You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Alessandro Furlan.
Most living organisms in both the plant and animal kingdoms have evolved processes to stay in tune with the alternation of day and night, and to optimize their physiology as a function of light supply. In mammals, a circadian clock relying on feedback loops between key transcription factors will thus control the temporally regulated pattern of expression of most genes. Modern ways of life have highly altered the synchronization of human activities with their circadian clocks. The circadian clock orchestrates most physiological events in living organisms and its deregulation in association with modern ways of life correlates with the rise of multiple pathologies in humans.
- circadian clock
- pathologies
- metabolic disorders
1. A Brief History of Circadian Rhythm Knowledge
Circadian rhythms in nature were observed for a long time, but their importance in physiology has come back into the spotlight recently. The circadian clock has gained much notoriety with the Nobel Prize in Physiology or Medicine awarded to Jeffrey Hall, Michael Rosbash, and Michael Young for their studies on the molecular mechanisms controlling the circadian rhythms [8,9,10][1][2][3].
In the plant kingdom, Androsthenes made the first report on circadian rhythmicity in the 4th century BC with the sleep movements of Tamaricus indicus’ leaves. Some two millennia occurred before some experimental work reinforced this concept of the plant circadian clock. In 1729, Jean-Jacques d’Ortous de Mairan actually noticed that the Mimosa leaves moved with a 24 h periodicity, even when he put the plant in a light-deprived environment [11][4]. This proved that an endogenous clock was able to impose a rhythm to processes, which could be discriminated from simple responses to daily stimuli.
Circadian rhythmicity is also obviously present in the behavioral patterns of animals (sleeping and feeding notably) and leads to the classification between diurnal and nocturnal animals. Besides, many biological processes including body temperature, hormone release, immune response, …, are now recognized to follow circadian evolutions.
