1. Introduction
Some of the most significant barriers to the widespread use of miRNA replacement/inhibition therapies for the treatment of cancer are consequences of systemic delivery and limitations in tumor-tissue specificity. Thus, because the therapeutic manipulation of miRNA expression is predominantly reliant on nucleic acid-based molecules, delivery vectors are needed to help them overcome not only the systemic barriers, such as rapid renal clearance, but also clinical barriers, such as biosafety
[1][13]. The following sections highlight the current state-of-the-art viral and nonviral delivery systems that have been demonstrated to deliver miRNA-based cancer therapies in vitro and in vivo, with a focus on nonviral nanotechnology-based miRNA delivery systems. The graphical depictions of these varied delivery systems, including their generalized modes of cell entry, as well as their strengths and weaknesses, can be found in
Figure 1 and
Table 1 below.
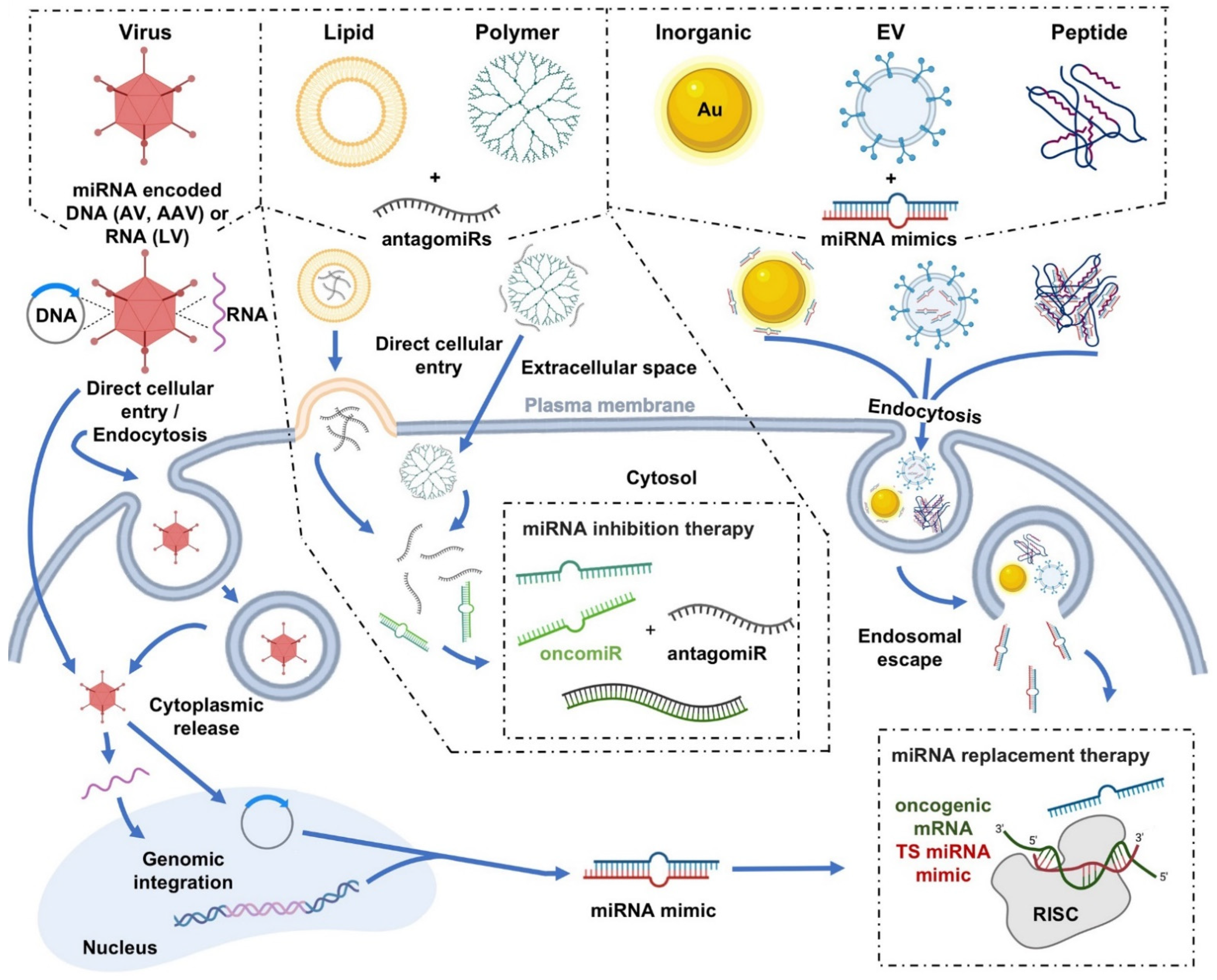
Figure 1. Graphical depictions highlighting the varied miRNA delivery platforms developed for cancer therapeutics, as well as their mechanisms of cellular internalization. Schematic diagram illustrating the generalized modes of cell entry for both viral and nonviral miRNA delivery systems. To cross the plasma membrane of the targeted cell, many of the delivery systems utilize multiple different cellular entry routes, but in general, utilize either direct cellular entry mechanisms or endocytosis-based uptake pathways
[2][3][70,71]. For example, viral vectors, such as adenovirus (AV), adeno-associated virus (AAV), and lentivirus (LV), can utilize either direct entry mechanisms or endocytosis-based uptake pathways in their delivery of miRNA mimic-encoded RNA/DNA cargo into cells. Other examples, in terms of nonviral delivery systems, can include direct cytoplasmic entry via lipid fusion of a lipid-based vector with the plasma membrane or direct cellular entry of a polymer (e.g., polyamidoamine (PAMAM))-based vector in the delivery of antagomiR cargo. Additionally depicted are examples of cellular internalizations via endocytosis of inorganic (e.g. gold (Au)), extracellular vesicle (EV), and peptide-based vectors in complex with miRNA mimic cargo. Select images within the figure were acquired from BioRender.com. OncomiR, oncogenic miRNA; TS miRNA, tumor suppressor miRNA.
Table 1. The highlighted advantages and disadvantages of both viral and nonviral delivery systems in their transport of miRNA-based therapeutics for the treatment of cancer.
2. Viral Delivery
