Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by James C. L. Chow and Version 2 by Rita Xu.
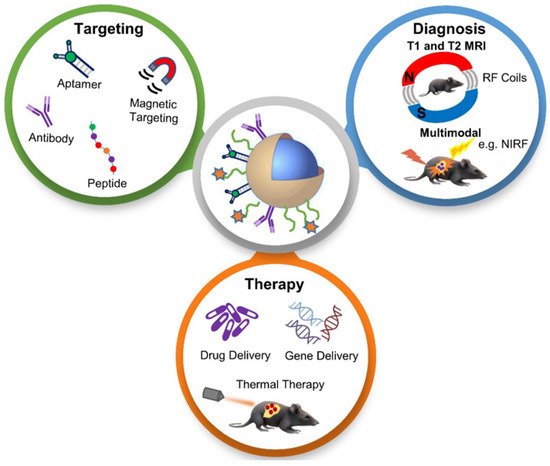
Cancer theranostics is the combination of diagnosis and therapeutic approaches for cancer, which is essential in personalized cancer treatment. The aims of the theranostics application of nanoparticles in cancer detection and therapy are to reduce delays in treatment and hence improve patient care. Recently, it has been found that the functionalization of nanoparticles can improve the efficiency, performance, specificity and sensitivity of the structure, and increase stability in the body and acidic environment. Moreover, functionalized nanoparticles have been found to possess a remarkable theranostic ability and have revolutionized cancer treatment.
- functionalized nanoparticles
- MRI-guided therapy
- molecular imaging
1. Introduction
Cancer treatment has gained considerable attention in biomedical research over the past few decades due to the serious threat it poses to human health. The mortality rate of cancer increases every year, which leads to the need for the development of more efficient cancer therapeutic strategies [1]. Even though there is a major advance in cancer therapy, it continues to be a significant challenge due to tolerability and adherence [2]. Theranostics is a term first used by John Funkhouser at the beginning of the 1990s. It is defined as a combination of diagnostic tools that are the most suitable for specific diseases [3]. Theranostics portrays a close connection between diagnostics and the consequent therapy, and the theranostic principle has attracted huge attention in personalized medicine, in particular oncology. This allowed tumours at the advanced stage to be treated accurately with fewer side effects. For decades theranostics have been used for the therapy of benign and malignant thyroid diseases; however, recently, theranostics have been applied to other malignancies [4]. Theranostics agents such as radioisotopes, liposomes, quantum dots and plasmonic nanobubbles can be attached to anticancer drugs, imaging agents and cancer cell markers with the support of imaging techniques, providing the potential to facilitate the diagnosis, treatment and management of cancer patients [5]. The development of highly sensitive imaging modalities such as SPECT and PET with the synthesis of novel radiolabelled molecules specific for different biochemical targets promoted nuclear medicine into a new era [6]. These molecular imaging modalities have been applied in cardiology, neuroscience, oncology, gene therapy and theranostics. Nanoparticles (NPs) have been used as therapeutic or imaging agents that enhance the efficacy and control biodistribution and reduce the toxicity of drugs. In 2014–2015, there were 51 FDA-approved nanomedicines that met the definition of nanomedicines as therapeutic or imaging agents, and 77 products in clinical trial [7]. One of the crucial characteristics of nanomaterials is their small size. Their high affinity, high specificity, high thermal stability, low off-target accumulation and good solubility are among many adventurous characteristics they possess in cancer therapy. They can penetrate dense tissues of the tumour very well [8]. Nanotechnology in medicine is currently developed for drug delivery, and many substances are under study for cancer therapy. Solid NPs can be used for drug targeting when they reach the intended diseased site in the body, and the toxicology of the drug nanocarriers has been evaluated [9]. Active targeting is accomplished by conjugating tumour-specific ligands to the NPs’ surface. It complements the enhanced permeability and retention effect (EPR). EPR is a universal pathophysiological phenomenon and mechanism where macromolecules with certain sizes above 40 kDa can progressively accumulate in the tumour vascularized area and achieve targeted delivery and retention of the anticancer compound into the solid tumour [10]. Some of the particles that are used to functionalize NPs are antibodies or antibody fragments, human transferrin protein, peptides, carbohydrates and vitamins. These biomarkers are recognized by their representative targeting ligands such as epidermal growth factor, human epidermal growth factor 2, Mucin-1, nucleolin, epithelial cell adhesion molecule and platelet-derived growth factor receptor 2. For anticancer drug delivery, Fu et al. [11] proposed to use aptamer-functionalized nanoparticles. This is because aptamers have favourable features such as a small size, very low immunogenicity, low cost of production and high affinity and specificity. The advantage of NPs as a theranostics agent is shown below in Figure 1 [12].
Figure 1. Advantages using nanoparticles in cancer theranostics.