Since almost all living organisms display circadian rhythms, this probably implies that evolution has selected the preservation of such rhythms. By allowing organisms to anticipate and prepare for circadian environmental changes, they would provide a selective advantage to living beings to optimize the light and food resources.
The molecular mechanisms responsible for these circadian rhythms remained elusive until the development of mutation screenings in the genome of the fruit fly a few decades ago. At that time, a seminal work highlighted the period (per) gene as critical for the periodicity of the fly activity [12][5]. Since then, researchers identified several other genes as core clock genes, driving circadian rhythms. This clock is based on an intricate network of intertwined transcriptional feedback loops acting as endogenous oscillators [13][6] (Figure 1).
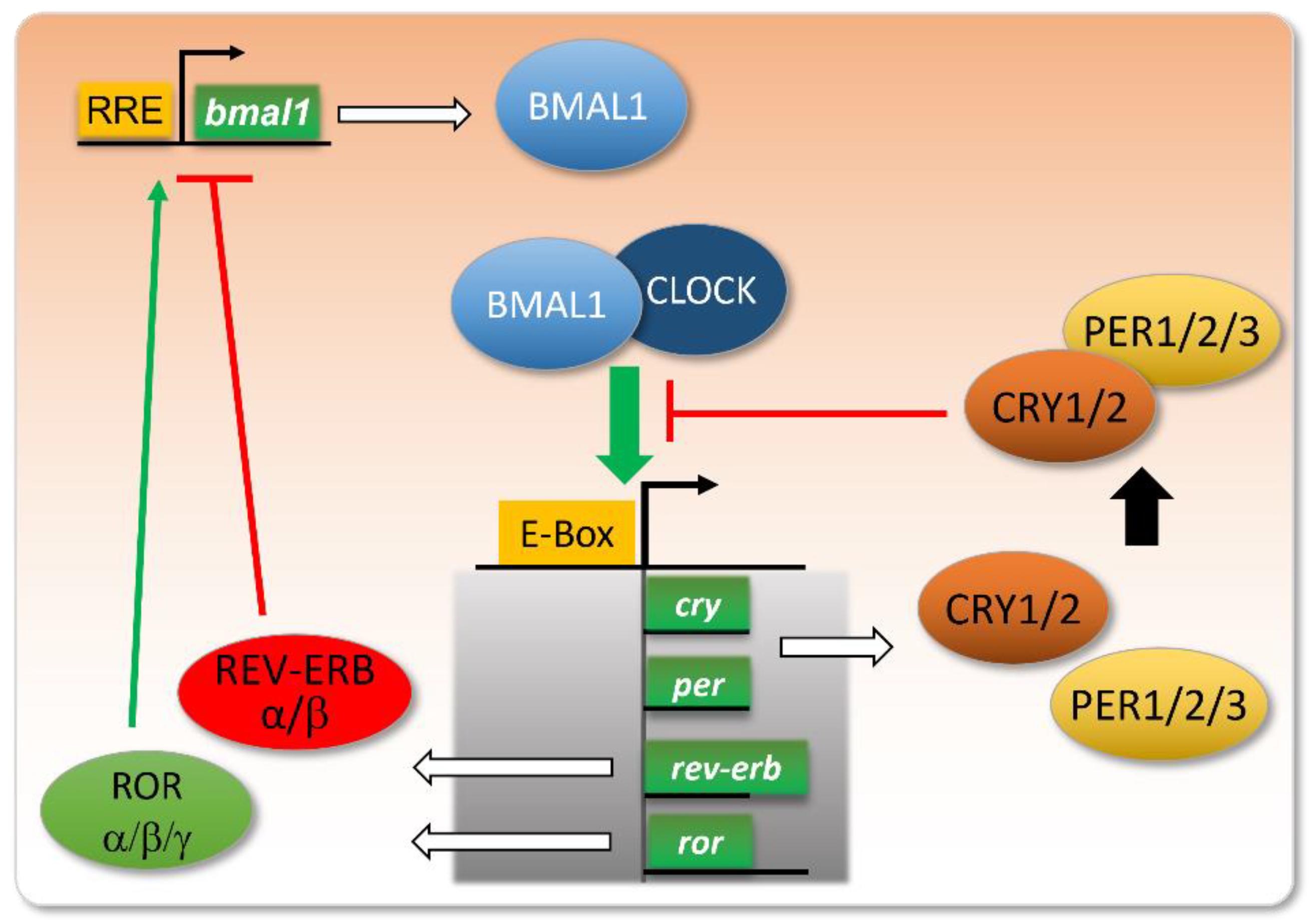
Figure 1. Core clock genes and their intertwined feedback loops.
The molecular core clock relies on the inter-regulation of several genes, including the couple Bmal1/Clock and their transcriptional targets Per, Cry, Ror, and Rev-Erb. When Bmal1 and Clock proteins dimerize, they bind to E-box-containing promoters and activate the transcription of their targets. Among these, PER and CRY proteins dimerize and repress the binding of BMAL1/CLOCK to E-boxes. The expression of REV-ERB will repress the transcription of the bmal1 gene, whereas ROR will stimulate this transcription, via their action on RRE (ROR Response Element) in the bmal1 gene promoter. These intertwined negative and positive feedback loops ensure proper circadian oscillations in the levels of these gene products, with a peak in BMAL1/CLOCK activity at the onset of the active phase, and a more or less delayed expression of its targets/regulators.
Nowadays, researchers evaluate that more than 40% of protein-coding genes display 24 h rhythmicity in mammals [14][7]. Moreover, it seems that beyond transcription, circadian rhythms of oxidation and reduction occur, as evidenced in red blood cells [15][8]. A link between these two kinds of regulation may be the Pentose Phosphate Pathway, with NADPH production regulating core clock transcriptional circuits [16][9].
