or any combination of the above [2]. IDHmut grade 4 astrocytomas are characterized by mutations of IDH1/2, ATRX, TP53, CDKN2A/B homozygous deletion, PDGFRA amplification or any combination of the above [2]. These genomic alterations of IDHwt gliomas are associated with fast growth rates and poor prognosis [2].
The standard of care treatment following diagnosis comprises maximal safe surgical resection of the tumor (debulking), followed by radiation therapy (RT) and concurrent and adjuvant chemotherapy with the alkylating agent temozolomide (TMZ) [3,4][3][4]. However, the survival rates of the patients diagnosed with GBM and receiving first-line treatment remain very low. The median overall survival (OS) is 12–15 months, while only 3% of patients have a progression-free survival (PFS) of more than 5 years [5]. The MGMT (O6-methylguanine–DNA methyltransferase) promoter is a well-established predictive marker of response in GBM patients receiving TMZ. The epigenetic silencing of the MGMT gene by promoter methylation compromises DNA repair, improving response to TMZ and leading to longer survival of glioblastoma patients [6]. Inevitably, all GBM patients receiving RT + TMZ and/or adjuvant TMZ therapy relapse; the median PFS upon completing the first line of treatment varies between 1, 5 and 6 months [7]. Lomustine (CCNU), an alkylating agent, is sometimes administered as ultimate treatment option to recurrent GBM patients with minor therapeutic benefit [8,9,10,11][8][9][10][11].
In 2009, the U.S. food and drug administration (FDA) approved bevacizumab for the treatment of GBM with relapse after prior RT + chemotherapy [12]. Bevacizumab is a recombinant humanized monoclonal antibody, with anti-angiogenic properties by blocking vascular endothelial growth factor A (VEGF-A). However, its moderate clinical benefit and unproven OS advantage to date have withheld approval by the European medicine agency (EMA) [13,14,15,16][13][14][15][16]. The most recent therapeutic approach for recurrent GBM, which received FDA approval in 2011, is a device known as tumor-treating fields (TTF) [17]. In 2015, the device was also granted FDA approval for newly diagnosed GBM [17]. The device delivers low intensity, alternating electric fields to the tumor, therewith inhibiting glioma cell proliferation [18,19][18][19]. Moderate improvements in the survival of newly diagnosed GBM patients have been observed by adding TTF as an adjuvant treatment upon completing the standard of care treatment [20,21][20][21]. In Europe, the use of TTF is very limited to date, as the appropriate usage and implementation of the device in daily clinical practice presents many challenges [22,23][22][23].
Despite these limited additions to the arsenal of treatments, the prognosis of GBM patients remains dismal [24,25][24][25]. Two key players are involved in failure of conventional and targeted therapies: (1) the tremendous intra- and inter-tumoral heterogeneity of GBM and (2) the blood–brain barrier (BBB) [26,27,28][26][27][28]. GBM heterogeneity contributes to drug resistance and treatment escape and comprises a complex and arduous obstacle to overcome [29,30,31][29][30][31]. Extensive genetic and epigenetic profiling led to the classification of GBM tumors into three distinct molecular subgroups (classical, mesenchymal and proneural) as well as to the characterization of distinct DNA methylation profiles and/or expression patterns within these GBM subgroups [32,33,34][32][33][34]. Additionally, single-cell RNA sequencing analysis revealed different molecular subtypes within each tumor that can dynamically adapt to micro-environmental cues [34,35,36,37][34][35][36][37]. To date, these findings provide a better understanding of the heterogeneous nature of GBM; however, their clinical relevance, in particular in relation to drug treatment, is still limited [38].
The second major obstacle in GBM treatment is the BBB, which prevents effective delivery of drugs to the central nervous system (CNS). Therefore, to achieve any therapeutic response, it is of utmost importance that drugs cross the BBB and reach the tumor region in therapeutically effective concentrations. Drug discovery tools have been developed to identify optimal drug candidates for CNS penetration based on their physicochemical properties [39,40][39][40]. Moreover, efforts are being directed towards assessing CNS penetration and actual target delivery of new agents, as noted in the increasing number of phase 0 trials for GBM [41,42,43][41][42][43]. In addition, new delivery techniques, such as focused ultrasound sonication (FUS) and/or the use of nanoparticles to encapsulate therapeutic molecules, are being used to enhance systemic drug delivery into the CNS [44,45,46][44][45][46]. Examples include chemotherapeutic agents widely used in clinical practice, such as paclitaxel, cytarabine, carboplatin, etoposide and daunorubicin [47,48,49,50,51,52,53][47][48][49][50][51][52][53].