2. Magnetic Resonance Imaging (MRI)
MRI is one of the most powerful means of clinical detection and prognosis observation [13][17]. MRI is an imaging modality that is non-invasive, and it provides comprehensive multi-parametric information generally used for brain imaging [14][18]. MRI benefits from the contrast agent that provides a more improved depiction of large and medium-sized vassals and can provide dynamic vascular/perfusional properties of tissues. Gadolinium (Gd)-based contrast agents are widely used in MRI [15][16][19,20]. MRI can be coupled with other therapy to provide image-guided therapy for better treatment outcomes and tumour-targeting ability [17][21]. A study synthesized a multifunctional Gd-DTPA-ONB lipid by adding the Gd-DTPA contrast agent to an o-nitro-benzyl ester lipid. It combines the MRI tracking ability with dual trigger release capabilities, which allow maximum sensitivity without reducing the drug encapsulation rate. It can be activated by both PH-trigger hydrolysis and photo treatment [18][22]. Another Gd nanocomposite was synthesized by decorating Gd NPs onto the graphene oxide, and then functionalized with polyethylene glycol and folic acid. It was used to load doxorubicin to accomplish targeted image-guided drug delivery with MRI [19][23]. Liposomes are a useful class of NPs due to their tunable properties and multiple liposomal drug formulation. They have been clinically approved for cancer treatment. A vast number of Gd-based liposomal MRI contrast agents have been developed that can be used for targeted image-guided drug delivery [20][24]. Chemical exchange saturation transfer MRI has important advantages such as its ability to detect diamagnetic compounds that are not detectable using conventional MRI. It makes a broad spectrum of bioorganic agents, nanocarriers and natural compounds directly MRI detectable with a high resolution. It is advantageous for image-guided drug delivery [21][25]. An in vivo study looked at amphiphilic polymer-coated magnetic iron oxide NPs that were conjugated with near-infrared (NIR) dye-labelled HER2 affibody and chemotherapy drugs. Cisplatin was the drug used as the chemotherapy drug. MRI-guided therapy and the optical imaging detection of the therapy-resistant tumour were examined in an orthotopic human ovarian cancer xenograft model with a high level of HER2 expression. The result shows it significant inhibited the primary tumour and peritoneal and lung metastases in the ovarian cancer model in mice [22][26]. Another study looked at the NP with a unique morphology, which consists of a superparamagnetic iron oxide core and star-shaped plasmonic shell with high aspect ratio branches. Its strong near-infrared responsive plasmonic properties and magnetic properties allow it to be used in multimodal quantitative imaging, which combines the advantageous functions of MRI, magnetic particle imaging (MPI) and photoacoustic imaging. It can be used for image-guided drug delivery with tunable drug release capacity [23][27]. Drug resistance in chemotherapy has been a challenge for a long time in pancreatic cancer due to the stomal barrier making it difficult to reach the tumour microenvironment. A study developed IGF1 receptor-directed multifunctional theragnostic NPs for the targeted delivery of Dos into IGF1R-expressing drug-resistant tumour cells and tumour-associated stromal cells. NPs were prepared by combining IGF1 with magnetic iron oxide NPs carrying dox. They provided an excellent theranostics platform and showed good tumour control in an in vivo study [24][28]. Superparamagnetic iron oxide NPs have also been widely used in MRI and nanotheranostics. They can be coated with a biocompatible polymer such as polyethylene glycol or dextran, which allows chemical conjugation. They have a very high potential in MRI-guided drug delivery [25][29]. Figure 2 shows superparamagnetic iron oxide NPs being used in liver imaging and lymph node imaging [26][30].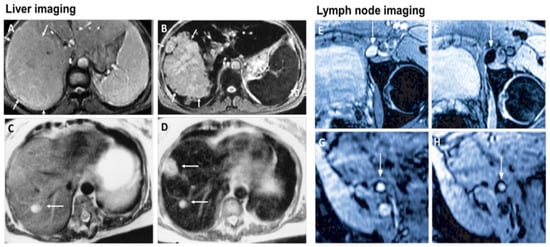
Figure 2. Superparamagnetic iron oxide NPs being used in liver imaging and lymph node imaging. (A,B): T2-weighted MR image of a liver with a large hepatocellular carcinoma before (A) and after (B) the administration of SPION. The lesion is demarcated with arrows. (C,D): Standard (C) and SPION-based contrast-enhanced (D) MR imaging of liver metastasis in a patient with colorectal cancer. After administration of ferumoxide SPION, a second metastasis becomes visible on T2-weighted MR image. (E,H): Lymph node in left iliac region (arrow), with and without metastatic infiltration. T2-weighted images before (E,G) and 24 h after (F,H) administration of ferumoxtran. Lymph node (arrow) appears bright before injection of UPIO (E,G). One day after injection, a signal loss in the lymph node (arrow) due to high UPIO macrophage uptake can be observed, thus indicating functionality and no metastasis (F). Conversely, in the lower panel, the lymph node (arrow) stays bright, indicating no trafficking of USPIO and thus metastatic colonization (H). Reprinted with permission from Ref. [26][30]. Copyright 2020 Elsevier.