2. Alterations in Day–Night Lifestyle Influence on Human Health
The central circadian clock in humans is localized in the SCN (suprachiasmatic nucleus), which receives light-driven inputs from specific retinal photoreceptors. This central clock then coordinates the whole-body activities, notably by releasing hormones such as melatonin and glucocorticoids and via neurotransmitters, thus entraining peripheral clocks in the various tissues of the body [17][10] (Figure 2). The SCN thus regulates food intake, and can as well impact metabolism independently of feeding [18,19][11][12]. In parallel, peripheral tissues are also entrained by food intake, and restricted feeding can uncouple peripheral oscillators from the SCN pacemaker [20][13]. In rare conditions, peripheral clocks can even modulate the central clock to a certain extent [21][14].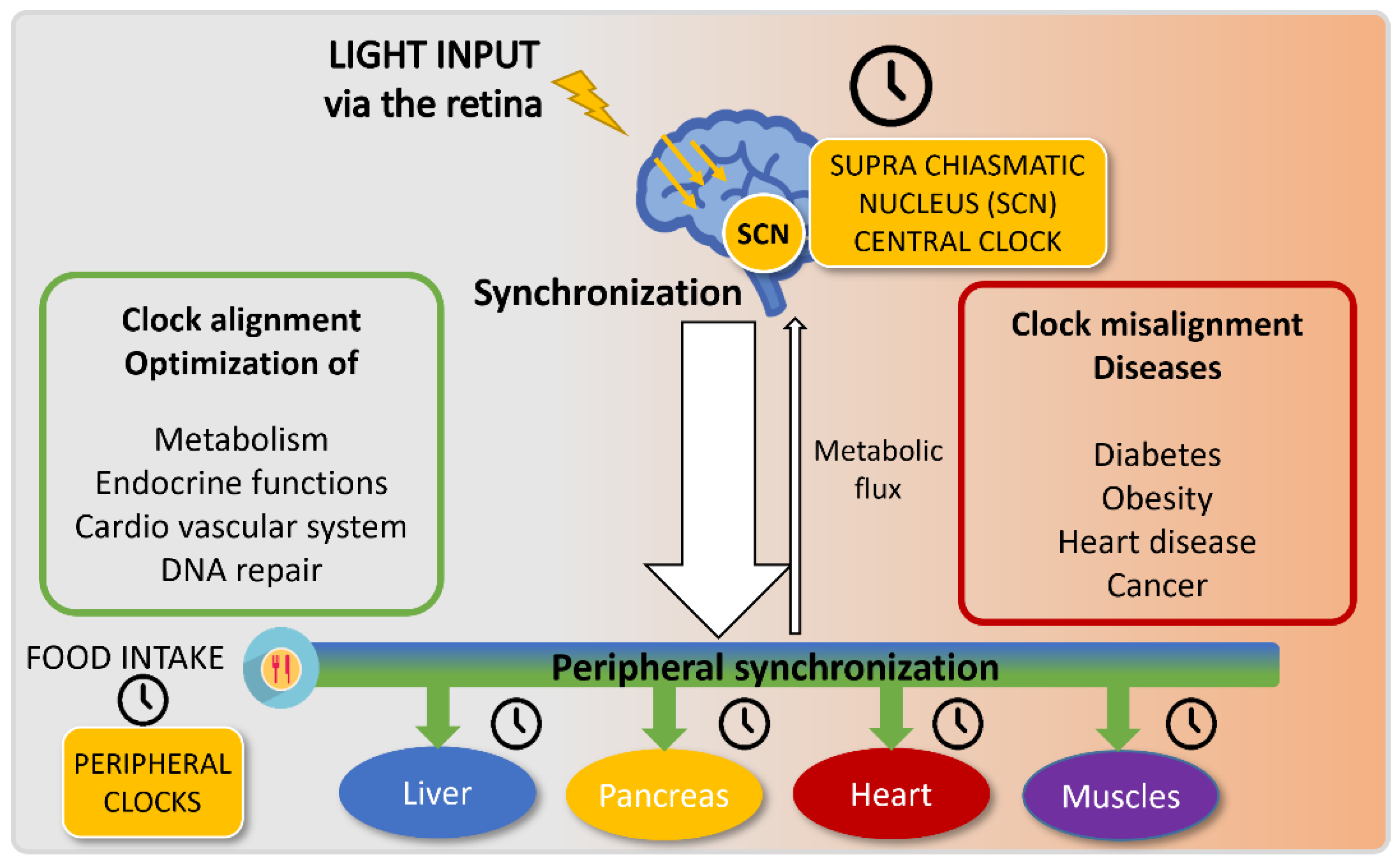
Figure 2. Circadian clock entrainment.
