Fly ash or coal fly ash causes major global pollution in the form of solid waste and is classified as a “hazardous waste”, which is a by-product of thermal power plants produced during electricity production. Si, Al, Fe Ca, and Mg alone form more than 85% of the chemical compounds and glasses of most fly ashes. Fly ash has a chemical composition of 70–90%, as well as glasses of ferrous, alumina, silica, and CaO. Therefore, fly ash could act as a reliable and alternative source for ferrous, alumina, and silica. The ferrous fractions can be recovered by a simple magnetic separation method, while alumina and silica can be extracted by chemical or biological approaches. Alumina extraction is possible using both alkali- and acid-based methods, while silica is extracted by strong alkali, such as NaOH. Chemical extraction has a higher yield than the biological approaches, but the bio-based approaches are more environmentally friendly. Fly ash can also be used for the synthesis of zeolites by NaOH treatment of variable types, as fly ash is rich in alumino-silicates. The present review work deals with the recent advances in the field of the recovery and synthesis of ferrous, alumina, and silica micro and nanoparticles from fly ash.
- fly ash
- aluminosilicate
- cenospheres
- plerospheres
- ferrospheres
1. Introduction
Fly ash or coal fly ash (CFA) is a spherical, glass-like, heterogeneous particle produced as a by-product from the combustion of pulverized coal during electricity production in thermal power plants (TPPs). Morphologically, fly ash particles are spherical in shape, with sizes varying from 200 nm to several microns, and structurally have ferrospheres, cenospheres, aluminosilicate spheres, or plerospheres, and irregular-shaped carbonaceous particles [1]. Fly ash has almost all the elements present in geological samples—that is, metals, heavy metals, and organic contents. Though the major composition of fly ash almost remains same throughout the world, the composition still varies based on the source of coal, their geographical origin, furnace temperature, and the operating conditions of the boiler [2]. As fly ash is derived from coal, which is rich in minerals, fly ash is also rich in silica, alumina, and ferrous [3], which are the three major contents of fly ash. Besides this, CFA also has minor oxides, such as rutile, K2O, CaO, Na2O, and phosphorous oxides, as well as traces of Cu, Cr, Zn, Ni, and Mo oxides [4]. In addition to this, fly ash is also loaded with several toxic heavy metals, such as Al, Ni, Co, Cr, Cd, Zn, Mo, As, and Hg, which categorizes fly ash into “hazardous materials” [5], and poses a potential threat to the flora, fauna, and the environment.
Every year, a million tonnes (MTs) of fly ash are produced around the globe, especially in the USA, China, France, and India [6]. Fly ash is not a serious concern for developed countries, but it poses a potential threat for developing countries [7]. This is because the fly ash utilization rate of some of developed countries is more than 90%; for instance, France utilizes almost 100% of fly ash, which indicates complete recycling of the fly ash [2]. At the same time, for a developing country, such as India, the fly ash utilization rate is 50–60%, whereas for other developing countries, it is below 40%. The more aggravating situation is the production of millions of tonnes of fly ash every year around the world. Even in the 20th century, 50% of global fly ash is dumped in the vicinity of TPPs. The dumping of fly ash on fertile agricultural land as landfills deteriorates hundreds of acres of land every year [8], which will ultimately lead to a negative impact on the environment. Moreover, the rainfall on piles of heavy metal-loaded fly ash leads to the leaching of heavy metals into the soil, groundwater, and ultimately rivers and other water bodies [9,10][9][10]. This will further lead to water pollution and also poses a potential threat to the aquatic flora and fauna, owing to the increased concentration of heavy metals.
The pollution arising from fly ash might be a negative side, but the presence of valuable minerals (silica, alumina, and ferrous) in higher compositions is the positive side of fly ash [11,12][11][12]. As fly ash is derived from coal, which has a high amount of silica, alumina, and ferrous, these elements are also common in the fly ash after combustion [3]. Today, with the continuous advancement of technology and research and development, these fly ashes have found applications in the fields of ceramics and construction, adsorbents, fertilizers, landfills, geopolymers, and metallurgy [9,10][9][10]. In ceramics and construction alone, they are used for making fly ash amended cement, tiles, pavement blocks, dike preparation, and embankments, among others [10]. Here, however, we are concerned with the recovery and synthesis of alumina, silica, and ferrous nanoparticles from fly ash. In the last decade, there has been a tremendous revolution in the field of nanotechnology and nanoparticles, which has helped it to find applications in the field of catalysis, drug delivery, medicine, and environmental clean-up [13]. However, as nanotechnology is still in its infancy stage, the synthesis of nanoparticles involves expensive precursor materials and sophisticated instruments, which makes the final nanoparticles very costly. Therefore, the nanotechnology replaces the expensive precursor material with waste materials such as agricultural waste (sugarcane bagasse, rice husk ash, citrus waste) and industrial waste, such as gypsum waste, egg-shell waste [14], red mud, and fly ash. If nanoparticles are synthesized from any of the above-mentioned waste, then the final product will be not only cost-effective, but also eco-friendly thanks to the minimization of the solid waste as pollution.
