Recent literature has stressed the importance of vitamin D (VD) in polycystic ovary syndrome (PCOS). Women with PCOS are deficient in VD, particularly those with a higher weight. Hypovitaminosis is a risk factor for glucose intolerance, and reduced levels of VD is associated with insulin resistance and increased diabetes risk. Since women with PCOS and hirsutism seem to have lower levels of VD than women with PCOS without hirsutism, a correlation between VD deficiency and hyperandrogenism may be suggested.
Interestingly, VD is crucial for many human physiological functions, including to counteract inflammation and oxidative stress. Some studies evaluated effects of VD supplementation on glucose homeostasis variables, hormonal status, lipid concentrations, and biomarkers of inflammation and oxidative stress among VD-deficient women. Moreover, VD has been shown to play a role in egg quality and fertility.
This review aims to show the relationship between VD and the endocrine and metabolic profile of PCOS patients, as well as its implications for their fertility. The supplement of VD to the common therapy can lead to an improvement of the insulin resistance and lipid metabolism, a reduction of circulating androgens, as well as a better response to the induction of ovulation in PCOS women.
- polycystic ovary syndrome (PCOS)
- vitamin D
- insulin resistance
1. Introduction
2. Vitamin D
2.1. Vitamin D Deficiency and PCOS Phenotypes
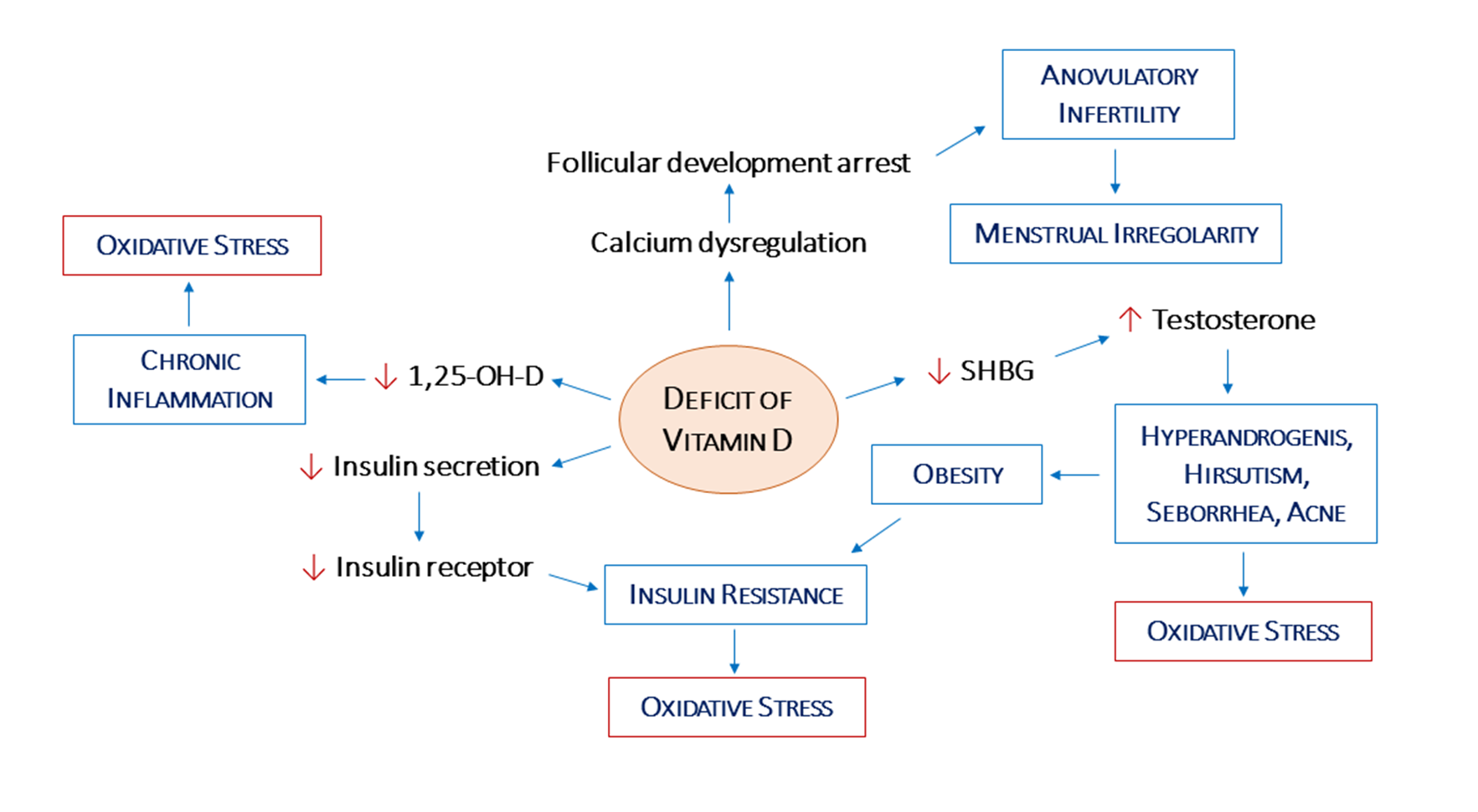
2.2. Vitamin D, Hypothalamic–Pituitary–Gonadal Axis, and Androgen Levels
2.3. Vitamin D, Ovarian Physiology, and Oxidative Stress
2.4. Vitamin D, Insulin Resistance, and Obesity
3. Relevance of Supplementation
3.1. Impact of Vitamin D Supplementation on Biomarkers of Oxidative Stress in PCOS
3.2. Vitamin D Supplementation and Fertility Outcomes in PCOS Women
In a prospective cohort study, the parathyroid hormone (PTH), the active form 1,25-hydroxy vitamin D3 (1,25OH-D3), and testosterone were measured in infertile women with PCOS undergoing clomiphene citrate stimulation [65]. ThIt is study d demonstrated that high PTH hormone levels correlated with low serum calcium and low 1,25OH-D3, whereas high PTH was associated with high body mass index and with higher testosterone serum levels. When comparing women who had developed a follicle with those who were resistant after stimulation with 50 mg of clomiphene citrate, lower 1,25OH-D3 serum levels were detected in resistant ones. Moreover, significantly improved pregnancy rate was highlighted in women with higher BMI and lower 1,25OH-D3 serum levels. Finally, the significant correlation between lower 1,25OH-D3 serum levels and lower follicle development after stimulation with 50 mg of clomiphene citrate may be ascribed to the well reported role of 1,25OH-D3 in ovarian activity.