Virus-mediated delivery of miRNAs has been shown to be highly efficacious, where viral vectors can be designed to deliver miRNAs at different stages of biogenesis (i.e., pri-miRNAs and pre-miRNAs). Driven by a viral promotor, pri/pre-miRNA cloned within a plasmid can be transcribed and further processed to the mature miRNA form, enabling it to subsequently act on the target mRNA
[4][57]. Adenoviruses (AdVs), particularly oncolytic AdVs (OAdVs), have been successful in the delivery of antimiRs and miRNA mimics. In fact, OAdV-mediated delivery of antimiRs in the form of long noncoding RNAs (lncRNAs), which has the therapeutic design advantage of targeting multiple copies of the same miRNA or different miRNAs within a single lncRNA molecule, has been shown to inhibit tumor growth in xenograft murine models of triple-negative breast cancer (TNBC) by simultaneously suppressing oncomiR levels of miR-9-5p, miR-10b-5p, miR-21-5p, miR-23a-3p, miR-29a-3p, miR-155-5p, miR-222-3p, miR-301a-3p, and miR-373-3p
[5][72] and to decrease sorafenib resistance in sorafenib-resistant hepatocellular carcinoma (HCC) by concomitantly targeting miR-21, miR-153, miR-216a, miR-217, miR-494, and miR-10a-5p
[6][73]. Virus-mediated overexpression of miRNAs can also have its advantages by suppressing particular oncogenes, as it has been shown that overexpression of miR-143 can inhibit cell growth and induce apoptosis by targeting KRAS in human colorectal cancer, in vitro
[7][74]. Moreover, virus-mediated overexpression of miR-199 and miR-34a has been found to lead to control of tumor growth by targeting mTOR, c-MET, HIF-1
α, and CD44, as well as complete tumor regression by targeting Bcl-2 and SIRT1 in xenograft murine models of HCC
[8][9][75,76], respectively. Similarly to AdV, adeno-associated virus (AAV)-mediated delivery systems have also had success in the treatment of HCC, as overexpression of miR-342-3p and miR-26a has each demonstrated anti-tumor effects in murine models
[10][11][77,78] by targeting MCT1 and cyclins D2 and E2, respectively. Additionally, Bhere and colleagues recently showed that AAV-mediated delivery of anti-miR-21 and miR-7 resulted in decreased cell proliferation, migration, and invasion of human prostate and colon cancer cells, in vitro, and a significant reduction in tumor burden in glioblastoma murine models
[12][79] through targeting of the PI3K/AKT and JAK/STAT3 signaling pathways (via anti-miR-21) and down-regulation of EGFR and p-AKT (via miR-7). Lentivirus-mediated delivery of miR-15a and miR-16 in murine models of chronic lymphoid leukemia
[13][80] and a miRNA sponge targeting miR-494 in murine models of breast cancer
[14][81] have also resulted in beneficial therapeutic effects; however, concerns of lentiviral integration into the host genome have limited their clinical application as a delivery vector.