One such precursor material for the synthesis of silica, alumina, and ferrous nanoparticles is fly ash. Fly ash is a rich source of ferrous (5–15%), silica (40–60%), alumina (20–40%), and calcium (0.5–15%), based on the types of coal used, geographical origin, and operating conditions for the combustion of coal in the thermal power plant [10]. Generally, class F fly ashes are rich sources of ferrous, alumina, and silica, as they are derived from the higher grades of coal—that is, anthracite and bituminous—whereas class C fly ashes have a lower content of ferro-alumino-silicate (FAS), as they are derived from the lower grades of coal—that is, sub-bituminous, lignite, and peat. As silica is present in the highest concentration in all of the fly ashes, most attempts have been made for the synthesis of silica nanoparticles (SiNPs) from various parts of the globe. The most preferred method for the synthesis of SiNPs from fly ash is the alkali dissolution method [15], where the fly ash is treated with 4–16 molarity of sodium hydroxides or potassium hydroxides at a temperature in the range of 90–100 °C for 1–3 h. Another method for silica nanoparticle synthesis is the alkali fusion method [16], where the fly ash is mixed with 4–16 M NaOH or KOH and fusion is done at higher temperatures of 600–1200 °C for 3–8 h in a muffle furnace. The high calcination temperature transforms the inert and crystalline minerals of fly ash into the reactive phase of Al and Si after reacting with sodium and potassium hydroxides [17]. The advantages of such a method is that the new products formed after calcination have high reactivity with acids and bases, which drastically increases the yield of silica. Further, as Al is amphoteric in nature, it can react with both acids and bases, and thus it can be extracted by treating the fly ash with concentrated mineral acids, such as sulphuric acid (H2SO4), hydrochloric acid (HCl), and nitric acid (HNO3), by keeping 4–16 molarity of acids, at temperatures of 100–130 °C for 1–3 h along with continuous stirring. Besides this, alumina can be extracted from fly ash by treating it with 4–16 M NaOH (keeping the solid-to-liquid ratio 1:5) at 90–100 °C for 1–3 h along with continuous stirring [18]. These procedures do not involve any pretreatment for the elimination of impurities in the form of Fe, Al, Na, Ca, etc., which may contribute, to some extent, to the final synthesized nanoparticles and make them undesirable.
2. Properties and Applications of Fly Ash
2.1. Morphological Properties of Fly Ash
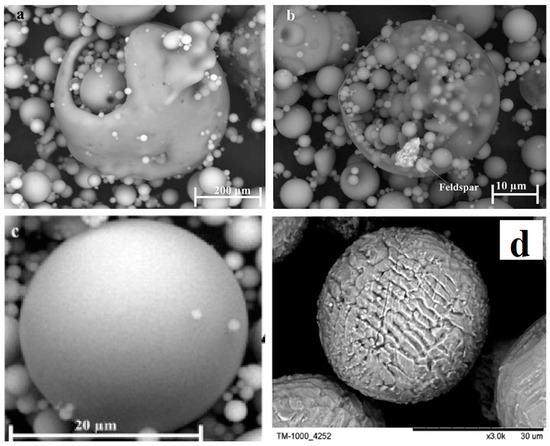
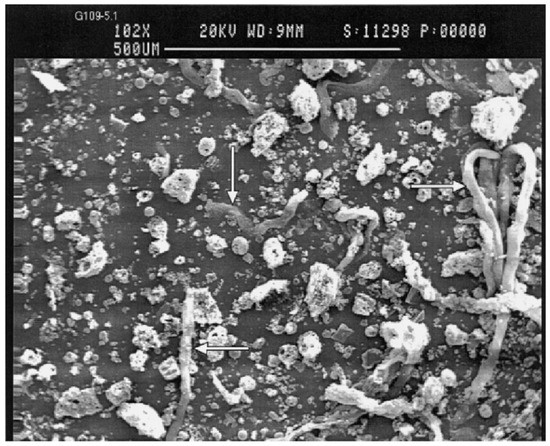
Fly ash is a sphere-shaped, micron-sized (0.01–100 µ) heterogeneous material, having depositions of mainly Al, Si, Fe and C in variable compositions on its surface, and closely resembles the volcanic ashes [25][19]. The fly ash particles can be either rough or smooth surfaced based on the type of depositions on their surface. Figure 1 show a typical fly ash particle, which is spherical in shape, whose sizes vary from 0.2 microns to several microns (6 µ). Morphologically, fly ash particles may have differently shaped particles, which also vary in their elemental composition viz. ferrospheres (ferrous rich spherical particles) [26][20], cenospheres or alumino-silicate spheres [27][21] (Al- and Si-rich particles), plerospheres [28][22] (larger spherical particles encapsulate smaller particles), plerospheres, and carbon nanomaterial [10]—i.e., soots, buck balls [29][23], fullerenes [30,31][24][25] and unburned carbon, including both organic and inorganic [2]. Figure 1a,b show fly ash plerospheres, which are thick- and thin-walled. Both the plerospheres have trapped numerous smaller sized spherical particles, along with gases and minerals. While Figure 1c depicts cenospheres which are spherical in shape, having mainly Al and Si, along with carbon, on their surface, Figure 1d shows ferrospheres, which have depositions of ferrous on their surface, due to which they have magnetic properties. The ferropsheres have rough surfaced and dendritic shape on their surface. In comparison to ferrospheres, cenospheres are lighter in weight [32][26] and have high mechanical strength, thermal resistance and have fireproof property [33][27]. The globular shape of such microspheres is due to the precipitation of crystalline phases during the cooling of iron aluminosilicate melt drops of complex composition [34][28]. The crystallite size and the composition of the iron-containing phases, that governs the magnetic properties of the microspheres, depend on both the melt composition and the thermal conditions of microsphere formation [35][29]. Cenospheres are more dominant structures in the fly ash [32][26], followed by the ferrospheres, which are spherical-shaped ferrous-rich particles, whose sizes fall in the micron range. The ferrospheres