Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Horacio Osorio and Version 2 by Catherine Yang.
Allicin, a sulfur compound naturally derived from garlic, has shown beneficial effects on several cardiovascular risk factors through the modulation of cellular mechanisms and signaling pathways. Garlic is especially rich in sulfur-containing compounds; thus, many of these compounds can be responsible for its therapeutic effects. Recent studies have shown that allicin, a garlic-derived sulfur compound, has beneficial effects on different cell types that could be useful for the management of CVD or its risk factors.
- allicin
- cardiovascular disease
- risk factors
- oxidative stress
- hypertension
- dyslipidemia
- endothelial dysfunction
- inflammation
- myocardial infarction
- apoptosis
1. Allicin
1.1. Garlic as a Natural Source of Allicin
Garlic is useful in the treatment of CVD, mainly due to its anti-inflammatory, anti-hypertensive, anti-platelet, and anti-diabetic effects [1][2][3][4][5][21,22,23,24,25]. However, recent studies suggest that the biological activities in garlic can be attributed to allicin, the compound formed in high proportion when a garlic clove is cut, macerated, or crushed. Therefore, it has been hypothesized that allicin is the main compound responsible for the beneficial effects of garlic consumption.
Raw garlic water content is approximately 50%, the rest consists of carbohydrates, lipids, proteins, fiber, vitamins, free amino acids, and minerals, and it is especially rich in sulfur compounds (Table 12) [2][3][4][6][22,23,24,26].
Garlic is odorless until the alliin compound [(+)-S-(2-propenyl)-L-cysteine sulfoxide] and the alliinase enzyme react. These components in intact garlic bulbs are stored in vesicles or vacuoles independently until mechanical stimuli such as cutting or maceration break it, thus, alliin and alliinase are released and the catalysis for allicin formation takes place (Figure 12A) [6][26]. There are other sulfur compounds in raw garlic, but the proportion of these is lower compared to the alliin.
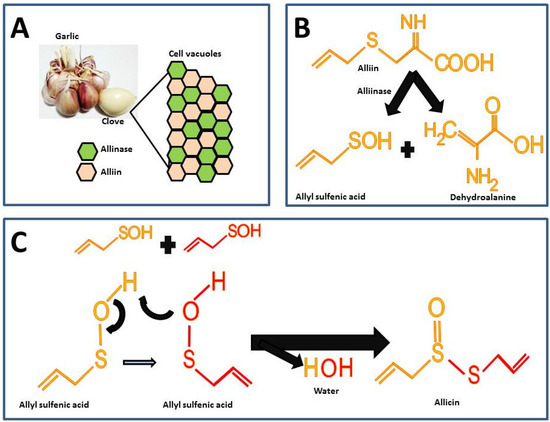
Figure 12. Chemical reactions for allicin synthesis in crushed garlic cloves and substrate formation for chemical synthesis: (A) Compartmentalization of alliin and alliinase in the intact garlic bulb; (B) First reaction in allicin formation; (C) Second reaction for the allicin formation.
The highest percentage of sulfur compounds in garlic is represented by γ-L-glutamyl-S-(2-propenyl)-L-cysteine (GSAC) an alliin precursor, which is transformed into alliin the substrate in the allicin synthesis reaction [6][26]. For the synthesis of allicin, alliin is hydrolyzed by the enzyme alliinase into allyl sulfenic acid and dehydroalanine (Figure 12B) [7][27]. Allicin is formed by the reaction from two allyl sulfenic acid molecules, resulting in allicin and one water molecule as a final product (Figure 12C).
The allicin synthesized represents 60 to 80% of all sulfur compounds (Table 12) and it gives the characteristic odor and hence is considered the active compound in garlic [4][24]. Furthermore, in spite of the fact that alliin and allicin have similar chemical residues, alliin does not show the biological activity of allicin [8][9][28,29]. In fact, a recent study assessed the effect of alliin on experimental obesity resulting in modest beneficial effects [9][29]. The latter supports the hypothesis that allicin is the active compound responsible for the biological activities in garlic.
Table 12. Composition of raw garlic (Allium sativum).
| Substance or Compound | Percent in 100 g Dry Weight | |
|---|---|---|
| Water | 50% | |
| Carbohydrates | 30% | |
| Proteins | 10% | |
| Alliinase | 10 mg/gr | |
| Free amino acid | 1.0% | |
| Lipids | 3.5% | |
| Fiber | 1.0% | |
| Kilocalories | 149 Kcal | |
| Vitamins | ||
| B1 | 0.16 mg | |
| B2 | 0.02 mg | |
| B6 | 0.32 mg | |
| Nicotinic Acid | 0.12 mg | |
| Ascorbic Acid | 14 mg | |
| Minerals | ||
| Potassium | 446 mg | |
| Phosphorous | 134 mg | |
| Sodium | 19 mg | |
| Calcium | 17 mg | |
| Iron | 1.2 mg | |
| Magnesium | 24.1 mg | |
| Zinc | 1.1 mg | |
| Iodine | 4.7 µg | |
| Selenium | 2 µg | |
| Sulfur compounds | 3.5% | |
| γ-glutamyl peptides: 80–85% | ||
| γ-L-glutamyl-S-(2-propenyl)-L-cysteine (GSAC) | 40–60% | |
| γ-L-glutamyl-S-(trans-1-propenil)-L-cysteine (GSPC) | 10–18% | |
| γ-L-glutamyl-S-methyl-L-cysteine (GSMC) | 10–18% | |
| Sulfoxides produced by the allinase action: | ||
| (+)-S-(2-propenyl)-L-cysteine sulfoxide (alliin) | 60–80% | |
| (+)-S-(trans-1-propenil)-L-cysteine sulfoxide (isoalline) | ||
| (+)-S-methyl-L-cysteine sulfoxide (methiine) | ||
| (1S, 3R, 5S) -5-methyl-1, 4-thiazan-3-carboxylic acid 1-oxide (cycloaliine). |
1.2. Synthetic Allicin
Allicin can also be obtained by chemical synthesis by a reverse process from the garlic decomposition cascade of natural synthesis. Briefly, diallyl disulfide dissolved in acetic acid is mixed with hydrogen peroxide to form allyl sulfenic acid, which, as described above, is the substrate for spontaneous condensation producing allicin (Figure 12C) that is further extracted from water with a polar organic solvent as dichloromethane. Through this synthetic procedure, allicin can be obtained with a high purity ranging from 90–92% [13][33]. Due to its hydrophobic nature, allicin can easily diffuse the phospholipid bilayer in cell membranes. Furthermore, it is known that allicin rapidly reacts with the free thiol or sulfhydryl groups of amino acids such as cysteine and the proteins on the cell membrane, as well as with other proteins in the cell compartments [14][15][34,35]. These characteristics could be important for the induction of the biological activities attributed to allicin.
