Sea cucumbers are considered a luxury food item and used locally in traditional medication due to their impressive nutritional profile and curative effects. Sea cucumbers contain a wide range of bioactive compounds, namely phenolics, polysaccharides, proteins (collagen and peptides), carotenoids, and saponins, demonstrating strong antioxidant and other activities. In particular, phenolic compounds, mainly phenolic acids and flavonoids, are abundant in this marine invertebrate and exhibit antioxidant activity. Protein hydrolysates and peptides obtained from sea cucumbers exhibit antioxidant potential, mainly dependent on the amino acid compositions and sequences as well as molecular weight, displayed for those of ≤20 kDa. Moreover, the antioxidant activity of sea cucumber polysaccharides, including fucosylated chondroitin sulfate and fucan, is a combination of numerous factors and is mostly associated with molecular weight, degree of sulfation, and type of major sugars. However, the activity of these bioactive compounds typically depends on the sea cucumber species, harvesting location, food habit, body part, and processing methods employed.
- sea cucumber
- antioxidants
- phenolics and polyphenolics
- protein hydrolysates and peptides
- polysaccharides
- carotenoids
1. Introduction
2. Bioactive Compounds of Sea Cucumbers and Their Antioxidant Activity
Sea cucumbers are a highly marketable echinoderm, which contains numerous bioactive compounds. These include proteins (collagen and peptides), polysaccharides, saponins, carotenoids, and phenolics with multiple biological and pharmacological properties, mainly antioxidant activity [2][6][2,7]. Antioxidants are substances that scavenge free radicals and hence prevent oxidation. The main mechanisms involved are hydrogen atom transfer (HAT), single electron transfer (SET), metal chelation, and reducing power. Therefore, the effectiveness of antioxidants in a specific medium is mainly dependent on the number and arrangement of the hydroxyl groups in the molecules of interest [7][8]. For example, phenolic antioxidants can donate hydrogen atoms from the hydroxyl groups to lipid radicals in order to neutralize the oxidation reaction, but the phenoxyl radicals that are produced are resonance stabilized and are therefore not involved in further oxidation, thus breaking the cycle of the generation of new radicals. Antioxidants with specific structures can also chelate metal ions (e.g., ferrous and copper), where metal ions can no longer act as an initiator of lipid oxidation due to the formation of a complex between the metal ions and antioxidants. Besides, synergistic effects can be observed among various antioxidants such as phenolics, α-tocopherol, β-carotene, and ascorbic acid [8][9][9,10]. Numerous techniques are available for determining antioxidant activity, including radical scavenging assays that include SET (e.g., ferric-reducing antioxidant power (FRAP), Trolox equivalent antioxidant capacity (TEAC), and the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay) and HAT (e.g., total radical-trapping antioxidant parameter (TRAP) and oxygen radical absorbance capacity (ORAC)) mechanisms [10][11][11,12]. However, each assay has a different mechanism of action, thus providing varied results for the antioxidant potential of the same sample [12][13]. On the other hand, due to lipid oxidation, the quality attributes of food, including flavor, color, and texture, deteriorate, which ultimately decreases the shelf life and nutritional value of food. Thus, antioxidants are widely used to control the rate and extent of lipid oxidation in foods. One of the main assays to measure the degree of lipid oxidation is the thiobarbituric acid (TBA) test that measures the TBA reactive substances (TBARS), which are then used to determine the secondary oxidation products, mainly the aldehydes, from among the others that are believed to produce rancid flavors and aromas [13][14]. Autoxidation is one of the main pathways of lipid oxidation in which PUFAs are involved in a free radical chain reaction under heat, light, or metal ions. Synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), tert-butylhydroquinone (TBHQ), and propyl gallate (PG) have been used as antioxidants in foods to prevent oxidation and off-flavor development. Nevertheless, due to the carcinogenic and toxicity characteristics of some synthetic antioxidants, researchers have shown much attention towards natural antioxidants [14][15][15,16]. For instance, sea cucumbers and their by-products are a good source of phenolic acids and flavonoids, which show strong antioxidant activity. The specific bioactive compounds in most common sea cucumbers and their antioxidant activities are detailed below (Figure 1).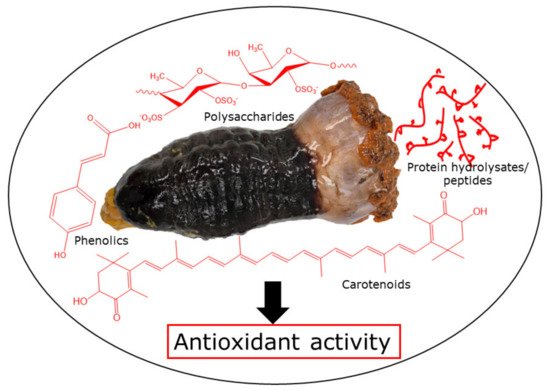