have high depositions of ferrous or Fe, which could be either rough, smooth, elliptical or molten drop-shaped, which are given in Section 4. Besides cenospheres and ferrospheres there is the third type of micron-sized spherical-shaped particles, called plerospheres, which are less frequent in fly ash in comparison to the other two forms. These plerospheres encapsulate several small fly ash particles, minerals and gases inside them during the formation from the molten slag at high temperature in the furnace [36,37][30][31]. Additionally, there are a large number of carbonaceous nanomaterials, such as fullerenes, graphene, soots and unburned irregular-shaped carbon particles in fly ash, formed due to the combustion of organic and inorganic carbon minerals present in the coal [38][32]. Such irregular or angular-shaped carbon-rich particles are shown in Figure 2, taken through Scanning Electron Micrograph (SEM), while the bright colored particles are electron-rich Fe, Al and Si rich region [39][33].
2.2. Elemental Properties of Fly Ash
The mineralogy and composition of fly ash is not constant, rather it varies from place to place, parent coal source, operating parameters and temperature of TPPs [42][36], the extent of coal preparation and cleaning, furnace design, usual climate storage [43][37] and handling. The mineralogical properties determine the crystalline phases of the fly ash, and their composition varies from 15–45% in the fly ash. Generally, fly ash has silica 40–60%, alumina 20–40% and ferrous 5–15% by weight fractions [44][38]. Almost all the fly ash has mullite, quartz, magnetite, hematite and calcite as the common crystalline minerals [45][39]. Based on mineral composition and sources of coal, fly ash is categorized into two classes—class F and class C. The major differences between these two classes of fly ash are described here. The source of class F fly ash is anthracite and bituminous coal, whereas for class C it is younger lignite and sub-bituminous coal. The lime content in class F is less than 20%, while class C has more than 20% of it. Ca in class F is mainly present in the form of Ca(OH)2, CaSO4 and glassy components, which is 1–12%, and in class C it is 30–40%. Class C has larger amount of crystalline content—i.e., 25–45%—than the class F, which has only 15–45% of the carbon [9,46,47][9][40][41]. The class F fly ash has a higher amount of alkali and sulfate than the class C fly ash. While, for cementing agent, class F requires Portland cement, hydrated lime and quicklime, whereas class C has self-cementing properties. Class F generally requires an addition of air entrainer, which is not required by the class C fly ash. When it comes to the application, class F is used in high SO43− exposure conditions, has high fly ash content concrete mixes and is explored for the structural and HP concretes. Whereas class C fly ash is not suitable for high sulfate conditions, limited to low fly ash content concrete mixes are mainly used for the residential construction.
2.3. Chemical Properties of Fly Ash
The pH of the fly ash tends to vary from acidic to alkaline (4.5 to 12.0), depending on the source of coal and the number of trace elements in them [48][42]. Fly ash produced from bituminous coal, is mostly acidic even though it has higher sulfur content, while alkaline fly ash is produced from the sub-bituminous coal, which has lower sulfur content, and has higher Ca and Mg content than that derived from bituminous coal [49][43]. Similarly the electrical conductivity (EC) of fly ash varies between 0.177 to 14 S/m, which directly corresponds to the quantitative concentration of soluble cations and anions in the fly ash [2,50][2][44]. Likewise, mineralogy and chemical composition too depend on the various parameters of coal combustion. Chemically, about 90–99% of the fly ash fraction constitutes oxides of silicon, aluminum, iron, calcium and titanium, (~0.5% to 3.5%), which are made up of oxides of sodium, potassium, phosphorus, manganese and sulfur [51][45], and the remaining fractions are the trace elements, including rare earth and radioactive elements. As per the universal rule, smaller particles with higher surface areas than the larger ones are also applicable to the fly ash particles—hence, smaller fly ash particles tend to accumulate a higher concentration of elements (As, Cd, Cu, Ga, Mo, Pb, S, Sb, Se, Ti and Zn) on their surface in comparison to the larger fly ash particles [52][46]. Fly ash particles have both crystalline and glassy amorphous materials. Silicates are present in crystalline form—i.e., sillimanite and mullite, while most of the silicates are present in the glass form. The average glass content in U.S. fly ash is 90%, while in Indian fly ash it varies from 49–69% by weight. This indicates that Indian fly ash has more crystalline content than the U.S. fly ash.