3. The Molecular Links between the Circadian Clock and Metabolism: The Food Connection
The energetic demands of our tissues drastically vary night and day, and metabolic activities are accordingly fluctuating in a circadian manner. It is therefore not surprising that food intake represents a critical zeitgeber for peripheral clocks involved in metabolism regulation. A clear proof of evidence of the link between circadian clock and metabolism came from studies showing that genetically disrupting either Clock or Bmal1 in mice induced the apparition of several traits associated with metabolic syndrome [36,37][29][30]. Some nuclear receptors such as REV-ERB, ROR, and peroxisome proliferation-activated receptors (PPARs) both regulate cellular metabolism and are components of the molecular core clock machinery. This presence at the crossroad of metabolism and circadian clock makes them good candidates to control metabolic storage and energetic consumption accordingly to circadian activities [38][31]. Hence, REV-ERB agonists, such as SR9009, represent interesting pharmacological compounds due to their pleiotropic activities in the regulation of the circadian clock and lipid metabolism [39][32]. The key role of REV-ERB in the integration of clock and food inputs was recently reinforced by the demonstration that REV-ERBα cyclically modifies the activity of OGT (O-GlcNAc transferase), a nutrient sensor, which regulates among others the expression of SREBP1c, a critical protein in lipogenesis [40][33]. REV-ERBα also controls mTOR [41][34], another protein well known for integrating nutrient and energy status in the cells and recently shown to affect the circadian clock [42][35]. To come back to the connection between metabolism and the clock, metabolite levels can as well modulate the clock and be controlled by it, as illustrated by the NAD+ (Nicotinamide Adenine Dinucleotide) example. NAD+ is a cofactor for SIRT1 deacetylase, which inhibits BMAL1 activity, and BMAL1/CLOCK conversely controls NAD+ level oscillations by controlling the NAD+ salvage pathway via NAMPT (nicotinamide phosphoribosyltransferase) expression control [43,44][36][37]. Moreover, SIRT1 deacetylates not only BMAL1 but also PGC1α, a transcriptional coactivator regulating energy homeostasis and the expression of core clock genes [45][38]. Since PGC1α activation depends on AMPK action before its subsequent deacetylation by SIRT1 [46][39], PGC1α stands as an integrator of both NAD+ and AMP energy-sensing inputs to control both circadian clock and metabolism. It is noteworthy that circadian fluctuations in metabolites are coordinated between the different tissues of an organism, reflecting a coherence among clocks [47][40]. Furthermore, meal timing was shown to play a role in peripheral clock synchronization in humans [48][41]. Given this tight link between metabolism and the circadian clock, it is not surprising to notice that clock alterations result in metabolic pathologies. In the Gremlins movie, Gizmo’s seller tells the hero not to give him food after midnight. Human beings should also pay more attention to the recommendation not to eat during their resting phase. Actually, it seems that many disorders occurring following the mishandled circadian clock may rely on improper food intake timing, resulting in physiopathological situations. Experimental studies with mice demonstrated that shifting their feeding time to their circadian rest phase induced metabolic syndrome in these mice [49][42]. Changing one’s diet can reciprocally affect the circadian clock [50][43]. Indeed, mice that are given a high-fat diet shift their locomotor activities [51][44]. Moreover, the nature of food can also alter the circadian clock, as illustrated by BMAL1 inhibition triggered by the saturated fatty acid palmitate [52][45]. When modern ways of life disrespect both food intake and the circadian clock, several metabolic pathologies arise.4. A Healthy Way of Life: Get Synchronized with Your Circadian Clock
