Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by lingyun Wang.
The biogenic aliphatic polyamines (spermine, spermidine, and putrescine) are responsible for numerous cell functions, including cell proliferation, the stabilization of nucleic acid conformations, cell division, homeostasis, gene expression, and protein synthesis in living organisms. The change of polyamine concentrations in the urine or blood is usually related to the presence of malignant tumors and is regarded as a biomarker for the early diagnosis of cancer. Therefore, the detection of polyamine levels in physiological fluids can provide valuable information in terms of cancer diagnosis and in monitoring therapeutic effects.
- polyamines
- detection
- suppressor strategies
1. Polyamine Detection Methods
Numerous measuring methods and instruments of analysis, such as the enzyme-linked immunosorbent assay (ELISA) [19][1], a combination of mass spectrometry (MS) with gas chromatography (GC) or liquid chromatography (LC) [20][2], high-performance liquid chromatography (HPLC) [21][3], an antibody-dependent assay [22][4] (ADA), and nuclear magnetic resonance (NMR) [23][5] have been developed. However, some drawbacks such as low selectivity, expensive equipment costs, the need for professional staff, and complicated sample pre-treatment requirements are frequently encountered. On the other hand, fluorescence-sensing technology has attracted more and more attention in the field of polyamine detection, due to its high sensitivity, simple operation, fast response speed, low cost, real-time visualization, and non-destructive monitoring. Therefore, we focus on fluorescent methods for polyamine detection in this review. Scheme 1 shows the roadmap for polyamine monitoring methods. Until now, small organic molecules [24[6][7][8][9][10],25,26,27,28], conjugated polymers [29[11][12][13][14],30,31,32], dye-assembled nanotubes [33][15], hydrogel hybrids [34][16], dye-embedded micelles [35[17][18],36], nanoparticles [37[19][20],38], nano-Au [39][21], or quantum dots [40][22] have been utilized to identify and detect polyamines. Most of them are involved in displacement or aggregation-based sensing mechanisms.
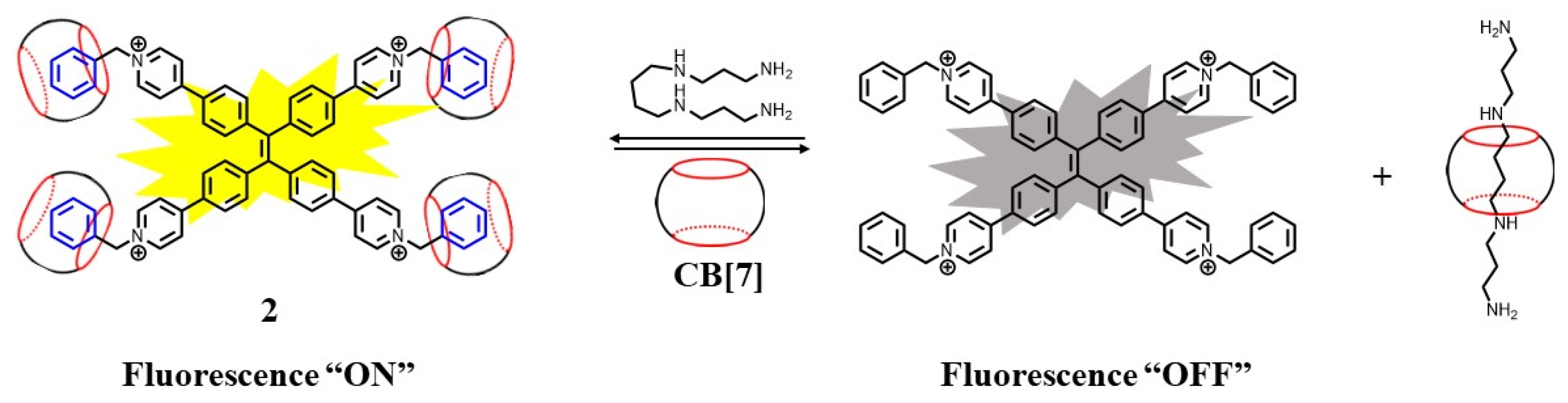
 The cationic pyridyl functionalized compound 2 was an aggregation-induced-emissive (AIE) active dye, which was non-emissive in solution. Upon complexation with CB[7] via host–guest interaction, a fluorescent supramolecular system was formed [45][27]. The binding constant between 2 and CB[7] was found to be 3.77 × 104, 2.22 × 104, and 2.86 × 104 M−1 at a pH of 3.0, 7.0, and 10.0, respectively. Since the binding constant (2.6 × 107 M−1) between spermine and CB[7] was 1000-fold higher than 2-CB[7], 2 was released from the supramolecular system. The fluorescence at 537 nm was turned off. The detection limit of spermine was found to be 1.0 μM (Figure 3).
The cationic pyridyl functionalized compound 2 was an aggregation-induced-emissive (AIE) active dye, which was non-emissive in solution. Upon complexation with CB[7] via host–guest interaction, a fluorescent supramolecular system was formed [45][27]. The binding constant between 2 and CB[7] was found to be 3.77 × 104, 2.22 × 104, and 2.86 × 104 M−1 at a pH of 3.0, 7.0, and 10.0, respectively. Since the binding constant (2.6 × 107 M−1) between spermine and CB[7] was 1000-fold higher than 2-CB[7], 2 was released from the supramolecular system. The fluorescence at 537 nm was turned off. The detection limit of spermine was found to be 1.0 μM (Figure 3).
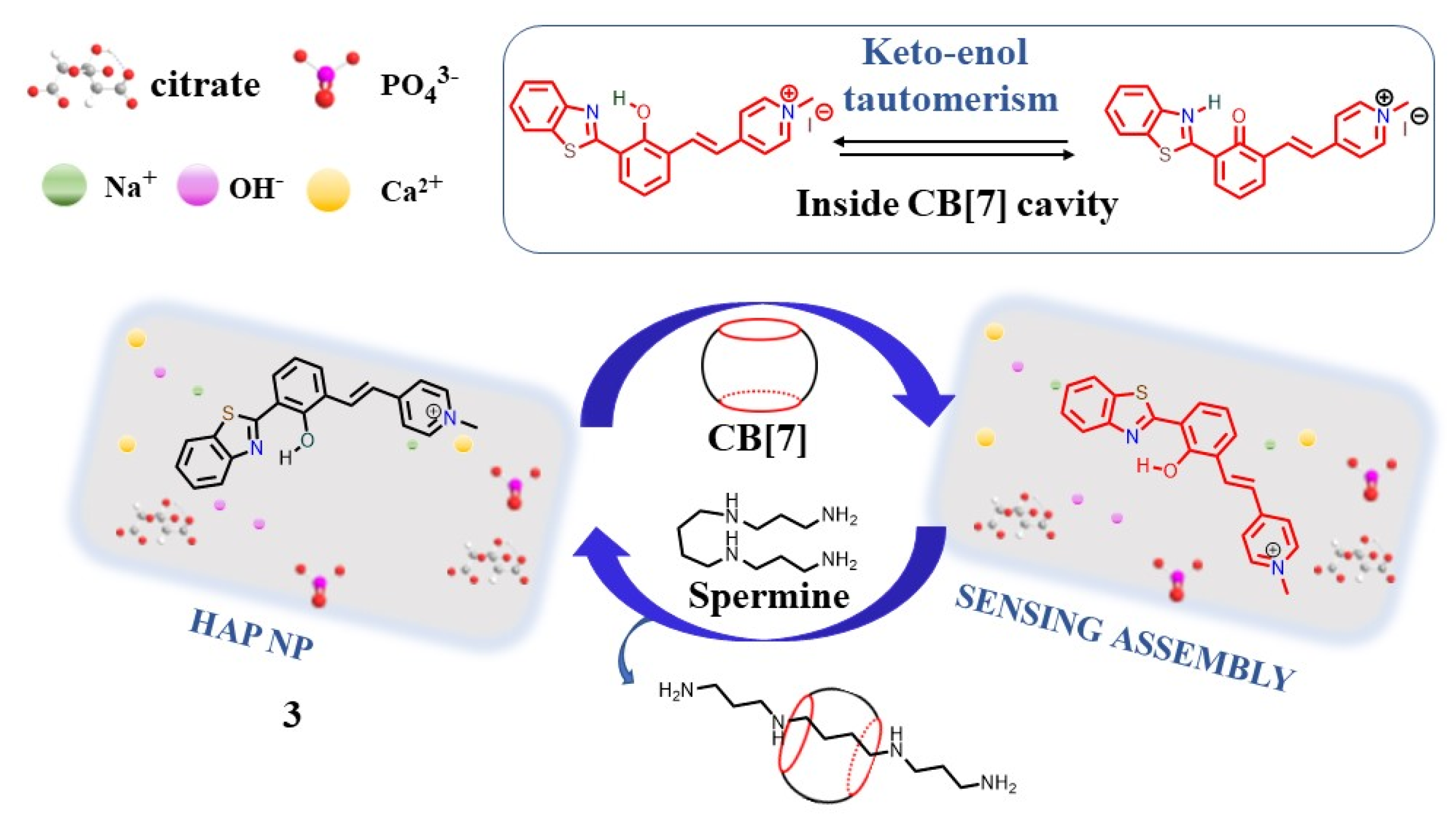