The chemical composition of the core or interior part of the fly ash is almost masked by the depositions of elements on the surface layer of fly ash particles [2]. Moreover, these surface layers get depositions of various elements during volatilization and condensation of molten slag in the furnace [55][47]. It has been reported that the concentration of some of the elements on the surface layer has many more folds than that of parent coal [56][48]. All fly ashes derived from different coal types, have oxides of Fe, Al, Si and varying carbon content. The chemical composition of fly ashes derived from different coal sources is given above in Table 1.
2.4. Physical Properties of Fly Ash
Based on the percentage of unburned carbon, fly ash color may vary from tan to grey or black [7]. The darker the color of fly ash, the higher the carbon content [57][49]. Based on the above fact, it is obvious that lower grades of coal (lignite, sub-bituminous) having a lesser amount of carbon, will produce light —i.e., tan to buff-colored fly ash [58][50]—while the higher grades of coal (anthracite and bituminous), being rich in carbon, will produce dark colored fly ash—i.e., grey to black. Moreover, calcium oxide content too contributes in the color of fly ash, as lower grades of coal have higher calcium content than the higher grades of coal, and provide white shade to the fly ash [55][47]. The specific surface area and the specific gravity of fly ash tend to vary in the range of 2000 to 6800 cm2 per gram and 2.1 to 3.0 g/cm3, respectively [9]. Regarding the particle sizes of fly ash, their composition varies from one geographical area to other, and for instance, the size of sandy particles is 2–0.5 mm and 4.75–0.075 mm in the U.S. and Indian fly ash, respectively, while the size of silt particles in U. S. fly ash vary from 0.05–0.002 mm and 0.075–0.002 mm in Indian fly ashes. However, the size of clay particles in both U.S. and Indian fly ashes are less than 0.002 mm. Sandy particles in U.S. fly ash are sub-divided into very coarse, coarse, medium, fine and very fine, and their total composition in fly ash is 32.4%, whereas in Indian, fly ash total composition of sandy particles is 35.69%, which indicates that the Indian fly ashes have 2–4% more sandy particles than the U.S. fly ashes. The percentage of silty particles in both U.S. fly and Indian fly ash are more than 60%; however, the U.S fly ash have marginally higher content of silty particles than the Indian fly ash. The average silty content in U.S. Fly ash is 63.2%, whereas in Indian fly ash it is 62.39%. Clay particles in U.S. fly ash are 4.3% in comparison to Indian fly ash having 1.91% of the clay. Hence, the U.S. fly ash has a 2–3% higher amount of clay particles than the Indian fly ash [7]. Variation in fly ash is also seen due to the different structural properties of the particles—i.e., cenospheres [59[51][52],60], plerospheres [61][53], ferrospheres [62][54] and irregular- or angular-shaped carbon particles [63][55], which are already briefly described in the introduction section. Cenospheres have a bulk density in the range of 0.4–0.6 ton/m3 and constitute up to 5% of the total weight of fly ash [32][26].
2.5. Applications of Fly Ash
Fly ash has great importance and numerous advantages either in the bulk form or in their separate natural nanostructured particles, which is depicted in the Figure 3. Besides, the fly ash also has a higher amount of Si, Al, and Fe that can be used in hydrometallurgy using the environmentally-friendly approach for the recovery of minerals at an economical cost [64,65][56][57]. The bulk form of fly ash can be potentially used as a biofertilizer, as it contains a rich source of plant nutrients such as, Na, Ca, K, P, Zn, Mg, Mn, Mo, etc. Moreover, the zeolites synthesized from fly ash can also be used for the sustained and controlled release of the N, P, K and other minerals to the plants [23,66][58][59]. In the field of agriculture, [67,68][60][61] the bulk fly ash can be used for resource conservation, reclamation of the contaminated sites and restoration of industrial sites [69][62]. Besides agriculture, the fly ash also finds application in civil engineering [70] [63] (bricks, tiles, cements, blocks), tiles [71[64][65],72], brick making, cements, geopolymer [73][66], landfills [74][67], mining [75][68], agriculture river embankments [76][69], fillers [77[70][71],78], panels and composite materials [79][72] and in metallurgy for the recovery of value-added minerals. The natural nanostructured form of fly ash—i.e., cenospheres, ferrospheres, carbonaceous particles and plerospheres, finds applications in nano-ceramics, mechanical engineering, construction of lightweight materials [80][73] and wastewater treatment. Besides, individual microspheres are also used for making thermoset plastics, concrete materials, nylon, material for coating [81][74], high-density polyethylene (HDPE), and others. In, hydrometallurgy, the high content of ferrous, alumina and silica in the fly ash, which is a waste, can possibly be considered as one of the most reliable materials for the recovery of ferrous, alumina and silica and their derivatives [82][75]. The recovery of such value-added minerals opens new horizons, as it not only reduces the global pollution in the form of solid waste but also acts as an alternative material for Si, aluminum and ferrous [83][76].