2.1. MRI-Guided NPs for Gene Therapy
Gene therapy has gained considerable attention over the years and the health community has gained much more new information and knowledge regarding gene therapy [27][31]. Gene therapy is a form of engineered viruses carrying a therapeutic agent or containing genetically modified cells such as when chimeric antigen receptors are introduced to the T lymphocytes for cancer therapy such as for leukemia [28][32]. New gene therapy has shown its potential to significantly improve the survival rate of cancer patients [29][33]. For cancer gene therapy, the therapeutic agent generally requires a carrier such as an NP. MRI allows the tracking of that carrier and allows image-guided therapy, which can significantly improve the outcome [30][34]. A study looked at low molecular weight poly (ethylenimine)-poly (ethylene glycol) nanogels loaded with transforming growth factor -β1 siRNA and ultra-small iron oxide NPs for gene therapy and a T1-weighted MRI of tumour and tumour metastasis in a mouse sarcoma model. The study result shows it enhances the MRI image and effectively delivers the siRNA and inhibits tumour growth in the subcutaneous sarcoma tumour model and lung metastasis by silencing the TGF-β1 gene [31][35]. Another study investigated shaped, controlled magnetic mesoporous silica NPs and their performances in magnetic resonance image-guided targeted hyperthermia-enhanced suicide gene therapy of hepatocellular carcinoma. They had a higher loading capacity and better magnetic hyperthermia properties. They also had decreased cytotoxicity [32][36]. A bowl-shaped Fe3O4 NP with a self-assembly concept and appropriately surface-functionalized was studied with the aim for it to be used as a multifunctional carrier in combination therapy and gene therapy. The in vivo result shows promising results in the mouse breast cancer model [33][37]. The catalytic deoxy ribozyme has great potential in gene therapy via gene regulation but requires the carrier to reach the tumour target. A study showed polydopamine-Mn2+ NPs to be effective carriers and together they can be used as a photothermal agent and contrast agent for photoacoustic and magnetic resonance imaging [34][38]. Another study developed Fe3O4@PDA NPs to transport siRNA for gene therapy. The NPs were coated with mesenchymal stem cells to form a membrane. The overall complex showed good transport ability and photothermal functionality, and enhanced MRI capability [35][39].2.2. MRI-Guided NPs for Thermal Therapy
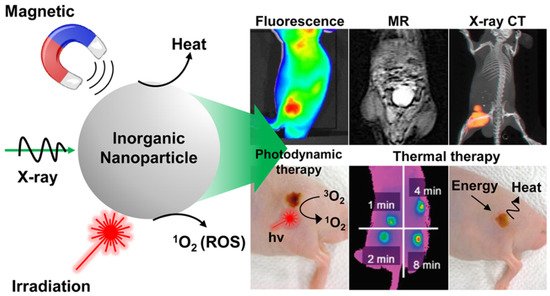
Light-activated therapies have been introduced for cancer treatment for numerous cancers. Two of the main methods are localizing chemical exchange on the tumour known as photodynamic therapy (PDT) and localized thermal damage to the tumour, also known as photothermal therapy (PTT) [36][40]. Inorganic NPs have gained significant attention in image-guided thermal therapy in recent years, and the applications of inorganic NPs in tumour imaging and therapy are shown in Figure 3. The NPs contain metal, a semiconductor, metal oxide, nanocrystal and lanthanide-doped up conversion NPs. They can generate heat and reactive oxygen species, so they are ideal for image-guided PTT [37][41]. The thermal energy also promotes the gasification of perfluoropentane to enable the visualization of cancer tissue in ultrasound imaging, as well as enhances MRI imaging, and makes it ideal for dual MRI ultrasound imaging [38][42]. Core/shell nanoparticles were investigated for MRI imaging, magnetic hyperthermia and PTT due to their surface being coated with a porous shell. It can entrap large quantities of water around the nanoparticles and allows enhanced and efficient water exchange, which provides an improved magnetic resonance contrast signal. It also helps with NIR absorbance of the core and can have an enhanced thermal effect via synergistic PTT and magnetic hyperthermia. The nanoparticles investigated for this purpose were MnFe2O4/PB [39][43]. Another study developed temperature-activated engineered neutrophils by combining indocyanine green-loaded magnetic silica NIR sensitive nanoparticles. It provides a platform for dual-targeted PTT. The combination of magnetic targeting and neutrophil targeting provides an enhanced accumulation of the photothermal agent at the tumour site [40][44]. A study wrapped together gadolinium-DTPA, indocyanine green and perfluoropentane in a poly (lactic-co-glycolic) acid shell membrane by a double emulsion approach. Under NIR the indocyanine green converts the optic energy into thermal energy and converts oxygen to singlet oxygen, which destroys cancer cells through PTT and PDT. Another nanotheranostics agent was prepared via the participation of hydrophilic CuS nanoparticles, styrene, methacrylic acid, N-isopropylacrylamide and a polymerizable rare earth complex. It had good biocompatibility with a high loading capacity for DOX-HCI. Drug release can be activated via PH or high temperature. All these properties make it ideal for PTT and chemotherapy. MRI can also be used on it for image-guided drug delivery [41][45]. CuS material shows poor MRI ability but excellent photo absorption ability, whereas Fe-based materials have good MRI ability. A study combined the two and made a CuxFeySz sample that includes CuFeS2, FeS2 and Cu5FeS4 nanomaterials. The study result shows it to have high potential in MRI-guided photothermal enhanced chemo dynamic therapy [42][46].