Based on these developments, a renewed interest in the available anticancer agents has arisen. With tools available to predict, enhance and assess drug delivery to CNS tumors as well as approaches to define markers of tumor sensitivity to specific compounds, the available arsenal of approved anticancer agents may be re-evaluated for potential GBM treatment. This approach, known as drug repurposing or drug repositioning, is a recognized strategy in drug discovery aiming to identify secondary indications for already approved drugs [54,55,56][54][55][56]. Given the unmet need for novel therapeutic options for GBM, drug repurposing may be a valuable tool, bypassing the delays and high costs of the novel drug development process and providing new drug candidates against GBM within a relatively short timeframe.
2. The Blood–Brain Barrier and Drug Efflux Pumps
The BBB is a neurovascular unit that, in physiological conditions, acts as a ‘gatekeeper’ [57]. The main task of the BBB is to maintain the brain homeostasis by controlling the passage of endogenous and exogenous molecules from the blood stream into the CNS [57,58][57][58]. Structurally, the BBB consists of endothelial cells interconnected with a complex network of proteins (tight junctions), while pericytes and astrocytic end-feet provide an additional structural support to the brain microvasculature (Figure 1) [57,59][57][59]. Tight junctions (TJs) are the key feature of the BBB and responsible for the impediment of polar solutes through intracellular and paracellular diffusion pathways [57]. TJs