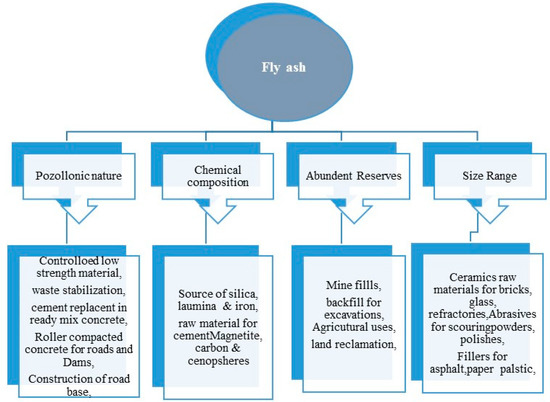
Figure 3. Broad areas of fly ash applications.
References
- Choudhary, N.; Yadav, V.K.; Malik, P.; Khan, S.H.; Inwati, G.K.; Suriyaprabha, R.; Singh, B.; Yadav, A.K.; Ravi, R.K. Recovery of Natural Nanostructured Minerals: Ferrospheres, Plerospheres, Cenospheres, and Carbonaceous Particles From Fly Ash. In Handbook of Research on Emerging Developments and Environmental Impacts of Ecological Chemistry; Gheorghe, D., Ashok, V., Eds.; IGI Global: Hershey, PA, USA, 2020; pp. 450–470.
- Ohenoja, K.; Pesonen, J.; Yliniemi, J.; Illikainen, M. Utilization of Fly Ashes from Fluidized Bed Combustion: A Review. Sustainability 2020, 12, 2988.
- Fuller, A.; Maier, J.; Karampinis, E.; Kalivodova, J.; Grammelis, P.; Kakaras, E.; Scheffknecht, G. Fly Ash Formation and Characteristics from (co-)Combustion of an Herbaceous Biomass and a Greek Lignite (Low-Rank Coal) in a Pulverized Fuel Pilot-Scale Test Facility. Energies 2018, 11, 1581.
- Wei, Q.; Song, W. Mineralogical and Chemical Characteristics of Coal Ashes from Two High-Sulfur Coal-Fired Power Plants in Wuhai, Inner Mongolia, China. Minerals 2020, 10, 323.
- Rodrigues, P.; Silvestre, J.D.; Flores-Colen, I.; Viegas, C.A.; Ahmed, H.H.; Kurda, R.; de Brito, J. Evaluation of the Ecotoxicological Potential of Fly Ash and Recycled Concrete Aggregates Use in Concrete. Appl. Sci. 2020, 10, 351.
- Alam, J.; Akhtar, M. Fly ash utilization in different sectors in Indian scenario. Int. J. Emerg. Trends Eng. Dev. 2011, 1, 1–14.
- Yadav, V.K.; Fulekar, M.H. The current scenario of thermal power plants and fly ash: Production and utilization with a focus in India. Int. J. Adv. Eng. Res. Dev. 2018, 5, 768–777.
- Nisham, K.; Sridhar, M.B.; Kumar, V. Experimental study on class F fly ash cement bricks using partial replacement of fly ash by metakaolin. Int. J. Chem. Sci. 2016, 14, 227–234.
- Yadav, V.K.; Pandita, P.R. Fly Ash Properties and Their Applications as a Soil Ameliorant. In Amelioration Technology for Soil Sustainability; Rathoure, A.K., Ed.; IGI Global: Hershey, PA, USA, 2019; pp. 59–89.
- Yadav, V.K.; Choudhary, N. An Introduction to Fly Ash: Natural Nanostructured Materials; Educreation: New Delhi, India, 2019; Volume 1, p. 162.
- Zhao, Y.; Soltani, A.; Taheri, A.; Karakus, M.; Deng, A. Application of Slag—Cement and Fly Ash for Strength Development in Cemented Paste Backfills. Minerals 2018, 9, 22.
- Valentim, B.; Białecka, B.; Gonçalves, A.P.; Guedes, A.; Guimarães, R.; Cruceru, M.; Całus-Moszko, J.; Popescu, G.L.; Predeanu, G.; Santos, C.A. Undifferentiated Inorganics in Coal Fly Ash and Bottom Ash: Calcispheres, Magnesiacalcispheres, and Magnesiaspheres. Minerals 2018, 8, 140.