2.1. Antioxidant Potential of Sea Cucumber Phenolics and Their Beneficial Effects on Human Health
Phenolic compounds are secondary metabolites that contain one or more aromatic rings and hydroxyl groups. Moreover, phenolic compounds play a key role in protecting plants by engaging in defense mechanisms against ultraviolet radiation, herbivory, and pathogen attacks. Phenolics are also involved in the plants for growth regulation and are responsible for the color, flavor, bitterness, and astringency of foods. Plant phenolics are mainly derived from phenylalanine and, in some cases, tyrosine. The formation of trans-cinnamic acid from phenylalanine is catalyzed by phenylalanine ammonia-lyase (PAL), whereas p-hydroxycinnamic acid from tyrosine is catalyzed by tyrosine ammonia-lyase (TAL) [16][17]. Phenolics can be categorized into different groups, namely phenolic acids, flavonoids, tannins, stilbenes, lignans, and coumarins [17][18]. Due to their antioxidant, antimicrobial, and coloring properties, phenolic compounds have received significant attention from several industries, especially from the food, pharmaceutical, cosmetics, packaging, and textile industries. In the food industry, phenolics are used as preservatives to inhibit the oxidation process and microbial growth of food products. Plant- and marine-based phenolic compounds are receiving increased attention due to their potential health benefits and multiple biological activities. Most of the phenolics have so far been researched from the terrestrial environment, but less attention has been paid to the marine environment, even though it provides many healthy foods due to its biodiversity. Sea cucumbers are one of the marine invertebrates that serves as a possible source of phenolic compounds with strong antioxidant activity. This could be due to the absorption of phenolics from phytoplankton, the primary food source for sea cucumbers. Phytoplankton is a rich source of phenolic compounds, including phenolic acids, flavonoids, and tannins [2][18][2,19]. For example, Ceesay et al. [19][20] reported that sea cucumbers contain catechins and flavonols as they feed mainly on seaweeds, rich sources of flavonoids. Various species of sea cucumbers have different levels of phenolic compounds and varied antioxidant activities. This might be due to the different geographic locations, food habits, and harvesting times. Therefore, suspension-feeding species, such as Cucumaria frondosa, may have more phenolics when compared to the deposit-feeding species. It has been reported that the different body parts of sea cucumbers, such as body wall, tentacles/flower, and viscera, contain a significant amount of phenolics with strong antioxidant activity. For example, Althunibat et al. [20][21] compared the antioxidant activity of three Malaysian sea cucumber species (Holothuria leucospilota, Holothuria scabra, and Stichopus chloronotus) without viscera, and reported that the extracts of H. leucospilota had higher total phenolic contents (TPC, 9.7 mg gallic acid equivalents (GAE)/g), but H. scabra contained a lower amount of TPC (1.53 mg GAE/g). S. chloronotus extracts showed a higher DPPH radical-scavenging capacity (80.58%) compared to the H. scabra (77.46%) and H. leucospilota (64.03%) extracts. Likewise, methanol extracts of H. scabra were found to be a good source of phenolics (30.52 mg GAE/g), dominated by 3- and 4-hydroxybenzaldehyde [21][22]. Wulandari et al. [22][23] cultured Holothuria scabra in an open pond system and found that antioxidant activity such as ABTS and hydroxyl radical-scavenging activities as well as ferric-reducing antioxidant power (FRAP) were related to the total flavonoid content (TFC). Besides, TPC and TFC were determined in the body wall of H. leucospilota, which contained 2,4-bis(1,1-dimethylethyl)-phenol [19][20]. Pre-treatments also affect the content of sea cucumber phenolic and