consist of claudins, occludins, junction adhesion molecules (JAMS) and various cytoplasmic accessory proteins, such as Zonula occludens-1, -2, -3 (ZO-1, ZO-2, ZO-3) (Figure 2) [60]. The transport of molecules across the BBB can be achieved by different routes, including passive diffusion, solute carriers (SLC), ATP-binding cassette (ABC) transporters, transcytosis and receptor-mediated transport [61,62][61][62]. Lipid soluble molecules can passively diffuse the BBB and reach the CNS at a rate that is linked to their physicochemical properties [40].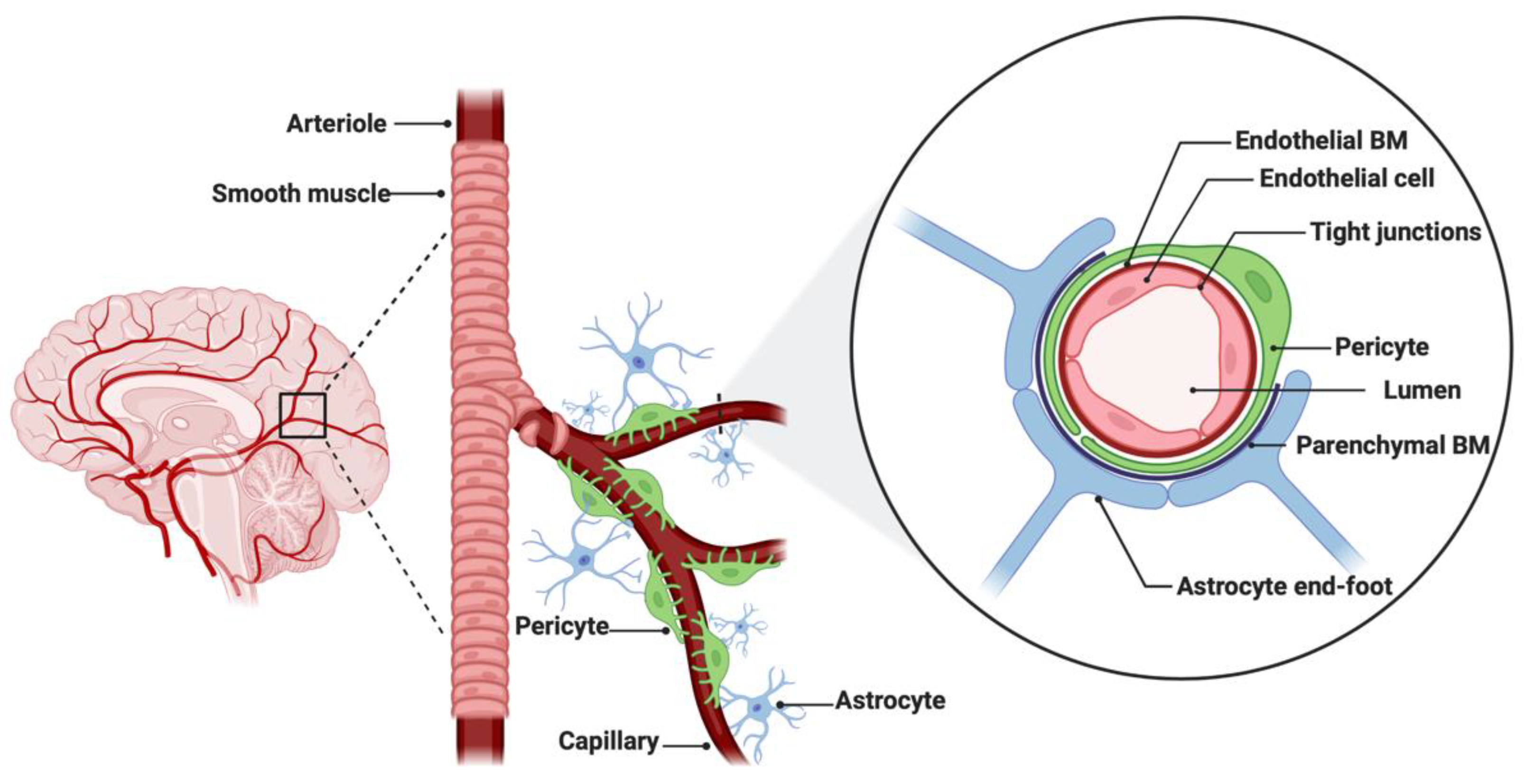
Figure 1. Anatomical features of the blood–brain barrier (BBB). The structure of the BBB in normal physiology consists of endothelial cells interconnected with a complex network of proteins (tight junctions), while mechanical support is provided by pericytes and astrocytic end-feet. The parenchymal and endothelial basal membranes (BM) provide additional strengthening to the cell attachments. Figure was created in BioRender.com (accessed on 5 July 2022).
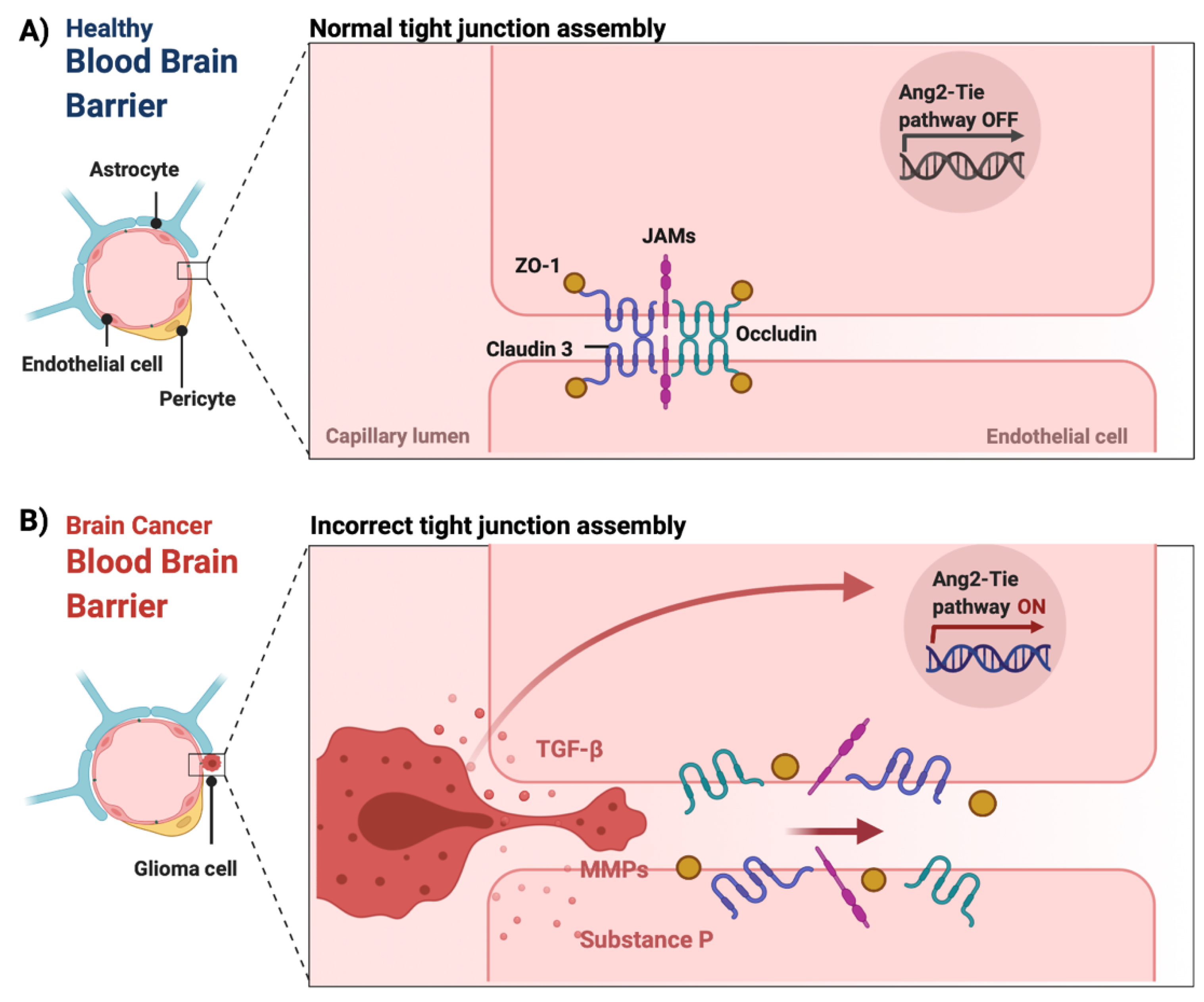
Figure 2. The integrity of the blood–brain barrier in physiological and malignant conditions. (A) In physiological conditions, tight junctions (claudin 3, occludin, junction adhesion molecules (JAMS) as well as cytoplasmic accessory proteins, such as Zonula occludens-1 (ZO-1)) of the endothelial cells remain intact maintaining the integrity of the BBB. (B) In CNS tumors, the release of chemical mediators by the tumor cells, such as substance P, matrix metalloproteinases (MMPs) and