- Li, S.; Qin, S.; Kang, L.; Liu, J.; Wang, J.; Li, Y. An Efficient Approach for Lithium and Aluminum Recovery from Coal Fly Ash by Pre-Desilication and Intensified Acid Leaching Processes. Metals 2017, 7, 272.
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J. Synthesis of Nano-Calcium Oxide from Waste Eggshell by Sol-Gel Method. Sustainability 2019, 11, 3196.
- Peng, X. Dynamic hydrothermal synthesis of xonotlite fibers by alkali silica extraction of fly ash. J. Eng. Fibers Fabr. 2019, 14, 155892501989034.
- Purnomo, C.; Wirawan, S.; Hinode, H. The utilization of bagasse fly ash for mesoporous silica synthesis. IOP Conf. Ser. Mater. Sci. Eng. 2019, 543, 012040.
- Guo, C.; Zou, J.; Ma, S.; Yang, J.; Wang, K. Alumina Extraction from Coal Fly Ash via Low-Temperature Potassium Bisulfate Calcination. Minerals 2019, 9, 585.
- Gong, Y.; Sun, J.; Sun, S.-Y.; Lu, G.; Zhang, T.-A. Enhanced Desilication of High Alumina Fly Ash by Combining Physical and Chemical Activation. Metals 2019, 9, 411.
- Langmann, B. Volcanic Ash versus Mineral Dust: Atmospheric Processing and Environmental and Climate Impacts. ISRN Atmos. Sci. 2013, 2013, 17.
- Sunjidmaa, D.; Batdemberel, G.; Takibai, S. A Study of Ferrospheres in the Coal Fly Ash. Open J. Appl. Sci. 2019, 9, 10–16.
- Murugesan, S.; Ramaswamy, J.; Parshwanath, R.; Sundararaj, J.; Jose, R. Evaluation of Suitability of Alumino-Silicate Precursor for Geopolymerization through Advance Analytical Techniques. Asian J. Chem. 2018, 30, 1771–1776.
- Valentim, B.; Flores, D.; Guedes, A.; Shreya, N.; Paul, B.; Ward, C.R. Notes on the occurrence of char plerospheres in fly ashes derived from Bokaro and Jharia coals (Jharkhand, India) and the influence of the combustion conditions on their genesis. Int. J. Coal Geol. 2016, 158, 29–43.
- Salah, N.; Al-Ghamdi, A.; Memic, A.; Habib, S.; Khan, Z. Formation of Carbon Nanotubes from Carbon Rich Fly Ash: Growth Parameters and Mechanism. Mater. Manuf. Process. 2015, 31, 150811005209005.
- Silva, L.; Martinello, K.; Mardon, S.; Hower, J.; Serra-Rodríguez, C. Fullerenes and Metallofullerenes in Coal-Fired Stoker Fly Ash. Coal Combust. Gasif. Prod. 2010, 2.
- Fu, B.; Hower, J.C.; Dai, S.; Mardon, S.M.; Liu, G. Determination of Chemical Speciation of Arsenic and Selenium in High-As Coal Combustion Ash by X-ray Photoelectron Spectroscopy: Examples from a Kentucky Stoker Ash. ACS Omega 2018, 3, 17637–17645.
- Yoriya, S.; Intana, T.; Tepsri, P. Separation of Cenospheres from Lignite Fly Ash Using Acetone-Water Mixture. Appl. Sci. 2019, 9, 3792.
- Choo, T.F.; Salleh, M.A.M.; Kok, K.Y.; Matori, K.A.; Rashid, S.A. Effect of Temperature on Morphology, Phase Transformations and Thermal Expansions of Coal Fly Ash Cenospheres. Crystals 2020, 10, 481.
- Hower, J.; Groppo, J.; Graham, U.; Ward, C.; Kostova, I.; Maroto-Valer, M.; Dai, S. Coal-derived unburned carbons in fly ash: A review. Int. J. Coal Geol. 2017, 179, 11–27.
- Bayukov, O.A.; Anshits, N.N.; Balaev, A.D.; Sharonova, O.M.; Rabchevskii, E.V.; Petrov, M.I.; Anshits, A.G. Mössbauer study of magnetic microspheres isolated from power plant fly ash. Inorg. Mater. 2005, 41, 50–59.
- Wang, P.; Massoudi, M. Slag Behavior in Gasifiers. Part I: Influence of Coal Properties and Gasification Conditions. Energies 2013, 6, 784–806.
- Krishnamoorthy, V.; Pisupati, S. A Critical Review of Mineral Matter Related Issues during Gasification of Coal in Fixed, Fluidized, and Entrained Flow Gasifiers. Energies 2015, 8, 10430–10463.