After having shown that the timing of feeding can trigger great metabolic repercussions, mice models also demonstrated that good feeding habits could restore a healthy situation. Time-restricted feeding actually proved to prevent the onset of metabolic alterations in mice given a high-fat diet, for an equivalent caloric intake [53][46]. This method was efficient as well to counteract the metabolic diseases of clock-deficient mice [54][47]. With this in mind, and although evidence is still scarce for food as a bona fide zeitgeber in humans [55][48], several strategies of chronological diet are nowadays proposed to improve human metabolism and health. These include fasting periods of 12H or more, caloric restriction, or fasting-mimicking diets based on high fat and low carbohydrate contents [56,57][49][50]. Such strategies display the advantage to improve weight maintenance without requiring pharmacological drugs, yet the importance of each intervention remains debated since they are often combined in the dietary protocols. The use of natural bioactive compounds as diet supplements also drove promising results [58][51]. Notably, Epigallocatechin-3-gallate (EGCG) can restore a proper clock and metabolism in mice fed with a high-fat and high fructose diet, when administered at specific times [59][52]. Circadian clock misalignments are highly associated with negative health impacts on shift workers, who represent a large population in our modern societies. Rotating night shift work was especially shown to increase the risk of developing type 2 diabetes when it was combined with several unhealthy lifestyles [60][53]. Several research groups are trying to identify strategies aimed at alleviating the health problems met by this category of workers. A study performed with a small cohort of 11 individuals simulating the night shift suggested that the absence of eating at night may be helpful to impair the metabolic disruption in shift workers [61][54]. Although measuring metabolic traits requires complicated logistics in such studies, sleep improvements represent useful hints to evidence reduced misalignments in shifted workers. A chronotype-adjusted shift schedule was implemented in a factory, by removing the highest shifts for extreme chronotypes, which led to an increase in sleep duration and quality [62][55]. While it may be difficult to apply in most factories or hospitals, another approach could be implemented more easily on a large scale, which relies on light-based interventions and was also successful in improving sleep, as well as reducing fatigue and working errors [63][56]. Exposure to artificial light at night reduces melatonin secretion and alters sleep and circadian rhythms [64][57]. An easy tip for most people would therefore be to reduce this exposure as much as possible, by limiting the use of computer screens late at night. When not possible due to work duties, softwares exist which modify the spectrum of screen light day and night, and especially decrease the emission of blue light at night, with the intent to preserve the circadian clock. The pharmacological use of melatonin to counteract its reduced secretion could represent another solution [65][58]. More globally, the impact of the visual and non-visual effects of light on human activity is gaining importance nowadays, giving rise to the human-centric lighting concept [66][59] and recommendations such as adapting light environments throughout the day [67][60].References
- Zehring, W.A.; Wheeler, D.A.; Reddy, P.; Konopka, R.J.; Kyriacou, C.P.; Rosbash, M.; Hall, J.C. P-Element Transformation with Period Locus DNA Restores Rhythmicity to Mutant, Arrhythmic Drosophila Melanogaster. Cell 1984, 39, 369–376.
- Reddy, P.; Zehring, W.A.; Wheeler, D.A.; Pirrotta, V.; Hadfield, C.; Hall, J.C.; Rosbash, M. Molecular Analysis of the Period Locus in Drosophila Melanogaster and Identification of a Transcript Involved in Biological Rhythms. Cell 1984, 38, 701–710.
- Baylies, M.K.; Bargiello, T.A.; Jackson, F.R.; Young, M.W. Changes in Abundance or Structure of the per Gene Product Can Alter Periodicity of the Drosophila Clock. Nature 1987, 326, 390–392.
- De Mairan, J.-J. Observation Botanique. Hist. Acad. R. Sci. 1729, 35–36. Available online: http://www.bibnum.education.fr/sciencesdelavie/biologie/observation-botanique (accessed on 4 May 2022).
- Konopka, R.J.; Benzer, S. Clock Mutants of Drosophila Melanogaster. Proc. Natl. Acad. Sci. USA 1971, 68, 2112–2116.
- Takahashi, J.S. Transcriptional Architecture of the Mammalian Circadian Clock. Nat. Rev. Genet. 2017, 18, 164–179.
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A Circadian Gene Expression Atlas in Mammals: Implications for Biology and Medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224.