transforming growth factor beta (TGF-β), can cause the loss of tight junctions, which leads to the dysfunction and disruption of the BBB [60,69,70,72][60][63][64][65]. Additionally, the overexpression of angiopoietin-2 (ANG-2) is linked to vascular malformations and pericyte detachment through the hypoxic upregulation of VEGF, which subsequently promotes angiogenesis at the tumor margin [68][66]. Figure was created in BioRender.com (accessed on 20 May 2022).
References
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996.
- WHO Classification of Tumours Editorial Board. World Health Organization Classification of Tumours of the Central Nervous System, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; Volume 6.
- Stupp, R.; Mason, W.P.; Bent, M.V.D.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996.
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol. 2014, 16, 896–913.
- Stupp, R.; Hegi, M.; Mason, W.P.; Bent, M.V.D.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466.
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003.
- Birzu, C.; French, P.; Caccese, M.; Cerretti, G.; Idbaih, A.; Zagonel, V.; Lombardi, G. Recurrent Glioblastoma: From Molecular Landscape to New Treatment Perspectives. Cancers 2020, 13, 47.
- Herrlinger, U.; Rieger, J.; Koch, D.; Loeser, S.; Blaschke, B.; Kortmann, R.-D.; Steinbach, J.P.; Hunds-berger, T.; Wick, W.; Meyermann, R.; et al. Phase II trial of lomustine plus temozolomide chemotherapy in addition to radiotherapy in newly diagnosed glioblastoma: UKT-03. J. Clin. Oncol. 2006, 24, 4412–4417.