 Bhosle et al. developed a three-component supramolecular sensing system containing a fluorescent compound, 3, CB[7], and hydroxyapatite nanoparticles (HAp NPs), which can be used to detect polyamines with high sensitivity [46][28]. In this case, compound 3 spontaneously interacted with HAp NPs by electrostatic interaction and with CB[7] via host–guest interaction. As a result, a three-component supramolecular system showed strong emissions at 615 nm (Figure 4). Spermine showed a higher binding ability to CB[7] than compound 3, leading to a higher binding constant (2.6 × 107 M−1 for spermine-CB[7] vs. 5.6 × 104 M−1 for 3-CB[7]). Upon the addition of polyamines, compound 3 was displaced from CB[7] and quenching fluorescence was observed. The low limits of detection of 1.4, 3.3, and 17.2 ppb for spermine, spermidine, and cadaverine were obtained, respectively.
Bhosle et al. developed a three-component supramolecular sensing system containing a fluorescent compound, 3, CB[7], and hydroxyapatite nanoparticles (HAp NPs), which can be used to detect polyamines with high sensitivity [46][28]. In this case, compound 3 spontaneously interacted with HAp NPs by electrostatic interaction and with CB[7] via host–guest interaction. As a result, a three-component supramolecular system showed strong emissions at 615 nm (Figure 4). Spermine showed a higher binding ability to CB[7] than compound 3, leading to a higher binding constant (2.6 × 107 M−1 for spermine-CB[7] vs. 5.6 × 104 M−1 for 3-CB[7]). Upon the addition of polyamines, compound 3 was displaced from CB[7] and quenching fluorescence was observed. The low limits of detection of 1.4, 3.3, and 17.2 ppb for spermine, spermidine, and cadaverine were obtained, respectively.
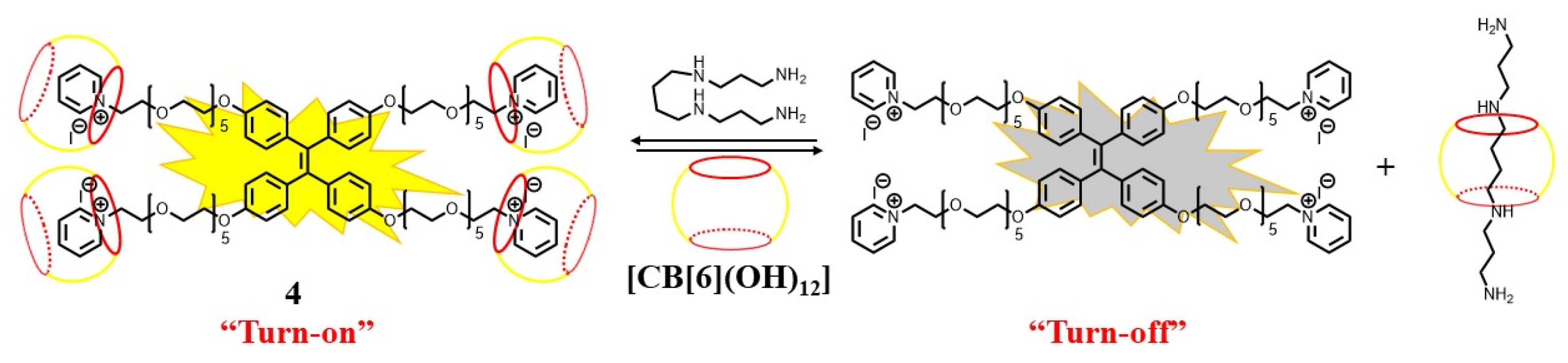
 Similarly, a three-component supramolecular sensing assembly based on the water-soluble TPE derivative (4), hydroxyl cucurbit[6]uril (CB[6]OH), and hydroxyapatite nanoparticles (HAp NPs) showed excellent sensing performance for the detection of spermine and spermidine in human urine and blood [47][29]. A binary conjugate via host−guest complexation between compound 4 and CB[6]OH) at a 1:4 ratio was formed. The further addition of HAp NPs to the binary conjugate led to a 13-fold enhancement by more effective aggregation. The corresponding three-component supramolecular assembly showed the fluorescence turn-off response at 474 nm to spermine and spermidine, but not to other amines. LOD values of 1.4 × 10−8 and 3.6 × 10−8 M for spermine and spermidine were reported, respectively. The much stronger affinity between spermine and CB[6]OH created compound 4 in monomeric form, leading to emission quenching (Figure 5).
Similarly, a three-component supramolecular sensing assembly based on the water-soluble TPE derivative (4), hydroxyl cucurbit[6]uril (CB[6]OH), and hydroxyapatite nanoparticles (HAp NPs) showed excellent sensing performance for the detection of spermine and spermidine in human urine and blood [47][29]. A binary conjugate via host−guest complexation between compound 4 and CB[6]OH) at a 1:4 ratio was formed. The further addition of HAp NPs to the binary conjugate led to a 13-fold enhancement by more effective aggregation. The corresponding three-component supramolecular assembly showed the fluorescence turn-off response at 474 nm to spermine and spermidine, but not to other amines. LOD values of 1.4 × 10−8 and 3.6 × 10−8 M for spermine and spermidine were reported, respectively. The much stronger affinity between spermine and CB[6]OH created compound 4 in monomeric form, leading to emission quenching (Figure 5).
 The ionic self-assembly of the benzimidazolium platform and ciprofloxacin-Tb3+ complex were developed for selective spermine detection with the fluorescence quenching response [48,49][30][31]. Guo et al. designed a supramolecular assembly of an amphiphilic sulfocalix[5]arene (SC5A12C) with lucigenin (LCG) via host–guest interaction [50][32]. The fluorescence of LCG was quenched due to the strong binding capacity between SC5A12C and LCG. In the presence of overexpressed spermine in cancer cells, the fluorescence was fully recovered due to the competition complex between spermine and SC5A12C. Co-assembled folate further promoted the cellular uptake by folate receptor-overexpressing cancer cells.