4. Conclusions
The in-depth knowledge of these mechanisms might potentially lead to this oral, rather safe and cost-effective vitamin becoming an adjunct treatment in therapies for PCOS patients. To this regard, in the clinical management of PCOS patients, the measurement of VD serum levels, along with other endocrine markers and the patient’s phospho-calcium metabolism, should be always recommended. This will lead the clinician to evaluate the oral VD dose necessary, in association with other more specific therapies. This approach may be an effective weapon in obese women and in the treatment of PCOS patients with insulin-resistance. Excluding kidney, liver, or internistic disease that modifies absorption, after evaluation of VD3 dose requirement, data from the literature suggest that the dose of 1000 IU per day corresponding to 25 mcg seems to be the most effective at raising 25-OH-D levels to sufficient amounts, during three months of therapy in PCOS women with VD deficiency [85].
References
- Azziz, R. Introduction: Determinants of Polycystic Ovary Syndrome. Fertil. Steril. 2016, 106, 4–5.
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The Prevalence and Phenotypic Features of Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. Oxf. Engl. 2016, 31, 2841–2855.
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Massaro, M.G.; Morgante, G.; Petraglia, F. Genetic, Hormonal and Metabolic Aspects of PCOS: An Update. Reprod. Biol. Endocrinol. RBE 2016, 14, 38.
- Teede, H.; Deeks, A.; Moran, L. Polycystic Ovary Syndrome: A Complex Condition with Psychological, Reproductive and Metabolic Manifestations That Impacts on Health across the Lifespan. BMC Med. 2010, 8, 41.
- De Groot, P.C.M.; Dekkers, O.M.; Romijn, J.A.; Dieben, S.W.M.; Helmerhorst, F.M. PCOS, Coronary Heart Disease, Stroke and the Influence of Obesity: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2011, 17, 495–500.
- Sangaraju, S.L.; Yepez, D.; Grandes, X.A.; Talanki Manjunatha, R.; Habib, S. Cardio-Metabolic Disease and Polycystic Ovarian Syndrome (PCOS): A Narrative Review. Cureus 2022, 14, e25076.
- Wehr, E.; Pilz, S.; Schweighofer, N.; Giuliani, A.; Kopera, D.; Pieber, T.R.; Obermayer-Pietsch, B. Association of Hypovitaminosis D with Metabolic Disturbances in Polycystic Ovary Syndrome. Eur. J. Endocrinol. 2009, 161, 575–582.
- Shi, X.-Y.; Huang, A.-P.; Xie, D.-W.; Yu, X.-L. Association of Vitamin D Receptor Gene Variants with Polycystic Ovary Syndrome: A Meta-Analysis. BMC Med. Genet. 2019, 20, 32.