their antioxidant activities. For example, free, esterified, and insoluble-bound phenolics from the body wall and internal organs of Atlantic sea cucumber (Cucumaria frondosa) were determined using high-pressure processing (HPP) pre-treatment [5][23][5,24]. Results demonstrated that HPP significantly improved the TPC, TFC, and antioxidant activity. TPC varied between 3.05 and 3.98 mg GAE/g for the body wall and 2.32–3.02 mg GAE/g for internal organs, while the TFC was 1.22–1.55 mg catechin equivalents (CE)/g and 1.01–1.24 mg CE/g for body wall and internal organs, respectively. Additionally, phenolic extracts obtained from the body wall and internal organs exhibited strong antioxidant activity in terms of DPPH, ABTS, and hydroxyl radical scavenging as well as metal chelation activities, which showed a strong positive correlation with TPC and TFC. Especially, TFC had a strong correlation with antioxidant activity, suggesting that sea cucumber phenolics are mostly flavonoids, which are responsible for the antioxidant effect. On the other hand, the antioxidant activity, TPC, and TFC were determined in extracts from different body parts (digestive tract, gonad, muscle, and respiratory apparatus) of C. frondosa [24][25]. The TPC varied from 0.22 to 2.36 mg GAE/g, while TFC ranged from 0.029 to 0.59 mg rutin equivalents (RE)/g and the oxygen radical absorbance capacity (ORAC) varied from 140 to 800 µmol Trolox equivalents (TE)/g. A higher TPC was also observed in the digestive tract when considering acetonitrile-rich fractions and ethyl acetate extracts, while the maximum TFC was obtained from the gonads using water-rich and acetonitrile-rich fractions. Similarly, Mamelona and Pelletier [25][26] determined the antioxidant activity (ORAC) of the viscera of C. frondosa using pressure liquid extraction (PLE) and observed that the ethanol extracts had higher ORAC values when compared to methanol, isopropanol, and water extracts at 60 °C extraction. Additionally, PLE allowed better extraction of TPC, total carotenoids, and α-tocopherol using ethanol followed by isopropanol, methanol, and water. In another study, the antioxidant property of fresh and processed C. frondosa with/without internal organs was evaluated [18][19]. The processed (rehydrated) samples, mainly those with internal organs, exhibited higher ORAC and DPPPH radical-scavenging activity, whereas fresh samples contained a significant amount of phenolics when compared to their rehydrated counterparts. However, Ridhowati et al. [26][27] reported that dried Stichopus variegatus contained a higher content of TPC (10.55–10.9 mg GAE/g) with strong DPPH radical-scavenging activity. Similarly, Husni et al. [27][28] stated that the TPC of Apostichopus japonicus body wall extract had a good correlation with antioxidant activity than the TFC, suggesting that phenolic compounds play an important role in exhibiting antioxidant activity. Sea cucumber phenolics are mainly phenolic acids and flavonoids. The most common phenolic compounds found in sea cucumbers are chlorogenic acid, gallic acid, p-coumaric acid, protocatechuic acid, ferulic acid, ellagic acid, cinnamic acid, catechin, rutin, quercetin, and pyrogallol (Figure 2).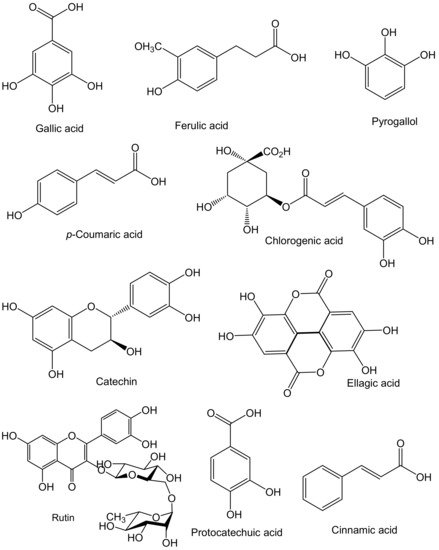
2.2. Antioxidant Potential of Protein Hydrolysates and Peptides and Their Health Benefits
Marine products and by-products are protein-rich and can be used to prepare protein hydrolysates, collagens, or peptides. The most common ways of producing protein hydrolysates include enzymatic (Flavourzyme, Alcalase, Protamex, papain, trypsin, chymotrypsin, and pepsin, among others), non-enzymatic (high-pressure processing, ultrasound, and supercritical fluids), organic solvents, and fermentation methods [28][29][41,42]. Among them, enzymatic procedures have received growing attention due to their higher efficiency, green nature, and lesser destruction than other techniques in order to produce value-added products for disease risk reduction and health promotion. In particular, microbial proteases, including Flavourzyme, Alcalase, and Corolase are favorable in industrial use due to their promising operational conditions [30][43]. However, the functionality of protein hydrolysates/peptides is mainly dependent on their amino acid compositions and sequences, molecular weight, and hydrophobicity/hydrophilicity, among