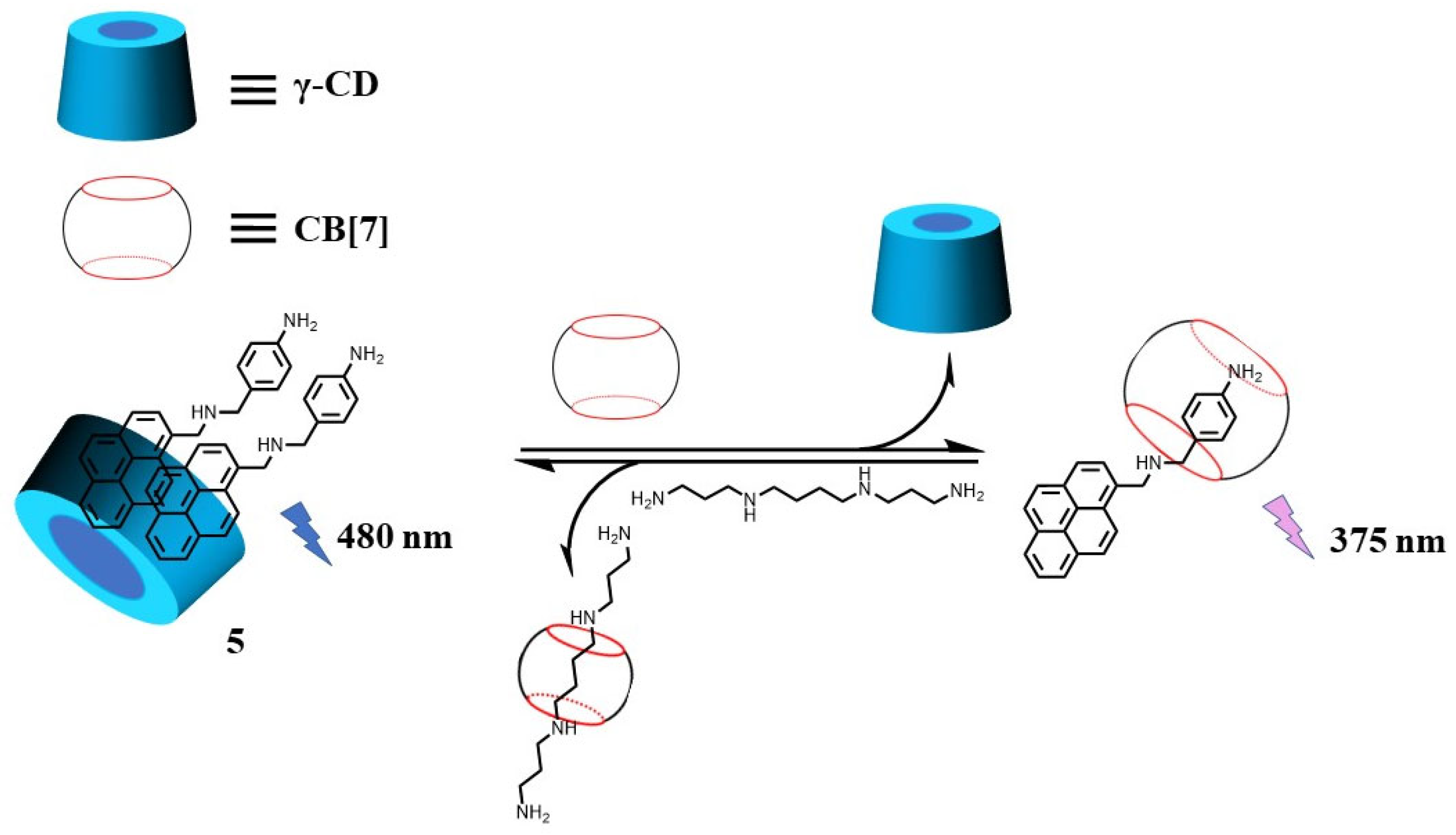
Yang’s group reported polyamine detection, based on pyrene excimer fluorescence, through the synergistic/competitive complexation among pyrene compounds (5), polyamines, γ-cyclodextrins (γ-CD), and CB[7] [51][33]. In this case, γ-CD accommodated compound 5 with binding constants of 5 × 106 M−1 for 1:2 binding modes, leading to the excimer emission of pyrene. Since a 1:1 host–guest complexation between 5 and CB[7] was identified, the addition of CB[7] yielded a decrease in the excimer fluorescence, accompanied by an increase in monomer fluorescence. On the other hand, polyamines can bind strongly to CB[7]. For example, spermine showed an association constant two orders higher of 1.28 × 106 M−1 than that of pyrene. The addition of urinary polyamines to a three-component supramolecular system (5, γ-CD, and CB[7]) competitively captured CB[7] and formed a complex of γ-CD-5 at 1:2 binding, enabling the recovery of the excimer fluorescence (Figure 6).
The ionic self-assembly of the benzimidazolium platform and ciprofloxacin-Tb3+ complex were developed for selective spermine detection with the fluorescence quenching response [48,49][30][31]. Guo et al. designed a supramolecular assembly of an amphiphilic sulfocalix[5]arene (SC5A12C) with lucigenin (LCG) via host–guest interaction [50][32]. The fluorescence of LCG was quenched due to the strong binding capacity between SC5A12C and LCG. In the presence of overexpressed spermine in cancer cells, the fluorescence was fully recovered due to the competition complex between spermine and SC5A12C. Co-assembled folate further promoted the cellular uptake by folate receptor-overexpressing cancer cells.
Yang’s group reported polyamine detection, based on pyrene excimer fluorescence, through the synergistic/competitive complexation among pyrene compounds (5), polyamines, γ-cyclodextrins (γ-CD), and CB[7] [51][33]. In this case, γ-CD accommodated compound 5 with binding constants of 5 × 106 M−1 for 1:2 binding modes, leading to the excimer emission of pyrene. Since a 1:1 host–guest complexation between 5 and CB[7] was identified, the addition of CB[7] yielded a decrease in the excimer fluorescence, accompanied by an increase in monomer fluorescence. On the other hand, polyamines can bind strongly to CB[7]. For example, spermine showed an association constant two orders higher of 1.28 × 106 M−1 than that of pyrene. The addition of urinary polyamines to a three-component supramolecular system (5, γ-CD, and CB[7]) competitively captured CB[7] and formed a complex of γ-CD-5 at 1:2 binding, enabling the recovery of the excimer fluorescence (Figure 6).