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110.
- Smirnov, V.V.; Beeraka, N.M.; Butko, D.Y.; Nikolenko, V.N.; Bondarev, S.A.; Achkasov, E.E.; Sinelnikov, M.Y.; Sinelnikov, P.R.H. Updates on Molecular Targets and Epigenetic-Based Therapies for PCOS. Reprod. Sci. Thousand Oaks Calif 2022. online ahead of print.
- Cappelli, V.; Musacchio, M.C.; Bulfoni, A.; Morgante, G.; De Leo, V. Natural Molecules for the Therapy of Hyperandrogenism and Metabolic Disorders in PCOS. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 15–29.
- DeLuca, H.F. The Metabolism and Functions of Vitamin D. Adv. Exp. Med. Biol. 1986, 196, 361–375.
- Bikle, D.D. Vitamin D: Newer Concepts of Its Metabolism and Function at the Basic and Clinical Level. J. Endocr. Soc. 2020, 4, bvz038.
- Grzesiak, M. Vitamin D3 Action within the Ovary-an Updated Review. Physiol. Res. 2020, 69, 371–378.
- Kinuta, K.; Tanaka, H.; Moriwake, T.; Aya, K.; Kato, S.; Seino, Y. Vitamin D Is an Important Factor in Estrogen Biosynthesis of Both Female and Male Gonads. Endocrinology 2000, 141, 1317–1324.
- Mahmoudi, T. Genetic Variation in the Vitamin D Receptor and Polycystic Ovary Syndrome Risk. Fertil. Steril. 2009, 92, 1381–1383.
- Vulcan, T.; Filip, G.A.; Lenghel, L.M.; Suciu, T.; Ilut, P.; Procopciuc, L.M. Polymorphisms of Vitamin D Receptor and the Effect on Metabolic AndEndocrine Abnormalities in Polycystic Ovary Syndrome: A Review. Horm. Metab. Res. 2021, 53, 645–653.
- He, C.; Lin, Z.; Robb, S.W.; Ezeamama, A.E. Serum Vitamin D Levels and Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 4555–4577.
- Davis, E.M.; Peck, J.D.; Hansen, K.R.; Neas, B.R.; Craig, L.B. Associations between Vitamin D Levels and Polycystic Ovary Syndrome Phenotypes. Minerva Endocrinol. 2019, 44, 176–184.
- Maktabi, M.; Chamani, M.; Asemi, Z. The Effects of Vitamin D Supplementation on Metabolic Status of Patients with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Horm. Metab. Res. 2017, 49, 493–498.
- Kuiri-Hänninen, T.; Sankilampi, U.; Dunkel, L. Activation of the Hypothalamic-Pituitary-Gonadal Axis in Infancy: Minipuberty. Horm. Res. Paediatr. 2014, 82, 73–80.
- Kılınç, S.; Atay, E.; Ceran, Ö.; Atay, Z. Evaluation of Vitamin D Status and Its Correlation with Gonadal Function in Children at Mini-Puberty. Clin. Endocrinol. (Oxf.) 2019, 90, 122–128.
- Chang, E.M.; Kim, Y.S.; Won, H.J.; Yoon, T.K.; Lee, W.S. Association between Sex Steroids, Ovarian Reserve, and Vitamin D Levels in Healthy Nonobese Women. J. Clin. Endocrinol. Metab. 2014, 99, 2526–2532.
- Hahn, S.; Haselhorst, U.; Tan, S.; Quadbeck, B.; Schmidt, M.; Roesler, S.; Kimmig, R.; Mann, K.; Janssen, O.E. Low Serum 25-Hydroxyvitamin D Concentrations Are Associated with Insulin Resistance and Obesity in Women with Polycystic Ovary Syndrome. Exp. Clin. Endocrinol. Diabetes 2006, 114, 577–583.
- Lundqvist, J. Vitamin D as a Regulator of Steroidogenic Enzymes. F1000Research 2014, 3, 155.
- Pal, L.; Berry, A.; Coraluzzi, L.; Kustan, E.; Danton, C.; Shaw, J.; Taylor, H. Therapeutic Implications of Vitamin D and Calcium in Overweight Women with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2012, 28, 965–968.
- Irani, M.; Merhi, Z. Role of Vitamin D in Ovarian Physiology and Its Implication in Reproduction: A Systematic Review. Fertil. Steril. 2014, 102, 460–468.e3.
- Bianchi, L.; Gagliardi, A.; Landi, C.; Focarelli, R.; De Leo, V.; Luddi, A.; Bini, L.; Piomboni, P. Protein Pathways Working in Human Follicular Fluid: The Future for Tailored IVF? Expert Rev. Mol. Med. 2016, 18, e9.
