Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Alexandru Achim.
Peripheral artery disease (PAD) increases the risk of diabetes, while diabetes increases the risk of PAD, and certain symptoms in each disease increase the risk of contracting the other. The phenotypic manifestations of atherosclerosis vary in each individual and throughout the body; it is not fully understood why plaque formation has such a heterogeneous distribution, although different arterial systems are correlated. Moreover, risk factors (such as diabetes mellitus, hypertension, smoking, etc.) undoubtedly aggravate atherogenesis and cardiovascular mortality through a dual risk: firstly, the intrinsic risk of the underlying disease; secondly, it increases the risk of atherosclerosis in various target organs.
- guidelines
- PAD
- antiplatelet therapy
1. Introduction
The phenotypic manifestations of atherosclerosis vary in each individual and throughout the body; it is not fully understood why plaque formation has such a heterogeneous distribution, although different arterial systems are correlated [1,2,3][1][2][3]. Moreover, risk factors (such as diabetes mellitus, hypertension, smoking, etc.) undoubtedly aggravate atherogenesis and cardiovascular mortality through a dual risk: firstly, the intrinsic risk of the underlying disease; secondly, it increases the risk of atherosclerosis in various target organs. In other words, the co-prevalence of two atherogenic conditions is not simply an association due to shared risk factors; one may in fact play a fundamental role in the pathophysiology of the other and vice versa. WResearchers know that there is a bidirectional relationship between the risk of cardiovascular disease and systemic diseases, such as diabetes mellitus or primary hypertension. Thus, the complex relationship between atherogenesis and risk factors intersects at several levels, and perhaps the severity of the patient’s clinical manifestations best demonstrates this harmful interplay [4,5][4][5].
An evocative example would be peripheral artery disease (PAD), which is a direct macrovascular disorder of diabetes mellitus (DM). PAD raises the risk of DM, and DM raises the risk of PAD. Each 1% increase in hemoglobin A1c (HbA1c) is associated with a nearly 30% increase in the risk of developing PAD during the follow-up period. PAD also manifests earlier in diabetics and progresses more rapidly to critical limb ischemia. In addition, a diabetic patient with PAD has a 14.2% increased risk of major cardiovascular events when HbA1c increases by 1% [6,7][6][7]. This deleterious interaction is self-evident, although the relationship between diabetes and vasculopathy has not been fully elucidated, despite considerable research efforts in molecular, animal, and translational models. Currently, the consequences of peripheral ischemia are thought to result from the interaction between hemodynamic, neurohumoral, and metabolic factors, leading to endothelial dysfunction. However, numerous fundamental questions remain unanswered. For instance, the phenomenon of the regression of microvascular disease following strict glycemic control is not observed in the larger vessels [5]. This situation highlights the need for an aggressive multidisciplinary approach to limb salvage in the diabetic population.
2. Management: Differences between Guidelines
The treatment of PAD in patients with DM has two main goals: to improve peripheral blood flow in symptomatic patients and to treat vascular risk factors and concomitant disorders, with an emphasis on coronary and cerebrovascular vascular diseases. Exercise training, such as structured walking, should be prescribed, while weight control must be advocated for overweight diabetics. A step-by-step schematic approach is shown in Figure 1.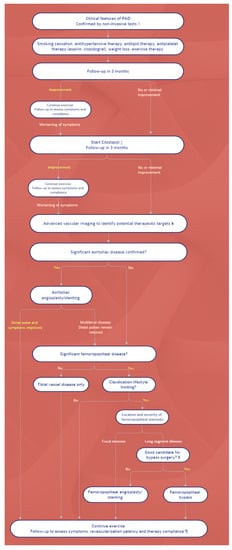
Figure 1. Algorithm for the management of PAD. Symbols: † Noninvasive studies include ankle-brachial pressure index, pulse volume recordings, partitioned pressures, and exercise testing. ‡ Cilostazol is recommended for patients with moderate-to-severe claudication. For patients with contraindications or for patients who do not tolerate cilostazol, pentoxifylline is an alternative. These can be prescribed simultaneously with the launching of an exercise program. ¥ CT or MR angiography. For patients with inflow occlusive disease (weak femoral pulse), conventional catheter-based arteriography may be performed initially, in expectation of possible intervention. § Optimal candidates for peripheral bypass surgery have favorable anatomy for bypass (target vessel, good runoff, and, ideally, vein conduit), are medically fit, and have an anticipated life expectancy that will allow the patient to benefit from the procedure. ¶ Following revascularization, antiplatelet therapy depends upon the nature of revascularization (i.e., the type of stent, type of bypass conduit); if initiated, cilostazol may be stopped.