- Wang, Y.-S.; Alrefaei, Y.; Dai, J.-G. Silico-Aluminophosphate and Alkali-Aluminosilicate Geopolymers: A Comparative Review. Front. Mater. 2019, 6, 106.
- Liu, H.; Sun, Q.; Wang, B.; Wang, P.; Zou, J. Morphology and Composition of Microspheres in Fly Ash from the Luohuang Power Plant, Chongqing, Southwestern China. Minerals 2016, 6, 30.
- Sharonova, O.; Anshits, N.; Fedorchak, M.; Zhizhaev, A.; Anshits, A. Characterization of Ferrospheres Recovered from High-Calcium Fly Ash. Energy Fuels 2015, 29, 5404–5414.
- Veranth, J.M.; Fletcher, T.H.; Pershing, D.W.; Sarofim, A.F. Measurement of soot and char in pulverized coal fly ash. Fuel 2000, 79, 1067–1075.
- Vassilev, S.; Menendez, R.; Borrego, A.; Díaz-Somoano, M.; Martínez-Tarazona, M. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 3. Characterization of magnetic and char concentrates. Fuel 2004, 83, 1563–1583.
- Gupta, D.K.; Rai, U.N.; Tripathi, R.D.; Inouhe, M. Impacts of fly-ash on soil and plant responses. J. Plant Res. 2002, 115, 401–409.
- Singh, G.B.; Subramaniam, K.V. Characterization of Indian fly ashes using different Experimental Techniques. Indian Concr. J. 2018, 92, 10–23.
- Šešlija, M.; Rosić, A.; Radović, N.; Vasić, M.; Đogo, M.; Jotić, M. Physiproperties of fly ash and slag from the power plants. Geol. Croat. 2016, 69, 317–324.
- Eisele, T.C.; Kawatra, S.K.; Nofal, A. Comparison of class C and class F fly-ashes as foundry sand binders and the effectiveness of accelerators in reducing curing time. Miner. Process. Extr. Metall. Rev. 2004, 25, 269–278.
- Vassilev, S.; Menendez, R.; Alvarez, D.; Díaz-Somoano, M.; Martínez-Tarazona, M. Phase-Mineral and Chemical Composition of Coal Fly Ashes as a Basis for Their Multicomponent Utilization. 1. Characterization of Feed Coals and Fly Ashes. Fuel 2003, 82, 1793–1811.
- Basu, M.; Pande, M.; Bhadoria, P.B.S.; Mahapatra, S.C. Potential fly-ash utilization in agriculture: A global review. Prog. Nat. Sci. 2009, 19, 1173–1186.
- Fulekar, M.H.; Dave, J.M. Heavy metals release from ash pond to soil water environment: A simulated technique. Environ. Int. 1992, 18, 283–295.
- Fulekar, M.H.; Yadav, V.K. Method for Separation of Ferrous, Alumina and Silica from Fly Ash. 201721035720, 7 October 2017.
- Papatzani, S.; Paine, K. A Step by Step Methodology for Building Sustainable Cementitious Matrices. Appl. Sci. 2020, 10, 2955.
- Davison, R.L.; Natusch, D.F.; Wallace, J.R.; Evans, C.A., Jr. Trace elements in fly ash. Dependence of concentration on particle size. Environ. Sci. Technol. 1974, 8, 1107–1113.
- Kleinhans, U.; Wieland, C.; Frandsen, F.J.; Spliethoff, H. Ash formation and deposition in coal and biomass fired combustion systems: Progress and challenges in the field of ash particle sticking and rebound behavior. Prog. Energy Combust. Sci. 2018, 68, 65–168.
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 20.
- Fulekar, M.H.; Naik, D.S.; Dave, J.M. Heavy metals in Indian coals and corresponding fly-ash and their relationship with particulate size. Int. J. Environ. Stud. 1983, 21, 179–182.
- Chou, M.-I.M. Fly Ash. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012; pp. 3820–3843.
- Ngu, L.-N.; Wu, H.; Zhang, D.-k. Characterization of Ash Cenospheres in Fly Ash from Australian Power Stations. Energy Fuels 2007, 21, 3437–3445.
- Żyrkowski, M.; Neto, R.C.; Santos, L.; Witkowski, K. Characterization of fly-ash cenospheres from coal-fired power plant unit. Fuel 2016, 174, 49–53.
- Fisher, G.L.; Chang, D.P.Y.; Brummer, M. Fly Ash Collected from Electrostatic Precipitators: Microcrystalline Structures and the Mystery of the Spheres. Science 1976, 192, 553–555.
- Zhao, Y.; Zhang, J.; Sun, J.; Bai, X.; Zheng, C. Mineralogy, Chemical Composition, and Microstructure of Ferrospheres in Fly Ashes from Coal Combustion. Energy Fuels 2006, 20, 1490–1497.