3. Nonviral Delivery
Despite being highly efficacious, virus-mediated miRNA-based therapeutic delivery platforms lack clinical desirability due to a number of biosafety concerns, including viral immunogenicity. Although classically thought of as inefficient compared to viral vectors, recent advancements in nonviral delivery platforms, however, are paving the way for nucleic acid-based therapies that make their application that much more feasible in the clinic
[1][13]. As such, the following sections highlight and discuss the pros/cons of the varied nonviral delivery technologies that have been developed for miRNA-based cancer therapeutics, including polymer nanoparticles, lipid-based nanoparticles, inorganic nanoparticles, extracellular vesicles, and an emerging technology - peptide carriers.
3.1. Polymer Nanoparticles
Polymeric delivery systems have found success as suitable vectors for delivery of nucleic acids due to their high stability and flexibility, and the facile ability to make substitutions and/or additions of functional groups
[15][82]. In fact, one particular polymer, poly lactic-co-gycolic acid, has gained FDA approval as a delivery vector and is in phase II clinical trials for the delivery of therapeutic small interfering RNA (siRNA) molecules (NCT01676259)
[16][83]. The ability to control the molecular weight, polymer composition, and architecture of polymers allows for the manipulation of size, morphology, charge, pKa, membrane interactions, and biodegradability
[17][84]. Moreover, polymer-based vectors are composed of a variety of materials, including natural polymers, such as collagen, gelatin, and chitosan, synthetic polymers, and combinations of natural and synthetic polymers. The following subsections pertaining to polymer nanoparticles detail polymer-based vectors that have found success in the delivery of specific miRNA-based cancer therapies.
Polyethylenimines
Polyethylenimines (PEI) are second-generation cationic polymers that are frequently utilized for therapeutic gene delivery
[17][84]. PEI is composed of many positively charged amino groups, allowing for complexation with anionic RNA molecules and shielding from degradation, as well as enabling the proton sponge effect, which promotes escape from endosomes after endocytosis
[18][85]. Indeed, PEI has been shown to be an effective delivery vector of miRNA for treatment of various cancers, including miR-33a and miR-145 for the treatment of colon cancer
[19][86] and miR-708-5p mimics for the treatment of metastatic non-small cell lung cancer
[20][87]. Although successful, PEI alone is not as desirable as other delivery vectors, due to the excess positive charge and low degradability due to the binding of serum proteins
[4][57]. As such, PEI in combination/conjugation with other lipids and polymers has been investigated to mitigate these undesirable effects and has found some success. For example, PEI-polyethylene glycol (PEG)-mediated delivery of miR-34a and miR-150 has been shown to be effective for treatment of HCC
[21][88] and to effectively reduce the cell viability of chronic myeloid leukemia cells
[22][89], respectively. Additionally, PEI-hyaluronic acid (HA)-PEG-mediated delivery of a plasmid encoding miR-125b has been demonstrated to inhibit tumor growth and induce apoptosis in a murine lung cancer model
[23][90]. Likewise, polyurethane-PEI-mediated delivery of a plasmid encoding miR-145 has shown success for the treatment of glioblastoma
[24][91], and PEI-antagomiR-126 complexes, loaded into liposomes, have been demonstrated to effectively target leukemic stem cells in vivo for the treatment of acute myeloid leukemia
[25][92].
Polyamidoamine
Polyamidoamine (PAMAM) is a hyperbranched synthetic polymer that is positively charged and biodegradable. While the overall positive charge of the polymer allows for complexation with nucleic acids, it promotes hepatic accumulation and toxicity
[26][93]. Consequently, PAMAM is frequently found in combination/conjugation with other lipids and polymers. For instance, PAMAM-PEG-mediated delivery of a miR-34a-expressing plasmid has been shown to have anti-tumor effects in non-small cell lung cancer
[27][94], and nanographene oxide (NGO)-PEG-PAMAM-mediated delivery of anti-miR-21 has proven effective in reducing migration and invasion of non-small-cell lung cancer A549 cells, in vitro
[28][95].