- O’Neill, J.S.; Reddy, A.B. Circadian Clocks in Human Red Blood Cells. Nature 2011, 469, 498–503.
- Rey, G.; Valekunja, U.K.; Feeney, K.A.; Wulund, L.; Milev, N.B.; Stangherlin, A.; Ansel-Bollepalli, L.; Velagapudi, V.; O’Neill, J.S.; Reddy, A.B. The Pentose Phosphate Pathway Regulates the Circadian Clock. Cell Metab. 2016, 24, 462–473.
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2010, 72, 517–549.
- Kent, B.A.; Rahman, S.A.; St Hilaire, M.A.; Grant, L.K.; Rüger, M.; Czeisler, C.A.; Lockley, S.W. Circadian Lipid and Hepatic Protein Rhythms Shift with a Phase Response Curve Different than Melatonin. Nat. Commun. 2022, 13, 681.
- Hong, F.; Pan, S.; Xu, P.; Xue, T.; Wang, J.; Guo, Y.; Jia, L.; Qiao, X.; Li, L.; Zhai, Y. Melatonin Orchestrates Lipid Homeostasis through the Hepatointestinal Circadian Clock and Microbiota during Constant Light Exposure. Cells 2020, 9, 489.
- Damiola, F.; Le Minh, N.; Preitner, N.; Kornmann, B.; Fleury-Olela, F.; Schibler, U. Restricted Feeding Uncouples Circadian Oscillators in Peripheral Tissues from the Central Pacemaker in the Suprachiasmatic Nucleus. Genes Dev. 2000, 14, 2950–2961.
- Sen, S.; Raingard, H.; Dumont, S.; Kalsbeek, A.; Vuillez, P.; Challet, E. Ultradian Feeding in Mice Not Only Affects the Peripheral Clock in the Liver, but Also the Master Clock in the Brain. Chronobiol. Int. 2017, 34, 17–36.
- Burke, T.M.; Markwald, R.R.; McHill, A.W.; Chinoy, E.D.; Snider, J.A.; Bessman, S.C.; Jung, C.M.; O’Neill, J.S.; Wright, K.P. Effects of Caffeine on the Human Circadian Clock in Vivo and in Vitro. Sci. Transl. Med. 2015, 7, 305ra146.
- Drake, C.; Roehrs, T.; Shambroom, J.; Roth, T. Caffeine Effects on Sleep Taken 0, 3, or 6 Hours before Going to Bed. J. Clin. Sleep Med. 2013, 9, 1195–1200.
- James, S.M.; Honn, K.A.; Gaddameedhi, S.; Van Dongen, H.P.A. Shift Work: Disrupted Circadian Rhythms and Sleep-Implications for Health and Well-Being. Curr. Sleep Med. Rep. 2017, 3, 104–112.
- Inokawa, H.; Umemura, Y.; Shimba, A.; Kawakami, E.; Koike, N.; Tsuchiya, Y.; Ohashi, M.; Minami, Y.; Cui, G.; Asahi, T.; et al. Chronic Circadian Misalignment Accelerates Immune Senescence and Abbreviates Lifespan in Mice. Sci. Rep. 2020, 10, 2569.
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A Marker for the End of Adolescence. Curr. Biol. 2004, 14, R1038–R1039.
- Hida, A.; Kitamura, S.; Ohsawa, Y.; Enomoto, M.; Katayose, Y.; Motomura, Y.; Moriguchi, Y.; Nozaki, K.; Watanabe, M.; Aritake, S.; et al. In Vitro Circadian Period Is Associated with Circadian/Sleep Preference. Sci. Rep. 2013, 3, 2074.
- Taillard, J.; Sagaspe, P.; Philip, P.; Bioulac, S. Sleep Timing, Chronotype and Social Jetlag: Impact on Cognitive Abilities and Psychiatric Disorders. Biochem. Pharm. 2021, 191, 114438.
- Goldin, A.P.; Sigman, M.; Braier, G.; Golombek, D.A.; Leone, M.J. Interplay of Chronotype and School Timing Predicts School Performance. Nat. Hum. Behav. 2020, 4, 387–396.