- Bakhshalizadeh, S.; Amidi, F.; Alleyassin, A.; Soleimani, M.; Shirazi, R.; Shabani Nashtaei, M. Modulation of Steroidogenesis by Vitamin D3 in Granulosa Cells of the Mouse Model of Polycystic Ovarian Syndrome. Syst. Biol. Reprod. Med. 2017, 63, 150–161.
- Yao, X.; Zhang, G.; Guo, Y.; Ei-Samahy, M.; Wang, S.; Wan, Y.; Han, L.; Liu, Z.; Wang, F.; Zhang, Y. Vitamin D Receptor Expression and Potential Role of Vitamin D on Cell Proliferation and Steroidogenesis in Goat Ovarian Granulosa Cells. Theriogenology 2017, 102, 162–173.
- Echchgadda, I.; Song, C.S.; Roy, A.K.; Chatterjee, B. Dehydroepiandrosterone Sulfotransferase Is a Target for Transcriptional Induction by the Vitamin D Receptor. Mol. Pharmacol. 2004, 65, 720–729.
- Baka, S.; Malamitsi-Puchner, A. Novel Follicular Fluid Factors Influencing Oocyte Developmental Potential in IVF: A Review. Reprod. Biomed. Online 2006, 12, 500–506.
- Masjedi, F.; Keshtgar, S.; Zal, F.; Talaei-Khozani, T.; Sameti, S.; Fallahi, S.; Kazeroni, M. Effects of Vitamin D on Steroidogenesis, Reactive Oxygen Species Production, and Enzymatic Antioxidant Defense in Human Granulosa Cells of Normal and Polycystic Ovaries. J. Steroid Biochem. Mol. Biol. 2020, 197, 105521.
- Merhi, Z.; Doswell, A.; Krebs, K.; Cipolla, M. Vitamin D Alters Genes Involved in Follicular Development and Steroidogenesis in Human Cumulus Granulosa Cells. J. Clin. Endocrinol. Metab. 2014, 99, E1137–E1145.
- Usluogullari, B.; Duvan, C.; Usluogullari, C. Use of Aromatase Inhibitors in Practice of Gynecology. J. Ovarian Res. 2015, 8, 4.
- Krishnan, A.V.; Swami, S.; Peng, L.; Wang, J.; Moreno, J.; Feldman, D. Tissue-Selective Regulation of Aromatase Expression by Calcitriol: Implications for Breast Cancer Therapy. Endocrinology 2010, 151, 32–42.
- Merhi, Z. Crosstalk between Advanced Glycation End Products and Vitamin D: A Compelling Paradigm for the Treatment of Ovarian Dysfunction in PCOS. Mol. Cell. Endocrinol. 2019, 479, 20–26.
- Merhi, Z.; Buyuk, E.; Cipolla, M.J. Advanced Glycation End Products Alter Steroidogenic Gene Expression by Granulosa Cells: An Effect Partially Reversible by Vitamin D. MHR Basic Sci. Reprod. Med. 2018, 24, 318–326.
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30.
- Mohammadi, M. Oxidative Stress and Polycystic Ovary Syndrome: A Brief Review. Int. J. Prev. Med. 2019, 10, 86.
- Morgante, G.; Massaro, M.G.; Scolaro, V.; Cappelli, V.; Luddi, A.; Troìa, L.; De Leo, V. Metformin Doses and Body Mass Index: Clinical Outcomes in Insulin Resistant Polycystic Ovary Syndrome Women. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8136–8142.
- De Leo, V.; Musacchio, M.C.; Morgante, G.; La Marca, A.; Petraglia, F. Polycystic Ovary Syndrome and Type 2 Diabetes Mellitus. Minerva Ginecol. 2004, 56, 53–62.
- Lerchbaum, E.; Obermayer-Pietsch, B. Vitamin D and Fertility: A Systematic Review. Eur. J. Endocrinol. 2012, 166, 765–778.
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The Role of Vitamin D and Calcium in Type 2 Diabetes. A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029.
- Milner, R.D.; Hales, C.N. The Role of Calcium and Magnesium in Insulin Secretion from Rabbit Pancreas Studied in Vitro. Diabetologia 1967, 3, 47–49.
- Lerchbaum, E.; Rabe, T. Vitamin D and Female Fertility. Curr. Opin. Obstet. Gynecol. 2014, 26, 145–150.
- Maestro, B.; Molero, S.; Bajo, S.; Dávila, N.; Calle, C. Transcriptional Activation of the Human Insulin Receptor Gene by 1,25-Dihydroxyvitamin D(3). Cell Biochem. Funct. 2002, 20, 227–232.