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.-D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688.
- Glas, M.; Happold, C.; Rieger, J.; Wiewrodt, D.; Bähr, O.; Steinbach, J.P.; Wick, W.; Kortmann, R.-D.; Reifenberger, G.; Weller, M.; et al. Long-term survival of patients with glioblastoma treated with radiotherapy and lomustine plus temozolomide. J. Clin. Oncol. 2009, 27, 1257–1261.
- Weller, M.; Le Rhun, E. How did lomustine become standard of care in recurrent glioblastoma? Cancer Treat. Rev. 2020, 87, 102029.
- Cohen, M.H.; Shen, Y.; Li, K.; Patricia, P.R. FDA drug approval summary: Bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 2009, 14, 1131–1138.
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.; Alfred, P.; Nina, N.; Martin, K.; et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740.
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E.; Marcello, J.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Sampson, J.; et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007, 25, 4722–4729.
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722.
- Wick, W.; Gorlia, T.; Bendszus, M.; Taphoorn, M.; Sahm, F.; Harting, I.; Brandes, A.A.; Taal, W.; Domont, J.; Idbaih, A.; et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N. Engl. J. Med. 2017, 377, 1954–1963.
- Mehta, M.; Wen, P.; Nishikawa, R.; Reardon, D.; Peters, K. Critical review of the addition of tumor treating fields (TTFields) to the existing standard of care for newly diagnosed glioblastoma patients. Crit. Rev. Oncol. 2017, 111, 60–65.
- Mun, E.J.; Babiker, H.M.; Weinberg, U.; Kirson, E.D.; Hoff, D.D.V. Tumor-Treating Fields: A Fourth Modality in Cancer Treatment. Clin. Cancer Res. 2018, 24, 266–275.
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004, 64, 3288–3295.
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543.
- Stupp, R.; Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316.
- Fabian, D.; Eibl, M.D.P.G.P.; Alnahhas, I.; Sebastian, N.; Giglio, P.; Puduvalli, V.; Gonzalez, J.; Palmer, J.D. Treatment of Glioblastoma (GBM) with the Addition of Tumor-Treating Fields (TTF): A Review. Cancers 2019, 11, 174.
- Lassman, A.B.; Joanta-Gomez, A.e.; Pan, P.C.; Wick, W. Current usage of tumor treating fields for glioblastoma. Neurooncol. Adv. 2020, 2, vdaa069.
- De Witt Hamer, P.C. Small molecule kinase inhibitors in glioblastoma: A systematic review of clinical studies. Neuro Oncol. 2010, 12, 304–316.
- Touat, M.; Idbaih, A.; Sanson, M.; Ligon, K.L. Glioblastoma targeted therapy: Updated approaches from recent biological insights. Ann. Oncol. 2017, 28, 1457–1472.
- Osuka, S.; Van Meir, E.G. Overcoming therapeutic resistance in glioblastoma: The way forward. J. Clin. Investig. 2017, 127, 415–426.
- Dirkse, A.; Golebiewska, A.; Buder, T.; Nazarov, P.V.; Muller, A.; Poovathingal, S.K.; Brons, N.H.C.; Leite, S.; Sauvageot, N.; Sarkisjan, D.; et al. Stem cell-associated heterogeneity in Glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat. Commun. 2019, 10, 1787.
- Qazi, M.A.; Vora, P.; Venugopal, C.; Sidhu, S.S.; Moffat, J.; Swanton, C.; Singh, S.K. Intratumoral heterogeneity: Pathways to treatment resistance and relapse in human glioblastoma. Ann. Oncol. 2017, 28, 1448–1456.
- Fabro, F.; Lamfers, M.L.M.; Leenstra, S. Advancements, Challenges, and Future Directions in Tackling Glioblastoma Resistance to Small Kinase Inhibitors. Cancers 2022, 14, 600.
- Yabo, Y.A.; Niclou, S.P.; Golebiewska, A. Cancer cell heterogeneity and plasticity: A paradigm shift in glioblastoma. Neuro-Oncology 2022, 24, 669–682.
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell 2020, 180, 188–204.e22.
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110.
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563.
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e6.
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401.