- Roenneberg, T.; Pilz, L.K.; Zerbini, G.; Winnebeck, E.C. Chronotype and Social Jetlag: A (Self-) Critical Review. Biology 2019, 8, 54.
- Islam, Z.; Hu, H.; Akter, S.; Kuwahara, K.; Kochi, T.; Eguchi, M.; Kurotani, K.; Nanri, A.; Kabe, I.; Mizoue, T. Social Jetlag Is Associated with an Increased Likelihood of Having Depressive Symptoms among the Japanese Working Population: The Furukawa Nutrition and Health Study. Sleep 2020, 43, zsz204.
- Makarem, N.; Paul, J.; Giardina, E.-G.V.; Liao, M.; Aggarwal, B. Evening Chronotype Is Associated with Poor Cardiovascular Health and Adverse Health Behaviors in a Diverse Population of Women. Chronobiol. Int. 2020, 37, 673–685.
- Knutson, K.L.; von Schantz, M. Associations between Chronotype, Morbidity and Mortality in the UK Biobank Cohort. Chronobiol. Int. 2018, 35, 1045–1053.
- Hood, S.; Amir, S. The Aging Clock: Circadian Rhythms and Later Life. J. Clin. Investig. 2017, 127, 437–446.
- Duffy, J.F.; Zitting, K.-M.; Chinoy, E.D. Aging and Circadian Rhythms. Sleep Med. Clin. 2015, 10, 423–434.
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and Metabolic Syndrome in Circadian Clock Mutant Mice. Science 2005, 308, 1043–1045.
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the Clock Components CLOCK and BMAL1 Leads to Hypoinsulinaemia and Diabetes. Nature 2010, 466, 627–631.
- Duez, H.; Staels, B. Nuclear Receptors Linking Circadian Rhythms and Cardiometabolic Control. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1529–1534.
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Kojetin, D.J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Noel, R.; et al. Regulation of Circadian Behaviour and Metabolism by Synthetic REV-ERB Agonists. Nature 2012, 485, 62–68.
- Berthier, A.; Vinod, M.; Porez, G.; Steenackers, A.; Alexandre, J.; Yamakawa, N.; Gheeraert, C.; Ploton, M.; Maréchal, X.; Dubois-Chevalier, J.; et al. Combinatorial Regulation of Hepatic Cytoplasmic Signaling and Nuclear Transcriptional Events by the OGT/REV-ERBα Complex. Proc. Natl. Acad. Sci. USA 2018, 115, E11033–E11042.
- Maayan, D.-F.; Chapnik, N.; Froy, O. REV-ERBα Activates the MTOR Signaling Pathway and Promotes Myotubes Differentiation. Biol. Cell 2020, 112, 213–221.
- Ramanathan, C.; Kathale, N.D.; Liu, D.; Lee, C.; Freeman, D.A.; Hogenesch, J.B.; Cao, R.; Liu, A.C. MTOR Signaling Regulates Central and Peripheral Circadian Clock Function. PLoS Genet. 2018, 14, e1007369.
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1. Science 2009, 324, 654–657.
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.-K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian Clock Feedback Cycle through NAMPT-Mediated NAD+ Biosynthesis. Science 2009, 324, 651–654.
- Liu, C.; Li, S.; Liu, T.; Borjigin, J.; Lin, J.D. Transcriptional Coactivator PGC-1alpha Integrates the Mammalian Clock and Energy Metabolism. Nature 2007, 447, 477–481.
- Cantó, C.; Jiang, L.Q.; Deshmukh, A.S.; Mataki, C.; Coste, A.; Lagouge, M.; Zierath, J.R.; Auwerx, J. Interdependence of AMPK and SIRT1 for Metabolic Adaptation to Fasting and Exercise in Skeletal Muscle. Cell Metab. 2010, 11, 213–219.
- Dyar, K.A.; Lutter, D.; Artati, A.; Ceglia, N.J.; Liu, Y.; Armenta, D.; Jastroch, M.; Schneider, S.; de Mateo, S.; Cervantes, M.; et al. Atlas of Circadian Metabolism Reveals System-Wide Coordination and Communication between Clocks. Cell 2018, 174, 1571–1585.e11.