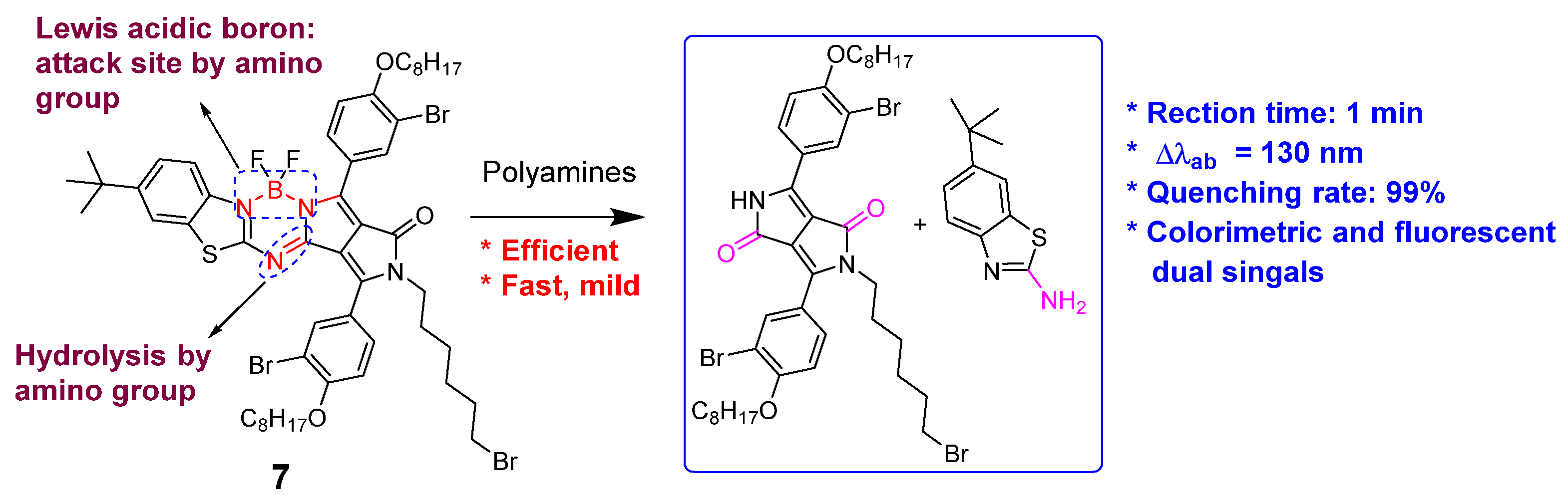
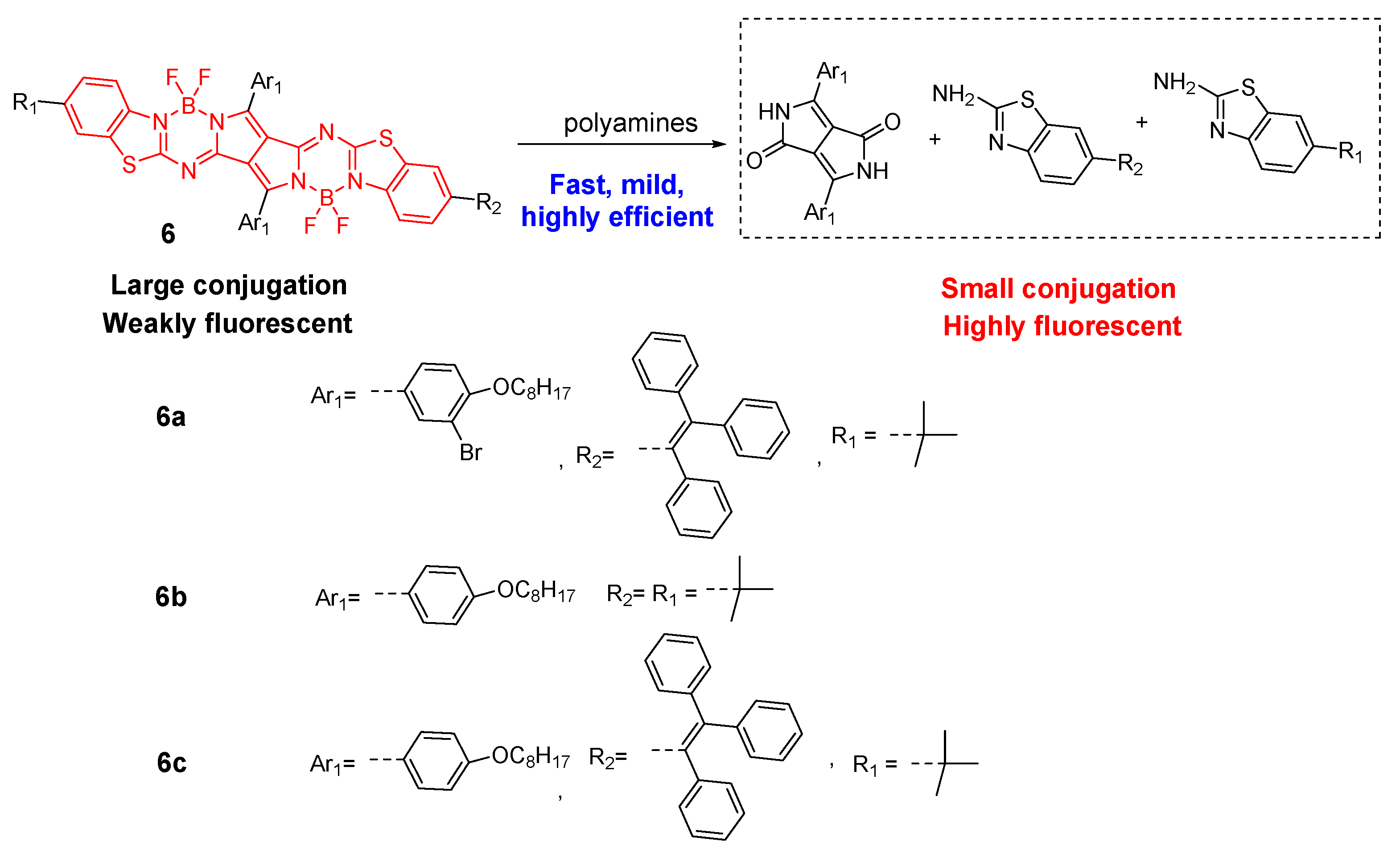 It is important to improve the reaction rate between fluorescent probes and polyamines. WOne group designed and synthesized a lactam-fused aza-BODIPY (compound 7), which contained fewer cleaved imine and B–N functional groups [63][45]. The presence of spermidine and spermine induced an obvious color change from pink to yellow (130 nm hypsochromic shift of the absorption peak) and a 99% fluorescence “turn-off” response within 1 minute. Other amines failed to yield the same optical phenomenon. The high pseudo-first-order rate constants (kobs) of 15.67 × 10−3 S−1 and 8.99 × 10−3 S−1 for spermine and spermidine were present, respectively. A similar proposed sensing mechanism was involved through the B–N bond cleavage and hydrolysis reaction, to yield much smaller conjugated molecules (Figure 8).
It is important to improve the reaction rate between fluorescent probes and polyamines. WOne group designed and synthesized a lactam-fused aza-BODIPY (compound 7), which contained fewer cleaved imine and B–N functional groups [63][45]. The presence of spermidine and spermine induced an obvious color change from pink to yellow (130 nm hypsochromic shift of the absorption peak) and a 99% fluorescence “turn-off” response within 1 minute. Other amines failed to yield the same optical phenomenon. The high pseudo-first-order rate constants (kobs) of 15.67 × 10−3 S−1 and 8.99 × 10−3 S−1 for spermine and spermidine were present, respectively. A similar proposed sensing mechanism was involved through the B–N bond cleavage and hydrolysis reaction, to yield much smaller conjugated molecules (Figure 8).