- Neftel, C.; Laffy, J.; Filbin, M.G.; Hara, T.; Shore, M.E.; Rahme, G.J.; Richman, A.R.; Silverbush, D.; Shaw, M.L.; Hebert, C.M.; et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019, 178, 835–849.e21.
- Bhaduri, A.; Lullo, D.E.; Jung, D.; Müller, S.; Crouch, E.E.; Espinosa, C.S.; Ozawa, T.; Al-varado, B.; Spatazza, J.; Cadwell, C.R.; et al. Outer Radial Glia-like Cancer Stem Cells Contribute to Heterogeneity of Glioblastoma. Cell Stem Cell 2020, 26, 48–63.e6.
- Weller, M.; Bent, M.V.D.; Preusser, M.; Rhun, L.E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2020, 18, 170–186.
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Moving beyond rules: The development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem. Neurosci. 2010, 1, 435–449.
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery. ACS Chem. Neurosci. 2016, 7, 767–775.
- Sanai, N. Phase 0 Clinical Trial Strategies for the Neurosurgical Oncologist. Neurosurgery 2019, 85, E967–E974.
- Sarapa, N. Exploratory IND: A new regulatory strategy for early clinical drug development in the United States. Ernst Scher. Res. Found Workshop 2007, 151–163.
- Yamashita, S.; Sugiyama, Y. New strategy for drug development with exploratory IND studies: Scientific basis and future directions. Adv. Drug Deliv. Rev. 2011, 63, 493.
- Bunevicius, A.; McDannold, N.J.; Golby, A.J. Focused Ultrasound Strategies for Brain Tumor Therapy. Oper. Neurosurg. 2020, 19, 9–18.
- Rathi, S.; Griffith, J.I.; Zhang, W.; Zhang, W.; Oh, J.-H.; Talele, S.; Sarkaria, J.N.; Elmquist, W.F. The influence of the blood–brain barrier in the treatment of brain tumours. J. Intern. Med. 2022, 292, 3–30.
- Juhairiyah, F.; de Lange, E.C.M. Understanding Drug Delivery to the Brain Using Liposome-Based Strategies: Studies that Provide Mechanistic Insights Are Essential. AAPS J. 2021, 23, 114.
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681.
- Markman, M.; Mekhail, T.M. Paclitaxel in cancer therapy. Expert Opin. Pharmacother. 2002, 3, 755–766.
- Krauss, A.C.; Gao, X.; Li, L.; Manning, M.L.; Patel, P.; Fu, W.; Janoria, K.G.; Gieser, G.; Bateman, D.A.; Przepiorka, D.; et al. FDA Approval Summary: (Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 2685–2690.
- Dicko, A.; Kwak, S.; Frazier, A.A.; Mayer, L.D.; Liboiron, B.D. Biophysical characterization of a liposomal formulation of cytarabine and daunorubicin. Int. J. Pharm. 2010, 391, 248–259.
- Wei, H.J.; Upadhyayula, P.S.; Pouliopoulos, A.N.; Englander, Z.K.; Zhang, X.; Jan, C.-I.; Guo, J.; Mela, A.-g.; Zhang, Z.; Wang, T.J.C.; et al. Focused Ultrasound-Mediated Blood-Brain Barrier Opening Increases Delivery and Efficacy of Etoposide for Glioblastoma Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 539–550.
- Dréan, A.; Lemaire, N.; Bouchoux, G.; Goldwirt, L.; Canney, M.; Goli, L.; Bouzidi, A.; Schmitt, C.; Guehennec, J.; Verreault, M.; et al. Temporary blood-brain barrier disruption by low intensity pulsed ultrasound increases carboplatin delivery and efficacy in preclinical models of glioblastoma. J. Neurooncol. 2019, 144, 33–41.
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801.
- Xue, H.; Li, J.; Xie, H.; Wang, Y. Review of Drug Repositioning Approaches and Resources. Int. J. Biol. Sci. 2018, 14, 1232–1244.
- Pushpakom, S.; Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58.
- Abbruzzese, C.; Matteoni, S.; Signore, M.; Cardone, L.; Nath, K.; Glickson, J.D.; Paggi, M.G. Drug repurposing for the treatment of glioblastoma multiforme. J. Exp. Clin. Cancer Res. 2017, 36, 169.
