Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by NKULU KABANGE ROLLY and Version 1 by NKULU KABANGE ROLLY.
Nitrogen is a gas present in the air with an atomic mass of 14.007, a boiling and melting points of 77.36 K and 63.15 K, respectively, and a density of 0.0012506 g·cm–3. N was first discovered in 1772 by the Scottish physician and chemist Daniel Rutherford. The multiple facets of N are described. A paradigm shift is important to shape to the future use of N-rich fertilizers in crop production and their contribution to the current global greenhouse gases (GHGs) budget would help tackle current global environmental challenges toward a sustainable agriculture.
- nitrogen
- agriculture
- environment
1. Basic Properties of Nitrogen and N-Containing Compounds
Nitrogen is the fifth most abundant element in the universe after hydrogen (H, first), helium (He, second), oxygen (O, third), and neon (Ne, fourth), and it makes up approximately 78.1% of the earth’s atmosphere. Reports indicate that an estimate of 4000 trillion tons of N can be found in the atmosphere in the form of N2 (https://pubchem.ncbi.gov/element/Nitrogen, accessed on 31 May 2022). The most popular use of N is for the production of ammonia (NH3) when combined with hydrogen (H), in a process called the “Haber process” [10]. Then, large amounts of NH3 are used to produce mineral fertilizers in a process known as the “Ostwald process”, among other uses [11]. N is found in all living systems as part of the makeup of biological compounds. During the decomposition of organic matter (OM), sodium nitrate (NaNO3) and potassium nitrate (KNO3) are formed. Other inorganic N compounds include HNO3, NH3, the oxides (nitric oxide (NO), nitrogen dioxide (NO2), N2O4, and nitrous oxide (N2O)), and cyanides (CN). NO2, NO, nitrous acid (HONO), and nitric acid (HNO3) belong to a group of highly reactive gases known as oxides of nitrogen or nitrogen oxides (NOx) [12].
NO2 is formed during nitrification and denitrification processes, in which N2O and NO are released, with N2O reported to be formed from NO3-dependent NO formation [13]. N2O is a potent greenhouse gas (GHG), with a global warming potential (GWP) 300 times greater than the mass of carbon dioxide (CO2) in the atmosphere, right before methane (CH4) and CO2 [14,15]. As for NO, reports indicate that this molecule is versatile and bioactive, with the potential to diffuse through biological membranes owing to its physiological properties. NO can act as a signaling molecule, which may involve a very wide web with reactive oxygen species (ROS). However, excessive accumulation of NO induces stressful conditions in plants [16,17]. NO and its derived molecules are reported to be involved in abiotic and biotic stress response mechanisms.
2. Historical Use of Nitrogen and N-Rich Fertilizers in Agriculture
The history of agriculture revealed that a number of plant species were domesticated from wild ancestors [18,19,20,21,22,23] during the Early Pre-Pottery Neolithic period, at various locations and different times between 10,500 and 10,100 years before common era, BCE [24,25,26]. During this period, crops exhibited a low productivity, poor yields, and poor quality, mainly attributed to their genetic makeup [25,27,28,29,30,31,32]. Since then, significant progress has been made using plant breeding and the establishment of plant nutrition schemes. According to Pennazio [33], the development of mineral nutrition of plants began between the 17th and 18th centuries. The patterns of mineral fertilizer applications in different cropping systems, as well as their impact on the environment, continue to nourish the debate globally [34,35,36,37,38,39,40,41,42,43,44,45,46,47]. However, the increase in food demands due to the rapid increase in the global population has shown the necessity to enhance the productivity of food crops. To achieve that, the common strategies used are crop improvement and the use of mineral fertilizers during crop cultivation, which have increased over the years [48,49]. Despite the recorded progress in plant breeding, N remains an indispensable macronutrient, among the 14 mineral elements (macro- and micronutrients) required by plants for optimum growth and development, high productivity, quality, fitness, and resistance toward environmental and biotic stresses [50,51].
Data indicate that the application of N-rich fertilizers increased over the years concomitant with the expansion of crop cultivation areas and the use of improved or high-yielding crop varieties that are demanding of nutrients, particularly observed in large-scale farming systems. This trend could be partially attributed to the increase in food demands subsequent to the increase in the world’s population. An estimate of the global population growth shows that the number of people on Earth increased by 2139.61% in 72 years, from about 2,536,431,149499,322,157 in 1950 to nearly 7,975,105,156 in 2022 (https://www.macrotrends.net/countries/WLD/world/population, accessed on 15 July 2022), in contrast to the decreasing pattern of the annual population growth rate during the same period (nearly 1.75% in 1951 down to 0.83% in 2022, a decrease by 47.4%). Available data on land coverage, as reported by the Food and Agriculture Organization of the United Nations (FAO) statistics (https://www.fao.org/faostat/en/#data/LC/visualize, accessed on 15 July 2022), suggest that herbaceous crops occupied about 1,877,418.8 ha of cultivated lands in 1992 compared to 1,904,136.4 ha in 2020 globally. In addition, the report on the use of nutrients for agricultural production during the last six decades indicates that nearly 4,155,951,874 metric tons of N was used between 1961 and 2020 (11,455,804.3 mt in 1961 and 113,291,696.7 mt in 2020) (https://www.fao.org/faostat/en/#compare, accessed on 15 July 2022). When compared with other macronutrients (phosphorus (P) and potassium (K)), N is by far the most abundantly used plant nutrient element in agriculture (nearly 4.15 billion tons (BT) within 59 years). For instance, a report by FAO showed that during 1961–2020, more than 1.9 BT of phosphate (P2O5) and 1.4 BT of potash (K2O) were used for agricultural production globally (Figure 1).