Chitosan
Chitosan is a biocompatible, natural polysaccharide. It is a deacetylated derivative of chitin, which is found in the exoskeleton of insects, crustaceans, and fungi, making it the second most abundant natural polymer
[29][96]. Chitosan consists of repeating units of β-1,4 linked N-acetyl-D-glucosamine and D-glucosamine
[30][97], and has been described as having a profound binding affinity for miRNAs
[31][10]. Chitosan has been shown to be an effective delivery vector in the treatment of breast cancer, through the complexation of miR-200c
[32][33][98,99], and in the treatment of prostate bone metastasis, through the complexation of miR-34a mimics
[34][100].
Poly Lactic-Co-Gycolic Acid
As previously mentioned, poly lactic-co-gycolic acid (PLGA) is an FDA-approved polymeric delivery vector. These polymers are polyesters and are negatively charged, biodegradable, and biocompatible. PLGA is also hydrophobic, which is thought to impair its miRNA delivery efficacy
[35][101]. As a result, PLGA in combination/conjugation with various lipids and polymers, both synthetic and natural, have been investigated, with some combinations proving effective in mediating miRNA delivery for the treatment of various cancers. In particular, PLGA-chitosan complexes containing miR-34a mimics have been shown to inhibit tumor growth of multiple myeloma xenografts and resulted in the greater survival of treated NOD-SCID tumor-bearing mice
[36][102]. Additionally, PLGA-HA-PEI in complex with a miR-145-encoding plasmid was shown to reduce tumor growth in a murine xenograft model of colon cancer
[37][58], and PLGA-PEG-anti-miR-21, PLGA-PEG-anti-miR-10b/21, and PLGA-PEG-miR-122 complexes were demonstrated to be effective in treating HCC
[38][103], breast cancer
[39][40][104,105], and colon cancer
[41][106], respectively.
3.2. Lipid-Based Nanoparticles
The ease of use and versatility of lipid-based nanoparticles in the form of liposomes have made them the most widely used nanoparticle for the delivery of nucleic acid-based therapies, which includes miRNAs. Liposomes are spherical structures with a hydrophilic core that is separated from the external environment by a phospholipid bilayer. Liposomes can accommodate hydrophobic molecules within the bilayer, hydrophilic molecules within the liposome core, and amphiphilic molecules at the interphase between the bilayer and core
[42][107]. Due to their phospholipid composition, liposomes can interact with cell membranes, which leads to efficient delivery of cargo.
Cationic Liposomes
The first generation of liposomes were cationic in nature, which allowed for electrostatic interactions with nucleic acid-based cargos, as well as with the negatively charged surfaces of cells
[43][44][108,109]. While advantageous for drug loading and delivery, this positive-charge property, however, was found to limit the cell specificity of cationic liposomes, allowed interactions with serum proteins, and increased the susceptibility of uptake by RES
[45][46][110,111]. Despite these challenges within the circulatory system, a significant tumor reduction in a xenograft tumor mouse model of colorectal cancer was still observed through cationic liposome-based nanoparticles loaded with miR-139-5p mimics, albeit with the liposomes also possessing other functionalized moieties
[47][112]. Decreased levels of miR-143 and miR-145, which are associated with colorectal carcinoma, and the delivery of miR-143 and miR-145 mimics using cationic liposomes to restore their levels have also been shown to reduce cell proliferation in a number of colorectal cancer cell lines
[48][113]. In addition to colorectal cancer, cationic liposome-mediated delivery of a miR-7-encoding plasmid and miR-29 mimics have been demonstrated to significantly reduce tumor sizes in xenograft tumor mouse models of lung cancer
[49][50][114,115].
Neutral Liposomes
To reduce the charge-associated shortcomings of cationic liposomes, neutral liposomes were developed by the inclusion of helper lipids, such as 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE)
[51][116], PEG
[52][117], phosphatidylcholines (PCs)
[53][118], and cholesterol
[54][119]. These modifications have led to reduced RES uptake, which allows for increased half-life of the neutral liposomes within the bloodstream
[45][46][110,111]. Taking advantage of the helper lipid phosphocholine, an intermediate of PC, Trang and colleagues found that neutral liposome-mediated delivery of miR-34a and let-7b mimics resulted in the significant reduction in tumor burden in a K-Ras-activated autochthonous mouse model of non-small cell lung cancer (NSCLC)
[55][120].