- Abbott, N.J.; Patabendige, A.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25.
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood-brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363.
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53.
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69.
- Theodorakis, P.E.; Müller, E.A.; Craster, R.V.; Matar, O.K. Physical insights into the blood-brain barrier translocation mechanisms. Phys. Biol. 2017, 14, 041001.
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7.
- de Vries, H.E.; Blom-Roosemalen, M.C.; van Oosten, M.; de Boer, A.G.; van Berkel, T.J.; Breimer, D.D.; Kuiper, J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J. Neuroimmunol. 1996, 64, 37–43.
- Schneider, S.W.; Ludwig, T.; Tatenhorst, L.; Braune, S.; Oberleithner, H.; Senner, V.; Paulus, W. Glioblastoma cells release factors that disrupt blood-brain barrier features. Acta Neuropathol. 2004, 107, 272–276.
- Ishihara, H.; Kubota, H.; Lindberg, R.L.; Leppert, D.; Gloor, S.M.; Errede, M.; Virgintino, D.; Fontana, A.; Yonekawa, Y.; Frei, K. Endothelial Cell Barrier Impairment Induced by Glioblastomas and Transforming Growth Factor β2Involves Matrix Metalloproteinases and Tight Junction Proteins. J. Neuropathol. Exp. Neurol. 2008, 67, 435–448.
- Thurston, G.; Daly, C. The Complex Role of Angiopoietin-2 in the Angiopoietin-Tie Signaling Pathway. Cold Spring Harb. Perspect. Med. 2012, 2, a006650.
- Kim, M.; Kizilbash, S.H.; Laramy, J.K.; Gampa, G.; Parrish, K.E.; Sarkaria, J.N.; Elmquist, W.F. Barriers to Effective Drug Treatment for Brain Metastases: A Multifactorial Problem in the Delivery of Precision Medicine. Pharm. Res. 2018, 35, 1–20.
- Nduom, E.; Yang, C.; Merrill, M.J.; Zhuang, Z.; Lonser, R.R. Characterization of the blood-brain barrier of metastatic and primary malignant neoplasms. J. Neurosurg. 2013, 119, 427–433.
- Brat, D.J.; Van Meir, E.G. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab. Investig. 2004, 84, 397–405.
- Zhao, C.; Wang, H.; Xiong, C.; Liu, Y. Hypoxic glioblastoma release exosomal VEGF-A induce the permeability of blood-brain barrier. Biochem. Biophys. Res. Commun. 2018, 502, 324–331.
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic targeting of the angiopoietin–TIE pathway. Nat. Rev. Drug Discov. 2017, 16, 635–661.
- Martin, T.A.; Jiang, W.G. Loss of tight junction barrier function and its role in cancer metastasis. Biochim. et Biophys. Acta (BBA)-Biomembr. 2009, 1788, 872–891.
- Mader, S.; Brimberg, L. Aquaporin-4 Water Channel in the Brain and Its Implication for Health and Disease. Cells 2019, 8, 90.
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Cancer 2019, 20, 26–41.
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.; Laramy, J.K.; et al. Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology 2017, 20, 184–191.
- Pacioni, S.; D’Alessandris, Q.G.; Buccarelli, M.; Boe, A.; Martini, M.; Larocca, L.M.; Bolasco, G.; Ricci-Vitiani, L.; Falchetti, M.L.; Pallini, R. Brain Invasion along Perivascular Spaces by Glioma Cells: Relationship with Blood–Brain Barrier. Cancers 2019, 12, 18.
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellisdavies, G.C.R.; Sontheimer, H. Disruption of astrocyte–vascular coupling and the blood–brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 1–15.
- de Gooijer, M.C.; Kemper, E.M.; Buil, L.C.M.; Citirikkaya, C.H.; Buckle, T.; Beijnen, J.H.; van Tellingen, O. ATP-binding cassette transporters restrict drug delivery and efficacy against brain tumors even when blood-brain barrier integrity is lost. Cell Rep. Med. 2021, 2, 100184.
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464.
- Hollenstein, K.; Dawson, R.; Locher, K.P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2007, 17, 412–418.