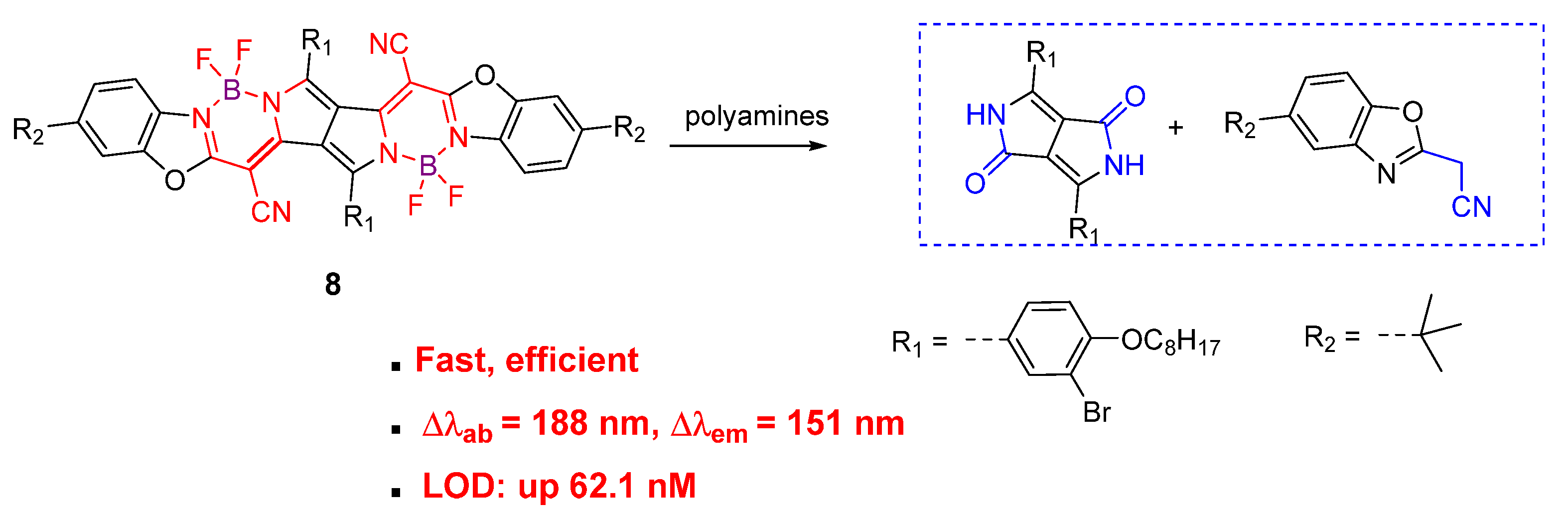
 The aza-Michael addition reaction between α,β-unsaturated nitrile and primary amines can proceed smoothly under mild conditions. The pyrrolopyrrole cyanine dye (8) contained α,β-unsaturated nitrile as Michael acceptors, which was further appended with a withdrawing boron atom to enhance the aza-Michael addition reactivity [64][46]. Through this strategy, a highly efficient fluorescent polyamine probe was developed. Compound 8 showed 158-fold higher kobs with putrescine than compound 6a. As an efficient chromophore reaction-based probe for polyamine detection, the synergistic aza-Michael addition, B–N detachment, and hydrolysis reaction were involved between compound 8 and polyamines. The resulting low-conjugated product generated synchronous colorimetric and fluorescence changes (Δλab = 188 nm and Δλem = 151 nm). A limit of detection up to the 62.1 nM level for spermine was obtained (Figure 9).
The aza-Michael addition reaction between α,β-unsaturated nitrile and primary amines can proceed smoothly under mild conditions. The pyrrolopyrrole cyanine dye (8) contained α,β-unsaturated nitrile as Michael acceptors, which was further appended with a withdrawing boron atom to enhance the aza-Michael addition reactivity [64][46]. Through this strategy, a highly efficient fluorescent polyamine probe was developed. Compound 8 showed 158-fold higher kobs with putrescine than compound 6a. As an efficient chromophore reaction-based probe for polyamine detection, the synergistic aza-Michael addition, B–N detachment, and hydrolysis reaction were involved between compound 8 and polyamines. The resulting low-conjugated product generated synchronous colorimetric and fluorescence changes (Δλab = 188 nm and Δλem = 151 nm). A limit of detection up to the 62.1 nM level for spermine was obtained (Figure 9).

Scheme 1.
A roadmap for the most common polyamine-monitoring methods.
2. Supramolecular Sensing System for Polyamine Detection
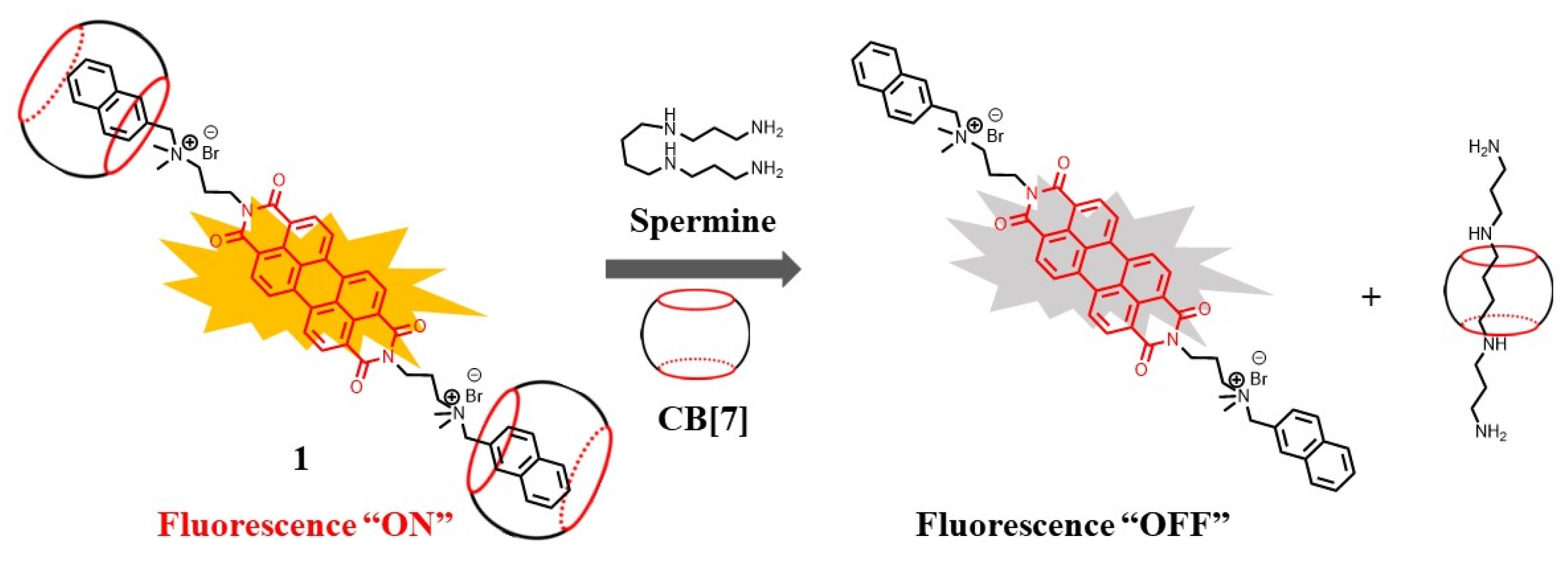
Recently, spermine detection, based on a supramolecular strategy, has received more scientific attention due to the strong complexation ability between the macrocyclic host and spermine [41][23]. In some cases, the modulation of the supramolecular system’s morphologies by spermine yielded obvious fluorescence signals [42][24]. By the use of the multi-cationic character of tetracationic spermine (pKa = 11.50, 10.95, 9.79, 8.90) and tricationic spermidine (pKa = 11.56, 10.80, 9.52), the generation of emissive excimers was investigated for the selective detection of spermine and spermidine [43][25]. Besides these strategies, the competitive binding induced by spermine is a widely utilized sensing mechanism [44,45,46,47][26][27][28][29]. The water-soluble cucurbit[7]uril (CB[7]) was selected as a popular host because of its strong binding ability toward polyamines. The highly emissive “dumbbell-shape” supraamphiphiles, based on perylene diimides (compound 1) and CB[7], were utilized as a supramolecular sensor for the fast and ultra-sensitive detection of spermine [44][26]. Due to its high affinity with CB[7], spermine can preferably bind to CB[7] (binding constant K = 2.6 × 107 M−1), leading to the dissociation of the supra-amphiphiles and generating fluorescence quenching. The supraamphiphiles showed good selectivity for spermine over other structural analogs (spermidine, putrescine, L-arginine, L-lysine, etc.). This high sensitivity was maintained even in the presence of a low concentration of spermine (about 10 nM) (Figure 2).
Figure 2. Schematic illustration of the mechanism for sensing spermine, based on supraamphiphiles [44].


Figure 4. Schematic representation of the three-component supramolecular sensing assembly for spermine, spermidine, and cadaverine [46].
Schematic representation of the three-component supramolecular sensing assembly for spermine, spermidine, and cadaverine [28].