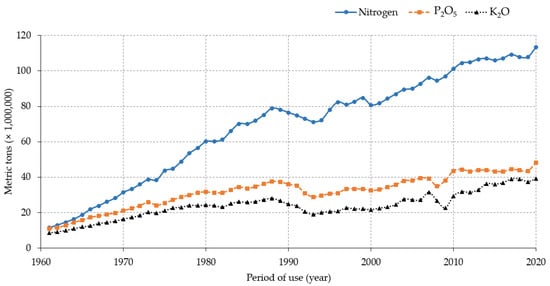
Figure 1. Pattern of global macronutrients use in agriculture from 1961 to 2020.
3. Essentiality of Nitrogen, Sources, and Availability
Arnon and Stout [52] proposed three criteria for the essentiality of a plant nutrient as follows: (1) a deficiency of the element makes it impossible for a plant to complete its lifecycle; (2) the deficiency is specific to the element in question; (3) the element is directly involved in the nutrition of the plant, such as a constituent of an essential metabolite or required for the action of an enzyme system. Other reports suggested that a more inclusive definition of the essentiality of a plant nutrient should be considered, which is not limited to those elements when they are deficient in the soil or unavailable, when they may cause symptoms of deficiency, and when their correction may involve an external supply through fertilization [53,54,55]. Therefore, on the basis of the abovementioned criteria, N and 15 other elements, namely, carbon (C), hydrogen (H), oxygen (O), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), molybdenum (Mo), boron (B), and chlorine (Cl), are considered essential for the growth of higher plants. Of this list, C, H, and O are acquired by the plant directly from the air and soil water, while the remaining 13 are supplied by the soil [56,57]. On the basis of the amounts in which the essential nutrients are acquired by the plant (except C, H, and O), they are categorized as primary (N, P, and K) and secondary macronutrients (Ca, Mg, and S), and micronutrients (Fe, Mn, Zn, Cu, B, Mo, and Cl).
Plants are sessile living organisms, which do not have the ability to move from one environment to another looking for food in the case of nutrient deficiency in their immediate environment [58,59]. Soil is the principal source of nutrients (including N) necessary for plants to complete their life cycle [60,61]. The availability of N is affected by various factors that may be extrinsic or intrinsic to the plant [62,63,64,65,66,67]. Crop productivity relies heavily on N fertilization [68,69,70]. N is found abundantly in the air but in a form that plants cannot absorb. The major sources of N are atmospheric nitrogen gas N2, exogenous N supply, and OM through fertilization. Atmospheric N2 gas (plentiful in the air but cannot be absorbed by plants in this form) is acquired through the nitrogen fixation cycle mediated by plant species belonging to Fabaceae (leguminous, along with a few non-leguminous plants containing nodules in their roots) in a symbiotic association with soil microorganisms known as nitrogen-fixing bacteria belonging to the genus Rhizobium. The latter mediate the conversion of atmospheric N2 to ammonium (NH4) in the soil, which in turn is converted to nitrate (NO3) during the nitrification process. According to Maier [71], the growth of many bacteria, either free-living in the environment or in symbiosis with plants, is promoted during N fixation in areas where fixed N is deficient in the soil. The root nodule bacteria have many O2-binding terminal oxidases, with a high O2 affinity, which are associated with N2 fixation and help maintain a steady O2 supply, coupled with ATP supply for high energy-demanding N2 fixation. The fertility of soil depends on several factors, including the quantity and quality of nutrients present in the soil, soil physical, biological, and chemical properties [72].
NO3 and NH4 are the major forms of N taken up by the plant, with NO3 being the most abundant [70]. However, roughly half of N (all N sources and forms considered) is used by the plant, while the remainder is either lost to groundwater (percolation or leaching), consumed by soil microorganisms (bacteria) involved in the decomposition of OM to humus, or converted back to atmospheric N2 via the denitrification process.
4. Nitrogen-Based Fertilizers
Nitrogen is commonly applied using commercial N-containing fertilizers or OM (composts, liquid organic fertilizers, and animal feces) [73,74,75,76,77]. One of the most abundant forms of N commercially available is urea 46% N ((CO(NH2)2) or CH4N2O) also known as carbamide, or it can be found together with P in the form of diammonium phosphate (DAP, (NH4)2HPO4). The latter is also referred to as ammonium monohydrogen phosphate, ammonium hydrogen phosphate, or ammonium phosphate dibasic, with the typical formulation of 18% N, 46% P2O5, 0% K2O) or triple superphosphate (TSP) referred to as calcium dihydrogen phosphate or monocalcium phosphate ((Ca(H2PO4)2·H2O), containing 45% phosphate (P2O5) (0–45–0), 15% Ca), whereas, K is supplied as potash (K2O) [67]. Together, they make up the trio widely known as NPK. In the soil, N is available to the plant and transported in the form of NO3 and NH4, with NO3 transport being predominant over NH4 and the major source N [78,79]. DAP is known as the world’s most widely used phosphorus fertilizer and one of the known water-soluble ammonium phosphate salts that can be produced when ammonia (NH3) reacts with phosphoric acid (H3PO4) ((NH4)2HPO4 (s) ⇌ NH3 (g) + (NH4)H2PO4 (s)) [80]. Reports indicate that, when applied as a source of N and P, DAP temporarily increases the soil pH but becomes more acidic over the long term upon nitrification of the NH4, and it is said to be incompatible with alkaline chemicals due to the high potential for the NH4 ion to convert to NH3 in a high-pH environment (pH 7.5–8.0).