- Dean, M. The Genetics of ATP-Binding Cassette Transporters. Methods Enzymol. 2005, 400, 409–429.
- Uitto, J. The gene family of ABC transporters—Novel mutations, new phenotypes. Trends Mol. Med. 2005, 11, 341–343.
- Kadioglu, O.; Saeed, M.E.M.; Munder, M.; Spuller, A.; Greten, H.J.; Efferth, T. Effect of ABC transporter expression and mutational status on survival rates of cancer patients. Biomed. Pharmacother. 2020, 131, 110718.
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Cancer 2010, 10, 147–156.
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Cancer 2002, 2, 48–58.
- Choi, C.-H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30.
- Choi, Y.H.; Yu, A.-M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807.
- Ambudkar, S.V.; Kim, I.-W.; Sauna, Z.E. The power of the pump: Mechanisms of action of P-glycoprotein (ABCB1). Eur. J. Pharm. Sci. 2006, 27, 392–400.
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559.
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.; Wesseling, P.; Wurdinger, T.; de Vries, H. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12.
- Mason, W.P. Blood-brain barrier-associated efflux transporters: A significant but underappreciated obstacle to drug development in glioblastoma. Neuro-Oncology 2015, 17, 1181–1182.
- Dréan, A.; Rosenberg, S.; Lejeune, F.-X.; Goli, L.; Nadaradjane, A.A.; Guehennec, J.; Schmitt, C.; Verreault, M.; Bielle, F.; Mokhtari, K.; et al. ATP binding cassette (ABC) transporters: Expression and clinical value in glioblastoma. J. Neuro-Oncol. 2018, 138, 479–486.
- Bronger, H.; König, J.; Kopplow, K.; Steiner, H.-H.; Ahmadi, R.; Herold-Mende, C.; Keppler, D.; Nies, A.T. ABCC Drug Efflux Pumps and Organic Anion Uptake Transporters in Human Gliomas and the Blood-Tumor Barrier. Cancer Res. 2005, 65, 11419–11428.
- Durmus, S.; Hendrikx, J.J.; Schinkel, A.H. Apical ABC Transporters and Cancer Chemotherapeutic Drug Disposition. Adv. Cancer Res. 2015, 125, 1–41.
- Bao, X.; Wu, J.; Xie, Y.; Kim, S.; Michelhaugh, S.; Jiang, J.; Mittal, S.; Sanai, N.; Li, J. Protein Expression and Functional Relevance of Efflux and Uptake Drug Transporters at the Blood–Brain Barrier of Human Brain and Glioblastoma. Clin. Pharmacol. Ther. 2020, 107, 1116–1127.
- de Trizio, I.; Errede, M.; d’Amati, A.; Girolamo, F.; Virgintino, D. Expression of P-gp in Glioblastoma: What we can Learn from Brain Development. Curr. Pharm. Des. 2020, 26, 1428–1437.
- Roy, L.-O.; Lemelin, M.; Poirier, M.-B.; Blanchette, M.; Fortin, D. SCDT-18. EXPRESSION OF ABC TRANSPORTERS AS PROGNOSTIC BIOMARKERS FOR GLIOBLASTOMA. Neuro-Oncology 2017, 19, vi268.
- Parrish, K.E.; Sarkaria, J.N.; Elmquist, W.F. Improving drug delivery to primary and metastatic brain tumors: Strategies to overcome the blood-brain barrier. Clin. Pharmacol. Ther. 2015, 97, 336–346.
- Arvanitis, C.D.; Askoxylakis, V.; Guo, Y.; Datta, M.; Kloepper, J.; Ferraro, G.B.; Bernabeu, M.O.; Fukumura, D.; McDannold, N.; Jain, R.K. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood–tumor barrier disruption. Proc. Natl. Acad. Sci. USA 2018, 115, E8717–E8726.
- Oberoi, R.K.; Parrish, K.E.; Sio, T.T.; Mittapalli, R.K.; Elmquist, W.F.; Sarkaria, J.N. Strategies to improve delivery of anticancer drugs across the blood–brain barrier to treat glioblastoma. Neuro-Oncology 2015, 18, 27–36.
More