Figure 5. Schematic representation of the three-component supramolecular sensing assay for spermine (and spermidine) [47].
Schematic representation of the three-component supramolecular sensing assay for spermine (and spermidine) [29].

3. Polyamine Detection Based on Chromophore Reaction
The chromophore-reaction-based fluorescent probes possess great advantages, such as a high selectivity to analytes and synchronous colorimetric and fluorescence changes [52,53,54,55,56,57][34][35][36][37][38][39]. Recently, wone rgroup reported a series of pyrrolopyrrole aza-BODIPY (PPAB)-based fluorescent probes for polyamine detection, wherein the chromophore reaction-sensing mechanism was involved [58,59,60,61][40][41][42][43]. For instance, three PPAB dyes (4a–4c) showed high selectivity and sensitivity toward polyamines by colorimetric changes from green to yellow and a fluorescent turn-on process [62][44]. There was a hypsochromic shift over 225 nm in the absorption maximum and a 12-fold fluorescence enhancement. The detection mechanism study revealed a B–N bond cleavage, and a transamination and hydrolysis reaction was involved that generated much smaller conjugated molecules. More interestingly, the limit of detection up to ppb level and the response time on the second timescale were demonstrated (Figure 7).
Figure 7.
The possible sensing mechanism between compound

Figure 8.
The possible sensing mechanism between compound

Figure 9.
The possible sensing mechanism between compound
4. Fluorescent Small Molecules for Polyamine Detection
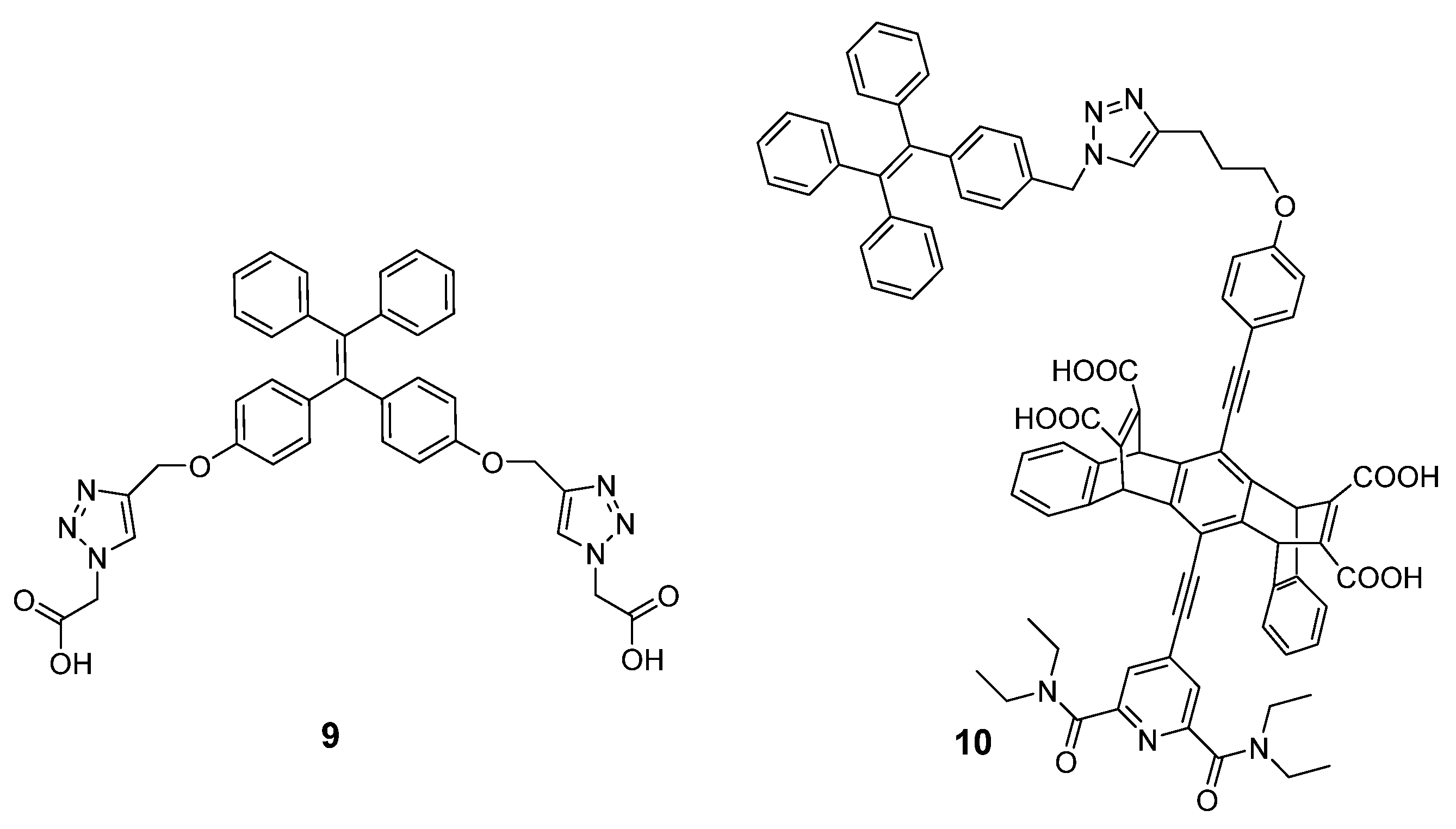
Barros et al. synthesized a tetraphenylethylene (TPE) derivative (9) containing two carboxylic acid groups as a fluorescent probe for the detection of spermine and spermidine [65][47]. The multi-cationic spermine and spermidine easily formed a complex with compound 9 by electrostatic- and hydrogen-bonding interactions at the physiological pH. As a result, a distinct emission enhancement at 473 nm (60- and 80-fold increase for spermine and spermidine, respectively) was shown because the restricted intramolecular rotations of compound 9 played a positive role. Conversely, diamines such as ethylenediamine, diethylenetriamine, cadaverine, and putrescine failed to generate such a fluorescence response. The LOD values of 0.70 µM and 1.17 µM for spermine and spermidine, respectively, were achieved in urine (Figure 10). Huang et al. synthesized an AIE-active compound, 10, based on TPE and pentiptycene, which showed a fluorescence “turn-on” response in the presence of spermine [66][48]. The sensing mechanism was ascribed to aggregation-induced fluorescence enhancement via electrostatic pairing and hydrogen bonding. For instance, the fluorescence intensity of compound 10 gave a 75-fold fluorescence enhancement at 480 nm upon the addition of 40 μM spermine. The detection limit regarding spermine was found to be 0.3 μM. The practical application for spermine detection in artificial urine was demonstrated (Figure 10).5. Fluorescent Nanoparticles for Polyamine Detection
A dual emissive nanoprobe was prepared from the combination of yellow emissive mercaptopropionic acid-capped CdTe quantum dots (YQDs) and blue emissive carbon dots (BCDs), wherein the former and the latter were employed as sensing and reference fluorophores, respectively [67][49]. Since spermine or spermidine could dramatically quench the fluorescence of YQDs (λem = 570 nm) but did not affect the emission of BCDs (λem = 450 nm), the presence of spermine or spermidine induced a decrease in the I570/I450 ratio. As a result, the green emission turned into a pink emission with the increase of spermine or spermidine, to yield a ratiometric signal. The low limits of detection were found to be 0.2 µM for spermine and 2.1 µM for spermidine, respectively. By using this sensing mechanism, a combinational logic-gate fluorescence sensor was achieved. Gluconate-stabilized AuNPs (Glu-AuNPs) possessed an anionic character, which was utilized as a nanosensor for selectively detecting spermine in urine samples [68][50]. In the presence of spermine, the absorption band at 518 nm gradually red-shifted to 618 nm due to aggregation, generating an obvious color change from pinkish-red to blue. The strong interaction between spermine and Glu-AuNPs was confirmed via its high binding constant (2.18 × 106 M−1). The ratio of A618/A518 showed a good linear relationship with spermine concentration. The LOD value was calculated to be 0.25 μM. When Glu-AuNPs were fabricated into a colorimetric strip-based kit, the sensing of spermine in urine samples was achieved. Two cobalt-based metal–organic frameworks (MOFs) were developed for polyamine detection, wherein a strong emission at about 332 nm was largely quenched by spermine, spermidine, or putrescine [69][51]. Since host–guest interactions between the polyamines and the MOFs were involved, the donor-acceptor electron transfer process from polyamines to MOFs generated photoinduced electron transfer (PET), leading to fluorescence quenching. Putrescine has higher HOMO energy levels than spermidine and putrescine (−4.81 vs. −4.96, −5.01 eV). The higher the energy difference between the HOMO energy levels of MOFs (−5.91 eV) and putrescine, the more effective the PET process. Thus, in terms of the lowest limit of detection (LOD) values (0.24 μM) for putrescine rather than the LOD for spermine, spermidine was shown.References
- Matsuoka, A.; Sakamoto, T. . Rinsho Byori Jpn. J. Clin. Pathol. 2004, 52, 328–331.