References
- Wong, N.D.; Gransar Shaw, L.; Polk, D.; Moon, J.H.; Miranda-Peats, R.; Hayes, S.W.; Thomson, L.E.; Rozanski, A.; Friedman, J.D.; Berman, D.S. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. JACC Cardiovasc. Imaging 2009, 2, 319–326.
- Achim, A.; Kákonyi, K.; Nagy, F.; Jambrik, Z.; Varga, A.; Nemes, A.; Chan, J.S.K.; Toth, G.G.; Ruzsa, Z. Radial Artery Calcification in Predicting Coronary Calcification and Atherosclerosis Burden. Cardiol. Res. Pract. 2022, 2022, 5108389.
- Homorodean, C.; Leucuta, D.C.; Ober, M.; Homorodean, R.; Spinu, M.; Olinic, M.; Tataru, D.; Olinic, D.M. Intravascular ultrasound insights into the unstable features of the coronary atherosclerotic plaques: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2022, 52, e13671.
- Achim, A.; Marc, M.; Ruzsa, Z. Surgical Turned-Downed CHIP Cases-Can PCI Save the Day? Front. Cardiovasc. Med. 2022, 9, 872398.
- The Diabetes Control and Complications Trial (DCCT) Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am. J. Cardiol. 1995, 75, 894–903.
- Jones, W.S.; Baumgartner, I.; Hiatt, W.R.; Heizer, G.; Conte, M.S.; White, C.J.; Berger, J.S.; Held, P.; Katona, B.G.; International Steering Committee and Investigators of the EUCLID Trial; et al. Ticagrelor Compared with Clopidogrel in Patients with Prior Lower Extremity Revascularization for Peripheral Artery Disease. Circulation 2017, 135, 241–250.
- Low Wang, C.C.; Blomster, J.I.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Fowkes, F.G.R.; Held, P.; Katona, B.G.; Norgren, L.; EUCLID Trial Executive Committee and Investigators; et al. Cardiovascular and Limb Outcomes in Patients with Diabetes and Peripheral Artery Disease: The EUCLID Trial. J. Am. Coll. Cardiol. 2018, 72, 3274–3284.
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e686–e725.
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; ESC Scientific Document Group; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816.
- Balletshofer, B.; Ito, W.; Lawall, H.; Malyar, N.; Oberländer, Y.; Reimer, P.; Rittig, K.; Zähringer, M. Position Paper on the Diagnosis and Treatment of Peripheral Arterial Disease (PAD) in People with Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127 (Suppl. 1), S105–S113.
- Törngren, K.; Eriksson, S.; Arvidsson, J.; Falkenberg, M.; Johnsson, Å.A.; Sjöberg, C.; Lagerstrand, K.; Nordanstig, J. A Reperfusion BOLD-MRI Tissue Perfusion Protocol Reliably Differentiate Patients with Peripheral Arterial Occlusive Disease from Healthy Controls. J. Clin. Med. 2021, 10, 3643.
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Chronic Lower Extremity Ischemia and Its Association with the Frailty Syndrome in Patients with Diabetes. Int. J. Environ. Res. Public Health 2020, 17, 9339.
- Olinic, D.M.; Stanek, A.; Tătaru, D.A.; Homorodean, C.; Olinic, M. Acute Limb Ischemia: An Update on Diagnosis and Management. J. Clin. Med. 2019, 8, 1215.
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; COMPASS Investigators; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330.
- Bonaca, M.P.; Bauersachs, R.M.; Anand, S.S.; Debus, E.S.; Nehler, M.R.; Patel, M.R.; Fanelli, F.; Capell, W.H.; Diao, L.; Jaeger, N.; et al. Rivaroxaban in Peripheral Artery Disease after Revascularization. N. Engl. J. Med. 2020, 382, 1994–2004.
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Wick, N.; Hantusch, B.; Novatchkova, M.; Taubenschmid, J.; Hämmerle, M.; Esk, C.; Bagley, J.A.; et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019, 565, 505–510.
More