Ionizable Liposomes
Further optimization of lipid-based nanoparticles has resulted in the generation of ionizable liposomes, which are cationic at low pH and neutral/anionic at neutral or higher pH levels. The ability to change charge states with respect to extracellular pH gives ionizable liposomes enhanced cell selectively characteristics that makes them more clinically translatable. In fact, an ionizable liposome-miRNA complex (comprising miR-34 mimics; MRX34) made it as far as phase I clinical trials for treatment of liver cancer and metastasis (NCT01829971)
[56][57][121,122]. However, this trial was stopped due to severe immune-related adverse events, which resulted in the death of four patients
[57][122]. Despite this unfortunate setback, another group has shown that ionizable liposomes could still hold promise as delivery vehicles for miRNA therapeutics, as they have found, at least at the preclinical level, that ionizable liposome-mediated delivery of miR-200c mimics could result in enhanced radiosensitivity in a xenograft mouse model of lung cancer
[58][123]. Additionally, another study found that delivery of a miR-199b-5p mimic using an ionizable cationic liposome
[59][15] could significantly impair Hes-1 (a downstream effector of the canonical Notch and noncanonical SHH pathways) and cancer stem cell markers in a number of different tumorigenic cell lines, including colon (HT-29, CaCo-2, and SW480), breast (MDA-MB231T and MCF-7), prostate (PC-3), glioblastoma (U-87), and medulloblastoma (Daoy, ONS-76, and UW-228) cancer cell lines
[60][124].
3.3. Inorganic Nanoparticles
Inorganic nanoparticles are desirable as delivery vectors because they can be designed to be biocompatible, nonimmunogenic, and nontoxic, and the size, shape, and porosity of particles can be controlled
[31][61][10,125]. Nevertheless, the use of inorganic materials for delivery of miRNAs still faces challenges, such as protection from degradation in vivo, as well as endosomal escape
[1][13]. The following subsections describe examples of common inorganic vectors utilized for delivery of miRNAs, however, the examples discussed herein are not a complete representation of all developed technologies.
Calcium Phosphate
Calcium phosphate (CaP) nanoparticles, composed of hydroxyapatite [Ca
5(PO
4)
3OH], the inorganic component of bone and teeth, are described as being the most successful inorganic vectors for miRNA therapeutics
[31][10], particularly for the treatment of colon/colorectal cancers. CaP owes this success to its unique in vivo characteristics, including its biocompatibility and biodegradability properties. Moreover, CaP’s susceptibility to acidic conditions allows for endocytic escape, where once these CaP nanoparticles are endocytosed, the acidic environment of the endosome dissolves them, resulting in subsequent increases in ionic strength that lead to osmotic swelling and the release of cargo
[62][126]. As previously mentioned, CaP nanoparticle-mediated delivery of miRNAs has been particularly successful in the treatment of colon/colorectal cancers, as delivery of miR-4711-5p
[63][127], miR-4689
[64][128], and miR-29b
[65][129] mimics were found to effectively inhibit tumor growth in xenograft colon/colorectal cancer mouse models.
Silica
Silica-based nanoparticle technologies are desirable due to their biocompatibility, large surface area, well-defined chemical properties, and ability to control characteristics, such as pore structure
[66][130]. One type of silica-based nanoparticle that has found success with miRNA delivery is the mesoporous silica nanoparticle (MSNP). MSNPs have a/an: (i) tunable particle size, which is important for endocytosis; (ii) stable and rigid framework, making them more resistant to heat, pH, mechanical stress, and hydrolysis-mediated degradation; (iii) uniform and tunable pore size, allowing for controlled drug loading; (iv) high surface area (>900 m
2/g) and large pore volume (>0.9 cm
3/g), which allows for increased drug loading; (v) interior and exterior surface, permitting selective functionalization of either surface; (vi) unique “honeycomb-like” porous structure, which aids in decreased premature drug release or leaking
[67][131]. Taking advantage of these MSNP properties, Bertucci and colleagues successfully induced apoptosis in temozolomide (TMZ)-resistant T98G glioblastoma cells, in vitro, by loading the MSNPs with the anti-cancer drug TMZ and decorating them on the surface with a polyarginine-peptide nucleic acid (R8-PNA) antimiR conjugate designed to target miR-221, a miRNA, whose downregulation was previously reported to sensitize glioma cells to TMZ
[68][69][132,133].