- Ibeto, C.N.; Obiefuna, C.J.; Ugwu, K.E. Environmental effects of concretes produced from partial replacement of cement and sand with coal ash. Int. J. Environ. Sci. Technol. 2020, 17, 2967–2976.
- Meer, I.; Nazir, R. Removal techniques for heavy metals from fly ash. J. Mater. Cycles Waste Manag. 2017, 20, 703–722.
- Zhang, W.; Noble, A.; Yang, X.; Honaker, R. A Comprehensive Review of Rare Earth Elements Recovery from Coal-Related Materials. Minerals 2020, 10, 451.
- Miricioiu, M.G.; Niculescu, V.C. Fly Ash, from Recycling to Potential Raw Material for Mesoporous Silica Synthesis. Nanomaterials (Basel) 2020, 10, 474.
- Kunecki, P.; Panek, R.; Wdowin, M.; Bień, T.; Franus, W. Influence of the fly ash fraction after grinding process on the hydrothermal synthesis efficiency of Na-A, Na-P1, Na-X and sodalite zeolite types. Int. J. Coal Sci. Technol. 2020.
- Kishor, P.; Ghosh, A.; Kumar, D. Use of Flyash in Agriculture: A Way to Improve Soil Fertility and its Productivity. Asian J. Agric. Res. 2010, 4, 1–14.
- Singh, R.P.; Gupta, A.K.; Ibrahim, M.H.; Mittal, A.K. Coal fly ash utilization in agriculture: Its potential benefits and risks. Rev. Environ. Sci. Bio/Technol. 2010, 9, 345–358.
- Behera, A.; Mohapatra, S.S. Challenges in Recovery of Valuable and Hazardous Elements from Bulk Fly Ash and Options for Increasing Fly Ash Utilization. In Coal Fly Ash Beneficiation—Treatment of Acid Mine Drainage with Coal Fly Ash; Akinyemi, S., Gitari, M.W., Eds.; IntechOpen: London, UK, 2018; pp. 19–39.
- Attarde, S.; Marathe, S.; Sil, A. Utilization of fly ash in construction industries for environment management. Int. J. Environ. Sci. 2014, 3, 117–121.
- Luo, Y.; Zheng, S.; Ma, S.; Liu, C.; Wang, X. Ceramic tiles derived from coal fly ash: Preparation and mechanical characterization. Ceram. Int. 2017, 43, 11953–11966.
- FİGen, A.; ÖZÇAy, Ü.; Pişkin, S. Manufacturing and Characterization of Roof Tiles a Mixture of Tile Waste and Coal Fly Ash. Süleyman Demirel Üniversitesi Fen Bilimleri Enstitüsü Derg. 2017, 21, 10.
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267.
- Nordin, N.; Abdullah, M.M.A.B.; Tahir, M.F.M.; Sandu, A.V.; Hussin, K. Utilization of fly ash waste as construction material. Int. J. Conserv. Sci. 2016, 7, 161–166.
- Tyson, S.; Blackstock, T. Coal combustion fly ash—Overview of applications and opportunities in the USA. Fuel Energy Abstr. 1996, 37, 422.
- Martin, J.; Collins, R.; Browning, J.; Bichl, J. Properties and Use of Fly Ashes for Embankments. J. Energy Eng. 1990, 116, 71–86.
- Baykal, G.; Edinçliler, A.; Saygılı, A. Highway embankment construction using fly ash in cold regions. Resour. Conserv. Recycl. 2004, 42, 209–222.
- Dasgupta, M.; Kar, S.; Gupta, S.D.; Mukhopadhyay, R.; Bandyopadhyay, A. Effect of Fly Ash as Filler in Rubber—A Comprehensive Study of the Vulcanisate Properties of Styrene-Butadiene Rubber Compounds. Prog. Rubber Plast. Recycl. Technol. 2013, 29, 151–168.
- Gaikwad, A.; Patel, B.K.; Verma, V.; Rai, A. Development of fly ash based new Bio-Composites Material as Wood Substitute. Int. J. Mech. Prod. Eng. Res. Dev. 2017, 7, 1–6.
- Nadesan, M.S.; Dinakar, P. Mix design and properties of fly ash waste lightweight aggregates in structural lightweight concrete. Case Stud. Constr. Mater. 2017, 7, 336–347.
- Rudić, O.; Ducman, V.; Malešev, M.; Radonjanin, V.; Draganić, S.; Šupić, S.; Radeka, M. Aggregates Obtained by Alkali Activation of Fly Ash: The Effect of Granulation, Pelletization Methods and Curing Regimes. Materials 2019, 12, 776.
- Valeev, D.; Kunilova, I.; Alpatov, A.; Varnavskaya, A.; Ju, D. Magnetite and Carbon Extraction from Coal Fly Ash Using Magnetic Separation and Flotation Methods. Minerals 2019, 9, 320.
- Brännvall, E.; Kumpiene, J. Fly ash in landfill top covers—A review. Environ. Sci. Process. Impacts 2016, 18, 11–21.