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775.e3.
- Mukherji, A.; Kobiita, A.; Damara, M.; Misra, N.; Meziane, H.; Champy, M.-F.; Chambon, P. Shifting Eating to the Circadian Rest Phase Misaligns the Peripheral Clocks with the Master SCN Clock and Leads to a Metabolic Syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, E6691–E6698.
- Eckel-Mahan, K.L.; Patel, V.R.; de Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the Circadian Clock by Nutritional Challenge. Cell 2013, 155, 1464–1478.
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421.
- Tal, Y.; Chapnik, N.; Froy, O. Non-Obesogenic Doses of Fatty Acids Modulate the Functionality of the Circadian Clock in the Liver. Cell. Mol. Life Sci. 2019, 76, 1795–1806.
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012, 15, 848–860.
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.W.; Panda, S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019, 29, 303–319.e4.
- Lewis, P.; Oster, H.; Korf, H.W.; Foster, R.G.; Erren, T.C. Food as a Circadian Time Cue-Evidence from Human Studies. Nat. Rev. Endocrinol. 2020, 16, 213–223.
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; de Cabo, R. A Time to Fast. Science 2018, 362, 770–775.
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059.
- Ibarz-Blanch, N.; Morales, D.; Calvo, E.; Ros-Medina, L.; Muguerza, B.; Bravo, F.I.; Suárez, M. Role of Chrononutrition in the Antihypertensive Effects of Natural Bioactive Compounds. Nutrients 2022, 14, 1920.
- Mi, Y.; Qi, G.; Fan, R.; Ji, X.; Liu, Z.; Liu, X. EGCG Ameliorates Diet-Induced Metabolic Syndrome Associating with the Circadian Clock. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1575–1589.
- Shan, Z.; Li, Y.; Zong, G.; Guo, Y.; Li, J.; Manson, J.E.; Hu, F.B.; Willett, W.C.; Schernhammer, E.S.; Bhupathiraju, S.N. Rotating Night Shift Work and Adherence to Unhealthy Lifestyle in Predicting Risk of Type 2 Diabetes: Results from Two Large US Cohorts of Female Nurses. BMJ 2018, 363, k4641.
- Grant, C.L.; Coates, A.M.; Dorrian, J.; Kennaway, D.J.; Wittert, G.A.; Heilbronn, L.K.; Pajcin, M.; Della Vedova, C.; Gupta, C.C.; Banks, S. Timing of Food Intake during Simulated Night Shift Impacts Glucose Metabolism: A Controlled Study. Chronobiol. Int. 2017, 34, 1003–1013.
- Vetter, C.; Fischer, D.; Matera, J.L.; Roenneberg, T. Aligning Work and Circadian Time in Shift Workers Improves Sleep and Reduces Circadian Disruption. Curr. Biol. 2015, 25, 907–911.
- Olson, J.A.; Artenie, D.Z.; Cyr, M.; Raz, A.; Lee, V. Developing a Light-Based Intervention to Reduce Fatigue and Improve Sleep in Rapidly Rotating Shift Workers. Chronobiol. Int. 2020, 37, 573–591.
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between Light at Night, Melatonin Secretion, Sleep Deprivation, and the Internal Clock: Health Impacts and Mechanisms of Circadian Disruption. Life Sci. 2017, 173, 94–106.
- Gunata, M.; Parlakpinar, H.; Acet, H.A. Melatonin: A Review of Its Potential Functions and Effects on Neurological Diseases. Rev. Neurol. 2020, 176, 148–165.
- Houser, K.W.; Esposito, T. Human-Centric Lighting: Foundational Considerations and a Five-Step Design Process. Front. Neurol. 2021, 12, 630553.
- Stefani, O.; Cajochen, C. Should We Re-Think Regulations and Standards for Lighting at Workplaces? A Practice Review on Existing Lighting Recommendations. Front. Psychiatry 2021, 12, 652161.
More