Gold
Gold (Au)-based nanoparticles (AuNPs) are well suited for delivery of nucleic acids, particularly after the addition of various functional groups. AuNPs have multifunctional monolayers, allowing for the addition of multiple functional moieties, which can control cytotoxicity, biodistribution, and excretion
[70][71][72][73][74][75][134,135,136,137,138,139]. AuNPs can also be easily scaled with low size dispersity
[76][140]. Due to these characteristics, AuNPs have found success in delivery of miRNAs for the treatment of various cancers. In particular, miR-375 mimic-coated AuNPs were observed to reduce tumor volume in primary and xenograft tumor mouse models of HCC
[77][141]. Additionally, AuNPs formulated with PEG were found to mediate the highly efficient cell uptake of miRNAs and could decrease cell proliferation upon delivery of a miR-31 mimic into neuroblastoma (NGP and SH-SY5Y) and ovarian (OVCAR8 and HEYA8) cancer cell lines
[78][142]. Moreover, Gilam and colleagues showed that in combination with the chemotherapy drug, cisplatin, AuNPs functionalized with PEG and a tumor-homing peptide, embedded within a hydrogel, could mediate the efficient local, selective, and sustained release of co-complexed miR-96 or miR-182 mimics, leading to the reduction in primary tumor size and metastasis in a breast cancer mouse model
[79][143].
3.4. Extracellular Vesicles
Extracellular vesicles (EVs) are cell-derived nanovesicles that transport DNA, RNA, proteins, and lipids for cellular communication and activation of signaling pathways
[80][144]. While EVs transport mRNA and other RNA species, such as lncRNAs and circular RNAs, miRNAs are perhaps the most abundant cargo molecule in EVs, particularly in exosomes
[81][145]. In fact, these exosome-associated miRNAs have significant roles in the post-transcriptional regulation of gene expression and participate in the mediation of inflammatory reactions, cell migration, proliferation, apoptosis, autophagy, and epithelial-mesenchymal transition
[81][145]. It, therefore, stands to reason that exploiting EVs for therapeutic miRNA delivery has its advantages over other delivery vectors. As a natural biomolecular carrier possessing specific ligands, EVs can be selectively delivered to cell types bearing specific surface receptors
[82][146]. Additionally, EV ligands, such as CD47, can aid in their protection from phagocytes
[83][147]. Moreover, lipid bilayer-encapsulated miRNA cargo is protected from RNase-mediated degradation, as well as from other circulatory system obstacles. This lipid bilayer also allows for direct fusion of the EV with the target cell membrane, with the subsequent release of cargo directly into the cytoplasm of the target cell, thus evading potential endosomal entrapment of the cargo; however, it should be noted that EVs can also undergo receptor-mediated endocytosis
[84][85][86][148,149,150]. Though a relatively new delivery platform for therapeutic nucleic acid-based drugs, EV-mediated delivery of miRNAs has already shown promising therapeutic responses in various cancers. For example, EV-mediated delivery of a chemically modified miR-143 mimic, a plasmid expressing miR-146b, and a miR-145 mimic has each been observed to have therapeutic effects in colon cancer
[87][151], glioma
[88][152], and lung cancer
[89][153], respectively. Other therapeutic uses of EVs have also been reported in the delivery of a plasmid expressing miR-122 in HCC
[90][154] and a chemically synthesized miR-199a-3p mimic in ovarian cancer
[91][155]. Similar to nonviral vectors discussed thus far, exosomes also have the advantage of being modified to contain different functional surface moieties. One interesting example of this type of modification was reported by Ohno and colleagues, where they engineered exosomes to express an epidermal growth factor receptor (EGFR)-specific targeting peptide, GE11, on their surfaces, which were then subsequently used to target a TS miRNA to EGFR-expressing breast cancer cells
[92][156]. In particular, when GE11-positive exosomes containing the TS miRNA, let-7a, were administered to EGFR-expressing breast cancer xenograft tumor-bearing mice, these GE11-positive let-7a-loaded exosomes were observed to not only target the tumors, but also impair their development
[92][156].
