The role of NLRP3 in the tumour microenvironment is elusive. In some cancers, the activation of NLRP3 causes a worse prognosis and in some cancers, NLRP3 increases chances of survivability. However, in many cases where NLRP3 has a protumorigenic role, inhibition of NLRP3 would be a crucial step in therapy. Consequently, activation of NLRP3 would be of essence when inflammation is required. Chitin and its derivatives are able to upregulate and downregulate the effect of the NLRP3 inflammasome based on its preparation, and these different reactions can be utilised to successfully target a broad range of cancers. Out of chitin, chitosan and chitooligosaccharide (COS), COS seems to be the best approach to actual products due its solubility being the highest, enabling it to be delivered more efficiently when compared to the other two. These specific preparations can be used on a case-by-case basis to help mitigate the negative effects of cancer and can potentially be used as a treatment or an adjuvant to cancer treatment.
1. Introduction
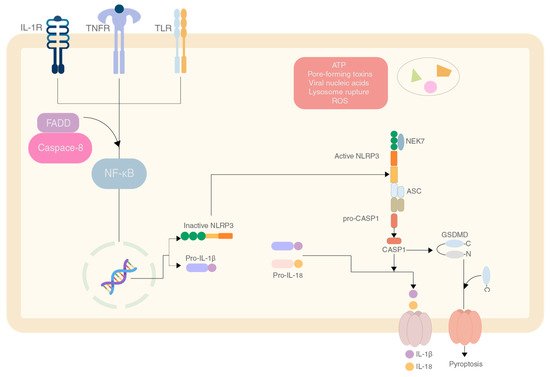
The NLRP3 inflammasome is an essential component of the innate immunity system. This inflammasome consists of three major proteins, the adaptor apoptosis-associated speck-like protein (ASC, which contains a caspase activation and recruitment domain, CARD), pro-caspase 1 (the effector molecule) and NLRP3 (containing pyrin domain 3)
[1]. The pyrin domain of the NLRP3 interacts with ASC to start the inflammasome assembly. The mechanism responsible for activation is shown in
Figure 1 [2].
Figure 1.
The mechanism of production and activation of the NLRP3 inflammasome by various effector molecules.
Current options for cancers treatment such as radiation therapy and chemotherapy, due to their non-selectivity and potency of the drugs can lead to detrimental long-term effects in patients. Even with breakthrough natural drugs such as paclitaxel, there are significant side effects such as hepatotoxicity, neurotoxicity and cardiotoxicity
[3][4]. However, studies on other natural extracts such as curcumin show positive results as an anticancer drug.
The abundance of natural extracts from plants, fungi and marine ecosystems provides an optimistic number of extracts that can be studied for their anti-cancer properties. Marine ecosystems in particular, with more than 80% of the oceans being undiscovered, can be a valuable resource for anti-cancer extracts. For example, chitin, an abundant polysaccharide in nature, mostly occurs in the exoskeletons of crustaceans
[4][5].
The possibility of using chitin and its derivatives as a potential anti-cancer drug due to its effects on the NLRP3 inflammasome and its components is underexplored. The authors suggest that due to the varying effects on the components of the inflammasome, chitin and its derivatives can be used as a potential anti-cancer drug or as an adjuvant.
2. The Role of Chitin and Its Derivatives
2.1. Effect of Chitin
Chitin is a very widely available polymer in nature, second to only cellulose. It is found mainly in fungal cell wall and exoskeleton of crustaceans. It is a long chain polymer of N-acetylglucosamine with β-(1–4) linkages
[5][62]. Although chitin is not found in mammals, there is a presence of chitinases in mammals
[6][63]. Chitin can also prove to be an avenue to recognise fungal pathogens due to lack of chitin in mammals normally. Studies conducted on human cancer cell lines have shown chitin to inhibit cell growth. Cytotoxicity was also shown against the Hep2 line at a concentration of 2000 μg/mL
[7][64].
Chitin has been shown to have pro-inflammatory and anti-inflammatory effects when introduced, which is based on the preparation and size of molecule
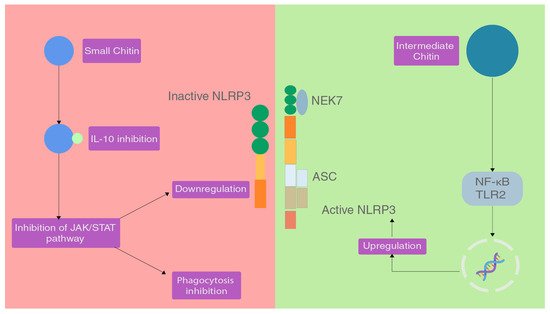
[8][65]. A study performed on C57BL/6 mice (TLR2 or TLR4 null mice and control mice) showed the effects of different sizes of chitin on the inflammatory response and categorised chitin as small chitin (SC) and intermediate chitin (IC)
[9][66].
Chitin molecules of size 40–70 µm are IC and chitin molecules of size lesser than 40 µm are SC. Chitin molecules larger than 70 µm are classified as big chitin (BC) and was found to be inert to the inflammatory response
[9][66]. Due to the size of BC, recognition and activity of any enzymes was ineffective so they are inert but if they get degraded by mammalian chitinase, that can generate IC and SC. In the same study, IC induced a pro-inflammatory response via TLR2 and NF-κB-dependent pathways for TNF production
[9][66].