- Rajakumar, K.; de las Heras, J.; Chen, T.C.; Lee, S.; Holick, M.F.; Arslanian, S.A. Vitamin D Status, Adiposity, and Lipids in Black American and Caucasian Children. J. Clin. Endocrinol. Metab. 2011, 96, 1560–1567.
- Oliveira, R.M.S.; Novaes, J.F.; Azeredo, L.M.; Azeredo, L.M.; Cândido, A.P.C.; Leite, I.C.G. Association of Vitamin D Insufficiency with Adiposity and Metabolic Disorders in Brazilian Adolescents. Public Health Nutr. 2014, 17, 787–794.
- Guasch, A.; Bulló, M.; Rabassa, A.; Bonada, A.; Del Castillo, D.; Sabench, F.; Salas-Salvadó, J. Plasma Vitamin D and Parathormone Are Associated with Obesity and Atherogenic Dyslipidemia: A Cross-Sectional Study. Cardiovasc. Diabetol. 2012, 11, 149.
- Ganie, M.A.; Marwaha, R.K.; Nisar, S.; Farooqi, K.J.; Jan, R.A.; Wani, S.A.; Gojwari, T.; Shah, Z.A. Impact of Hypovitaminosis D on Clinical, Hormonal and Insulin Sensitivity Parameters in Normal Body Mass Index Polycystic Ovary Syndrome Women. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2016, 36, 508–512.
- Alvarez, J.A.; Ashraf, A. Role of Vitamin d in Insulin Secretion and Insulin Sensitivity for Glucose Homeostasis. Int. J. Endocrinol. 2010, 2010, 351385.
- Li, H.W.R.; Brereton, R.E.; Anderson, R.A.; Wallace, A.M.; Ho, C.K.M. Vitamin D Deficiency Is Common and Associated with Metabolic Risk Factors in Patients with Polycystic Ovary Syndrome. Metabolism. 2011, 60, 1475–1481.
- Ngo, D.T.M.; Chan, W.P.; Rajendran, S.; Heresztyn, T.; Amarasekera, A.; Sverdlov, A.L.; O’Loughlin, P.D.; Morris, H.A.; Chirkov, Y.Y.; Norman, R.J.; et al. Determinants of Insulin Responsiveness in Young Women: Impact of Polycystic Ovarian Syndrome, Nitric Oxide, and Vitamin D. Nitric Oxide Biol. Chem. 2011, 25, 326–330.
- Menichini, D.; Facchinetti, F. Effects of Vitamin D Supplementation in Women with Polycystic Ovary Syndrome: A Review. Gynecol. Endocrinol. 2020, 36, 1–5.
- De Leo, V.; Cappelli, V.; Morgante, G.; Di Sabatino, A. The role of vitamin D in assisted reproduction techniques. Minerva Ginecol. 2018, 70, 268–285.
- Belenchia, A.M.; Tosh, A.K.; Hillman, L.S.; Peterson, C.A. Correcting Vitamin D Insufficiency Improves Insulin Sensitivity in Obese Adolescents: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2013, 97, 774–781.
- Yildizhan, R.; Kurdoglu, M.; Adali, E.; Kolusari, A.; Yildizhan, B.; Sahin, H.G.; Kamaci, M. Serum 25-Hydroxyvitamin D Concentrations in Obese and Non-Obese Women with Polycystic Ovary Syndrome. Arch. Gynecol. Obstet. 2009, 280, 559–563.
- Ott, J.; Wattar, L.; Kurz, C.; Seemann, R.; Huber, J.C.; Mayerhofer, K.; Vytiska-Binstorfer, E. Parameters for Calcium Metabolism in Women with Polycystic Ovary Syndrome Who Undergo Clomiphene Citrate Stimulation: A Prospective Cohort Study. Eur. J. Endocrinol. 2012, 166, 897–902.
- Gallea, M.; Granzotto, M.; Azzolini, S.; Faggian, D.; Mozzanega, B.; Vettor, R.; Mioni, R. Insulin and Body Weight but Not Hyperandrogenism Seem Involved in Seasonal Serum 25-OH-Vitamin D3 Levels in Subjects Affected by PCOS. Gynecol. Endocrinol. 2014, 30, 739–745.
- Maestro, B.; Dávila, N.; Carranza, M.C.; Calle, C. Identification of a Vitamin D Response Element in the Human Insulin Receptor Gene Promoter. J. Steroid Biochem. Mol. Biol. 2003, 84, 223–230.
- Bikle, D. Nonclassic Actions of Vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34.
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology 2007, 132, 2169–2180.