- Yu, Z.; Huang, H.; Zhang, H.; Kessler, B.M. Improved Profiling of Polyamines Using Two-Dimensional Gas Chromatography Mass Spectrometry. Talanta 2019, 199, 184–188.
- Balcerzak, W.; Pokajewicz, K.; Wieczorek, P. A Useful Procedure for Detection of Polyamines in Biological Samples as a Potential Diagnostic Tool in Cancer Diagnosis. Appl. Cancer Res. 2017, 37, 23.
- Venlinen, M.; Roine, A.N.; Hkkinen, M.; Vepslinen, J.; Rantanen, T.K. Altered Polyamine Profiles in Colorectal Cancer. Anticancer Res. 2018, 38, 3601–3607.
- Takahashi, Y.; Horio, H.; Sakaguchi, K.; Hiramatsu, K.; Kawakita, M. Significant Correlation between Urinary N1, N12-Diacetylspermine and Tumor Invasiveness in Patients with Clinical Stage Ia Non-Small Cell Lung Cancer. BMC Cancer 2015, 15, 65.
- Patin, F.; Corcia, P.; Vourc’h, P.; Baranek, T.; Goossens, J.F.; Marouillat, S.; Dessein, A.F.; Descat, A.; Bruno, C.; Leman, S.; et al. Omics to Explore Amyotrophic Lateral Sclerosis Evolution: The Central Role of Arginine and Proline Metabolism. Mol. Neurobiol. 2017, 54, 5361–5374.
- Nohta, H.; Satozono, H.; Koiso, K.; Yoshida, H.; Ishida, J.; Yamaguchi, M. Highly Selective Fluorometric Determination of Polyamines Based on Intramolecular Excimer-Forming Derivatization with a Pyrene-Labeling Reagent. Anal. Chem. 2000, 72, 4199–4204.
- Lee, B.; Scopelliti, R.; Severin, K. A Molecular Probe for the Optical Detection of Biogenic Amines. Chem. Commun. 2011, 47, 9639–9641.
- Nakamura, M.; Sanji, T.; Tanaka, M. Fluorometric Sensing of Biogenic Amines with Aggregation-Induced Emission-Active Tetraphenylethenes. Chem.-Eur. J. 2011, 17, 5344–5349.
- Singh, G.; Mangat, S.; Sharma, H.; Singh, J.; Arora, A.; Singh Pannu, A.; Singh, N. Design and Syntheses of Novel Fluorescent Organosilicon-Based Chemosensors through Click Silylation: Detection of Biogenic Amines. RSC Adv. 2014, 4, 36834.
- Fletcher, J.T.; Bruck, B.S. Spermine Detection Via Metal-Mediated Ethynylarene ’Turn-on’ Fluorescence Signaling. Sens. Actuators B 2015, 207, 843–848.
- Satrijo, A.; Swager, T.M. Anthryl-Doped Conjugated Polyelectrolytes as Aggregation-Based Sensors for Nonquenching Multicationic Analytes. J. Am. Chem. Soc. 2007, 129, 16020–16028.
- Bao, B.; Yuwen, L.; Zheng, X.; Weng, L.; Zhu, X.; Zhan, X.; Wang, L. A Fluorescent Conjugated Polymer for Trace Detection of Diamines and Biogenic Polyamines. J. Mater. Chem. B 2010, 20, 9628–9634.
- Wang, J.; Zhang, Q.; Zhong, D.L.; Cheng, Z.H. Calf Thymus DNA-Stabilized Polythiophene Fluorescence Probe for Label-Free Detection of Spermine. Analyst 2012, 137, 5565–5570.
- Malik, A.H.; Hussain, S.; Iyer, P.K. Aggregation-Induced Fret Via Polymer-Surfactant Complexation: A New Strategy for the Detection of Spermine. Anal. Chem. 2016, 88, 7358–7363.
- Hu, Y.; Ma, X.; Zhang, Y.; Che, Y.; Zhao, J. Detection of Amines with Fluorescent Nanotubes: Applications in the Assessment of Meat Spoilage. ACS Sens. 2015, 1, 22–25.
- Ikeda, M.; Yoshii, T.; Matsui, T.; Tanida, T.; Hamachi, I. Montmorillonite-Supramolecular Hydrogel Hybrid for Fluorocolorimetric Sensing of Polyamines. J. Am. Chem. Soc. 2011, 133, 1670–1673.
- Koestereli, Z.; Severin, K. Fluorescence Sensing of Spermine with a Frustrated Amphiphile. Chem. Commun. 2012, 48, 5841–5843.
- Tu, J.; Sun, S.; Xu, Y. A Novel Self-Assembled Platform for Ratiometric Fluorescent Detection of Spermine. Chem. Commun. 2015, 52, 1040–1043.
- Chopra, S.; Singh, J.; Kaur, H.; Singh, H.; Singh, N.; Kaur, N. Selective Chemosensing of Spermidine Based on Fluorescent Organic Nanoparticles in Aqueous Media Via a Fe3+ Displacement Assay. New J. Chem. 2015, 39, 3507–3512.
- Kim, T.I.; Park, J.; Kim, Y. A Gold Nanoparticle-Based Fluorescence Turn-on Probe for Highly Sensitive Detection of Polyamines. Chem.-Eur. J. 2011, 17, 11978–11982.
- Dan, Y.; Liu, J.J.; Zhi, Z.H.; Wang, N.; Zou, H.Y.; Huang, C.Z.; Wang, J. Highly Selective Detection of Spermine in Human Urine Via a Nanometal Surface Energy Transfer Platform. Talanta 2018, 188, 218–224.
- Bhamore, J.R.; Murthy, P.; Kailasa, S.K. Fluorescence Turn-Off Detection of Spermine in Biofluids Using Pepsin Mediated Synthesis of Gold Nanoclusters as a Probe. J. Mol. Liq. 2019, 280, 18–24.
- Tawfik, S.M.; Shim, J.; Biechele-Speziale, D.; Sharipov, M.; Lee, Y.I. Novel “Turn Off-on” Sensors for Highly Selective and Sensitive Detection of Spermine Based on Heparin-Quenching of Fluorescence CdTe Quantum Dots-Coated Amphiphilic Thiophene Copolymers. Sens. Actuators B 2018, 257, 734–744.
- Zhou, Y.; Tang, H.; Li, Z.H.; Xu, L.; Wang, L.; Cao, D. Bio-Inspired AIE PillarArene Probe with Multiple Binding Sites to Discriminate Alkanediamines. Chem. Commun. 2021, 57, 13114–13117.