SC was able to induce an anti-inflammatory response via the production of IL-10 which is an anti-inflammatory cytokine
[9][66]. IL-10 can suppress the immune response via binding to the heterodimeric receptors IL-10R1 and IL-10R2, which when activated triggers the JAK/STAT signalling pathway that serves to inhibit phagocytosis, presenting receptors and inhibition of release of pro-inflammatory cytokines
[10][11][67,68] (
Figure 23).
Figure 23.
The effect of different sizes of chitin on the NLRP3 inflammasome.
Chitin is mostly just recognised in mammalian cells due to the absence of chitin normally. The LYSMD3 domain is a pattern recognition receptor that is capable of binding chitin and sensing it. Other pattern recognition receptors that have affinity to chitin and derivatives of chitin are FIBCD1, NKR-P1, RegIIIγ and galectin-3
[12][13][14][15][16][69,70,71,72,73].
Chitin can occur in three types of crystal structures, α-, β- and γ-forms. The α-form is the most abundant in nature. However, due to the arrangement of these crystals, there is more inter-molecular bonding, albeit these bonds stabilise the structure, they hinder the ability of chitin to dissolve in many common solvents
[17][74]. This prompts the usage of chitosan instead of chitin for pharmaceutical use as chitosan can dissolve in more solvents depending on the degree of deacetylation
[18][75].
2.2. Effect of Chitosan
Chitosan is a polymer of chitin that is formed when chitin is deacetylated, yielding β-1,4-D-glucosamine
[19][76]. It can be synthesised via biological and chemical methods, with chemical methods being employed more often in the industry due to its increased bioactivity and easier processing
[20][77]. The chemical methods of production via by-products of crustaceans involve a three-step process that includes demineralisation, deproteination and deacetylation to finally yield chitosan
[19][76].
The purity and molecular weight of chitosan strongly influences the immune response that it induces
[21][22][78,79] and having a high degree of purity while using chitin or its derivatives is a must.
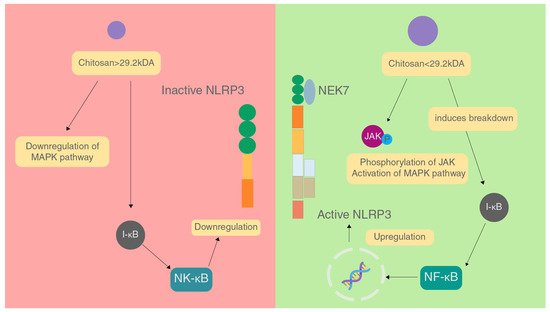
The molecular weight of chitosan is also a major factor in affecting the immune response caused. In a study conducted, it was found that chitosan with molecular weight greater than 29.2 kDa inhibited inflammation by downregulating the MAPK signalling pathway, inhibiting NF-κB activation and prevents expression of iNOS which brings down NO levels, and stopping the production of TNF-α and IL-6
[22][79]. These factors combined reduce the activation of the NLRP3 inflammasome (
Figure 34).
Figure 34.
Effect of different sizes of chitosan on the NLRP3 inflammasome.
On the contrary, chitosan of molecular weight lesser than 29.2 kDa acts in a pro-inflammatory manner by increasing phosphorylation of JNK of the MAPK pathway, enhancing NF-κB activation and iNOS expression causing an increase in the levels of NO, and increasing the production of TNF-α and IL-6 by binding to CR3, TLR4 and CD14 on macrophages. All these factors combined increase the activation of the NLRP3 inflammasome
[22][79]. In a study on Hep2 and RD cell lines, chitosan showed cytotoxicity against these cell lines, and had a better IC than chitin and irradiated chitin
[7][64].
Studies on chitosan often only characterise MW or DDA in high detail and leave out the other and fail to fully characterise the features of chitosan. This can lead to ambiguity in results. Studies have found that DDA does not make a difference in the immunoreactive properties of chitosan
[19][76]. It is also suggested that endotoxin presence can interfere with the properties of chitosan. Another factor to consider is that as the DDA of chitosan approaches 100% its solubility increases that can play a role in being more bioavailable and affecting its properties during delivery
[23][80].
Further treatment of the chitosan polymer can lead to the formation of chitosan nanoparticles and chitosan scaffolds. Although the role of DDA in the effect of chitosan polymer affecting the immune system has contradictory and ambiguous results, there are studies investigating the properties of the scaffolds and the nanoparticle showing a correlation between DDA and the properties of the material.
2.3. Effect of Chitooligosaccharides
Chitooligosaccharides (COS) are degraded products of chitosan by enzymatic or chemical methods. Chitosan, like its precursor is still insoluble and not easily absorbed into the body. However, COS is soluble and easily absorbed by the gastrointestinal tract, after which it affects the target cells. This implies that COS can be utilised for a myriad of applications by retaining the bioactive properties of chitosan
[24][84].
As explored previously, the antitumoral effect of COS is attributed to recruitment of T-cells and immunoregulation.
TWe propose that alongside this, there is an inflammation/inflammasome-dependent manner by which COS and variations of COS are able to affect cancer
[4][5]. It is likely that a combination of the two of these factors leads to the potent anti-cancer properties that these molecules possess.
This hypothesis can be further supported by studies conducted on the types of cancers described previously. A study conducted by Shen et al. showed that the use of COS with 23.99 kDa molecular weight, markedly reduced the proliferation of HepG2 cells by inhibiting the NF-κB
[25][26][85,86]. Studies were conducted on colorectal cancer cells showing that utilisation of COS between 1 and 3 kDa reduced pro-inflammatory cytokine production by the inhibition of MM2 and iNOS, which led to a preventative effect
[24][84].