others. Generally, bioactive peptides contain 3–20 amino acid units and show antioxidant activity [31][44]. Notably, the antioxidant activity of the bioactive peptides can improve in the presence of amino acids such as tyrosine, phenylalanine, proline, glutamic acid, histidine, and arginine. For example, proline is very common in collagen, shielding cells from the oxidation induced by free radicals [32][45]. The physicochemical and antioxidant properties of the protein hydrolysates of C. frondosa and its processing discards were evaluated using Alcalase, Flavourzyme, and Corolase as well as their combination [30][43]. The hydrolysates prepared with combination of enzymes were predominant in glutamic acid and displayed the highest radical-scavenging activity against ABTS and DPPH radicals as well as metal-chelation activity. In addition, hydrolysates were able to inhibit TBARS production in a meat model system and beta-carotene bleaching in an oil-in-water emulsion. They also noted that the level of free amino acids after hydrolysis, the degree of hydrolysis, amino acid sequence, and molecular weight played the main role in rendering radical-scavenging activity. Similarly, Yan et al. [33][46] prepared enzymatic hydrolysates from C. frondosa viscera using Alcalase, Flavourzyme, Neutrase, trypsin, papain, and bromelain and observed that Alcalase, Flavourzyme, and trypsin were major enzymes that resulted in strong antioxidant activity, possibly related to a high amount of hydrophobic amino acids in the hydrolysates. The choice of proteases in the preparation of protein hydrolysates plays a significant role in the resultant antioxidant potency and bioactivity of peptides. For example, Alcalase-produced protein hydrolysates obtained from C. frondosa exhibited up to 35% higher in vitro antioxidant activity (e.g., DPPH radical, hydroxyl radical, and superoxide radical anion-scavenging properties) than the trypsin-produced hydrolysates, suggesting that the amino acid composition and structural conformation of the peptides played main roles in determining antioxidant activity [34][47]. This is because trypsin-produced peptide fractions were not the same as Alcalase-produced peptide fractions, mainly when the sizes of the peptides were small (≤10 kDa). Moreover, in silico docking for in vivo function prediction demonstrated a better inhibitory activity in myeloperoxidase (a marker of in vivo oxidative stress) by Alcalase than by trypsin. Likewise, protein hydrolysates obtained from Atlantic sea cucumber viscera showed antioxidant activities in lipid oxidation tests and the ORAC assay, which could be related to the releasing of antioxidative peptides upon hydrolysis [35][48]. The findings of Wang et al. [36][49] suggest that C. frondosa internal organs hydrolysates have the potential to show anti-diabetic activity through insulin resistance and lipid metabolism syndromes. Besides, enzymatic hydrolysates prepared from C. frondosa by-products (aquapharyngeal bulb and internal organs) using nine different proteases, including Alcalase, bromelain, Flavourzyme, fungal protease, neutral protease, papain, peptidase AM (Aspergillus melleus), peptidase AO (Aspergillus oryzae), and Protamex, were tested against Herpes Simplex virus 1 (HSV-1) [37][50]. Results suggested that papain was the most efficient enzyme in demonstrating antiviral activities. Lin et al. [38][51] reported the anti-aging effect of sea cucumber (C. frondosa) hydrolysates, which was mainly linked to the low-molecular-weight (~<3 kDa) peptides. The anti-aging mechanism could be related to the up-regulated Klotho expression, down-regulated acetylcholinesterase activity, increased SOD and GSH-Px activities, and lipid and protein oxidation inhibition.2.3. Antioxidant Potential of Sea Cucumber Polysaccharides
Sea cucumber, mainly the body wall, is a good source of polysaccharides, including sulfated polysaccharides (fucosylated chondroitin sulfate, FCS) and fucan (Figure 3).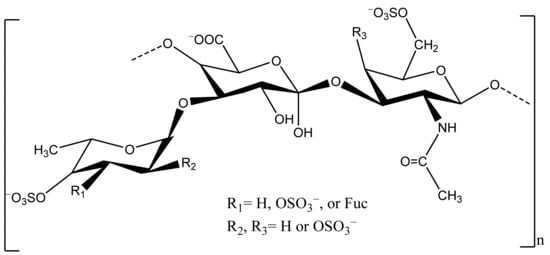
2.4. Antioxidant Potential of Carotenoids and Physiological Effects of PUFAs
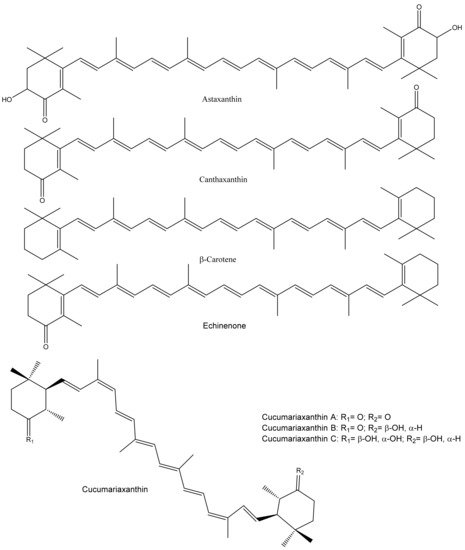
Marine animals, including sea cucumbers, are a good source of carotenoids that show structural diversity. Sea cucumbers, mainly their gonads and aquapharyngeal bulb/tentacles, contain various carotenoids (Figure 4).