- Kim, Y.; Kim, T. Analyte-Directed Formation of Emissive Excimers for the Selective Detection of Polyamines. Chem. Commun. 2016, 52, 10648.
- Jiang, G.; Zhu, W.; Chen, Q.; Li, X.; Zhang, G. Selective Fluorescent Probes for Spermine and 1-Adamantanamine Based on the Supramolecular Structure Formed between AIE-Active Molecule and CucurbitUrils. Sens. Actuators B 2018, 261, 602.
- Bhosle, A.; Banerjee, M.; Barooah, N.; Bhasikuttan, A.C.; Kadu, K.; Ramanan, S.R.; Chatterjee, A. ESIPT-Active -HAP Nps Based Supramolecular Sensing Assembly for Spermine, Spermidine and Cadaverine: Application in Monitoring Cancer Biomarkers and Food Spoilage. J. Photochem. Photobiol. A 2022, 426, 113770.
- Naik, V.G.; Kumar, V.; Bhasikuttan, A.C.; Kadu, K.; Ramanan, S.R.; Bhosle, A.A.; Banerjee, M.; Chatterjee, A. Solid-supported amplification of aggregation emission: A tetraphenylethylene–cucurbit supramolecular sensing assembly for the detection of spermine and spermidine in human urine and blood. ACS Appl. Bio Mater. 2021, 4, 1813–1822.
- Tripathi, N.; Singh, P.; Luxami, V.; Mahajan, D.; Kumar, S. Spermine Detection from Urine and Blood Serum Using Ionic Self-assembly of Benzimidazolium Based Dipod and Dodecylsulfate. Sens. Actuators B 2018, 270, 552–561.
- Nguyen, N.N.; Huy, B.T.; Phong, P.T.; Han, J.S.; Kwon, D.H.; Lee, Y. Simple Fluorescence Optosensing Probe for Spermine Based on Ciprofloxacin-Tb3+ Complexation. PLoS ONE. 2021, 16, e0251306.
- Tian, H.; Chang, Y.; Hu, X.; Li, X.; Guo, D. Supramolecular Imaging of Spermine in Cancer Cells. Nanoscale 2021, 13, 15362–15368.
- Tian, H.; Yu, X.; Yao, J.; Gao, G.; Wu, W.; Yang, C. Supramolecular Spectral/Visual Detection of Urinary Polyamines through Synergetic/Competitive Complexation with Gamma-CD and CB. Chem. Commun. 2021, 57, 1806–1809.
- Wang, L.; Ran, X.; Tang, H.; Cao, D. Recent Advances on Reaction-Based Amine Fluorescent Probes. Dyes Pigm. 2021, 194, 109634.
- Du, J.; Hu, M.; Fan, J.; Peng, X. Fluorescent Chemodosimeters Using “Mild” Chemical Events for the Detection of Small Anions and Cations in Biological and Environmental Media. Chem. Soc. Rev. 2012, 41, 4511–4535.
- Asanuma, D.; Sakabe, M.; Kamiya, M.; Yamamoto, K.; Urano, Y. Sensitive Β-Galactosidase-Targeting Fluorescence Probe for Visualizing Small Peritoneal Metastatic Tumours in Vivo. Nat. Commun. 2015, 6, 6463.
- Uno, S.N.; Kamiya, M.; Yoshihara, T.; Sugawara, K.; Okabe, K.; Tarhan, M.C.; Fujita, H.; Funatsu, T.; Okada, Y.; Tobita, S. A Spontaneously Blinking Fluorophore Based on Intramolecular Spirocyclization for Live-Cell Super-Resolution Imaging. Nat. Chem. 2015, 6, 681–689.
- Hu, D.; Zhang, T.; Li, S.; Yu, T.; Zhang, X.; Hu, R.; Feng, J.; Wang, S.; Liang, T.; Chen, J. Ultrasensitive Reversible Chromophore Reaction of Bodipy Functions as High Ratio Double Turn on Probe. Nat. Commun. 2018, 9, 362.
- Wang, L.; Wu, S.; Tang, H.; Cao, D. An efficient probe for sensing different concentration ranges of glutathione based on AIE-active Schiff base nanoaggregates with distinct reaction mechanism. Sens. Actuators B 2018, 273, 1085–1090.
- Li, L.; Li, W.; Ran, X.; Wang, L.; Tang, H.; Cao, D. A Highly Efficient, Colorimetric and Fluorescent Probe for Recognition of Aliphatic Primary Amines Based on a Unique Cascade Chromophore Reaction. Chem. Commun. 2019, 55, 9789–9792.
- Wang, L.; Xiong, Z.; Ran, X.; Tang, H.; Cao, D. Recent Advances of Nir Dyes of Pyrrolopyrrole Cyanine and Pyrrolopyrrole Aza-Bodipy: Synthesis and Application. Dyes Pigm. 2022, 198, 110040.
- Shimizu, S.; Lino, T.; Araki, Y.; Kobayashi, N. Pyrrolopyrrole Aza-Bodipy Analogues: A Facile Synthesis and Intense Fluorescence. Chem. Commun. 2013, 49, 1621–1623.
- Shimizu, S.; Iino, T.; Saeki, A.; Seki, S.; Kobayashi, N. Rational Molecular Design Towards Vis/Nir Absorption and Fluorescence by Using Pyrrolopyrrole Aza -Bodipy and Its Highly Conjugated Structures for Organic Photovoltaics. Chem.-Eur. J. 2014, 21, 2893–2904.
- Li, L.; Li, W.; Wang, L.; Tang, H.; Ran, X. Pyrrolopyrrole Aza-Bodipy Dyes for Ultrasensitive and Highly Selective Biogenic Diamine Detection. Sens. Actuators B 2020, 312, 127953.
- Wang, L.; Ding, H.; Tang, H.; Cao, D.; Ran, X. A Novel and Efficient Chromophore Reaction Based on a Lactam-Fused Aza-Bodipy for Polyamine Detection. Anal. Chim. Acta 2020, 1135, 38–46.
- Wang, L.; Xin, S.; Zhang, C.; Ran, X.; Tang, H.; Cao, D. Development of a Novel Chromophore Reaction-Based Fluorescent Probe for Biogenic Amines Detection. J. Mater. Chem. B 2021, 9, 9383–9394.
- Barros, M.; Ceballos, S.; Arroyo, P.; Sáez, J.A.; Parra, M.; Gil, S.; Costero, A.M.; Gaviña, P. Spermine and Spermidine Detection through Restricted Intramolecular Rotations in a Tetraphenylethylene Derivative. Chemosensors 2022, 10, 8.
- Huang, J.; Ye, W.; Zha, S.; Tao, Y.; Yang, M.; Huang, K.; Liu, J.; Fung, J.; Li, Y.; Zhu, L.; et al. Sensitive and Responsive Pentiptycene-based Molecular Fluorescence Chemosensor for Detection of Polyamines. J. Lumin. 2021, 232, 117856.
- Samira Abbasi-Moayed, S.; Bigdeli, A.; Hormozi-Nezhad, M.R. Determination of Spermine and Spermidine in Meat with a Ratiometric Fluorescence Nanoprobe and a Combinational Logic Gate. Food Chem. 2022, 384, 132459.
- Khan, S.A.; Misra, T.K. Novel Gluconate Stabilized Gold Nanoparticles as a Colorimetric Sensor for Quantitative Evaluation of Spermine. Colloids Surf. A 2022, 648, 129146.
- Leelasree, T.; Aggarwa, H. MOF Sensors for Food Safety: Ultralow Detection of Putrescine and Cadaverine in Protein Rich Foods. J. Mater. Chem. C. 2022, 10, 2121–2127.
More