3.5. Peptides
The use of peptides as a delivery vector of nucleic acid-based therapeutics was initially described over two decades ago
[93][94][95][157,158,159], but has only recently begun to gain popularity. Peptides are favorable delivery vectors because of the diversity of their physiochemical properties and functions
[96][160]. The controlled ability to modify their amino acid sequences and ease of synthesis allows for the production of peptides that can overcome many of the systemic circulation-associated barriers faced by nucleic acid-based therapies. As such, many different classes of peptide carriers exist, one of which, the cell-penetrating peptides (CPPs), are proving to be highly efficacious and clinically translatable for the treatment of various cancers, as suggested by their presence in phase I and II clinical trials
[97][98][99][100][101][161,162,163,164,165], as either a therapeutic agent alone or as a delivery agent for macromolecular therapeutics
[102][166]. CPPs are typically 4–40 amino acid residues in length
[103][167], can penetrate the plasma membrane of a cell and facilitate the delivery of different cargos
[104][168], and are considered by some to be the most promising nonviral delivery platform for improvement of intracellular trafficking of nucleic acid-based cargos
[105][169], which have included DNA, RNA, siRNA
[106][170], and more recently, even those associated with miRNA-based therapeutics. For instance, one particular peptide carrier, named FA-R9-FP
cas3, comprising a folate receptor-targeting ligand, folic acid (FA), a nona-arginine CPP (R9), and a Caspase-3-sensitive imaging probe (FP
cas3), was used to form a multi-functional peptide-miRNA nanocomplex consisting of the miR-34a mimic that was capable of suppressing tumor growth upon tail vein injection into living mice bearing subcutaneous HeLa tumors
[107][171]. Moreover, because molecular imaging is such a powerful tool for visualization and quantification of pathological processes, such as cancer, Yang and colleagues recently demonstrated that a CPP, PepFect6, could also be used in complex with a radioactively-labeled antisense miRNA oligonucleotide (AMO) designed to target the oncomiR, miR-21, to successfully image miR-21 expression in A549 lung adenocarcinoma xenografts, thus demonstrating a promising method for the noninvasive imaging of miRNA expression levels in vivo
[108][172]. Additionally, although not examples of cancer-related applications, peptides such as LMWP and PepFect6 have also been shown to successfully deliver miRNA mimics, including miR-29b, to stem cells to promote osteoblastic differentiation
[109][173] and miR-146a (a known anti-inflammatory miRNA) to inhibit inflammatory responses in a murine model of irritant contact dermatitis
[110][174], respectively. Lastly, as noted above, it has also been reported that CPPs can be effective carriers of therapeutic small noncoding RNAs, particularly siRNAs, which are similar to miRNAs in function in that they both can induce post-transcriptional gene silencing, but differ in that siRNAs typically inhibit the expression of a single mRNA target, whereas miRNAs normally regulate the expression of multiple mRNA targets
[111][43]. More specifically, researchers have demonstrated that a CPP, named 599, could enhance the intracellular delivery and bioavailability of siRNAs in oral cancer cells in vitro, as well as induce oncogene silencing upon intratumoral administration, resulting in significant inhibition of tumor growth in an orthotopic oral cancer mouse model
[112][113][54,175]. In subsequent work, it was demonstrated that co-complexation of 599 with a cancer cell-targeting peptide could synergistically mediate the effective targeting/delivery of siRNAs to xenograft oral cancer tumors upon systemic administration and significantly enhance silencing of the targeted oncogene
[114][176]. More recently, in an effort to improve upon the efficacy of siRNA uptake and gene silencing mediated by 599, it was further found that a 599 peptide variant, RD3AD, which exhibited enhanced siRNA uptake and gene silencing in comparison to 599, also directed siRNAs to specific cell-surface protrusions, identified as filopodia
[115][177]. Intriguingly, filopodia are highly dynamic, elongated, and thin cellular processes that have been reported to facilitate the highly efficient cell entry of viruses, bacteria, activated receptors, lipo/polyplexes, and exosomes by mediating their retrograde transport and/or “surfing” along the structures toward the cell body
[116][117][118][119][120][178,179,180,181,182], where, at the filopodial base, endocytic hotspots potentially allow for easier cell entry
[117][179]. Of particular relevance regarding exosomes, which are known transporters of miRNAs
[121][183], is that they can utilize filopodia to “surf” toward endocytic hotspots at the filopodial base, internalize, and then traffic within endosomes to the ER
[116][178], which coincidentally is the central nucleation site of siRNA and miRNA-mediated RNA silencing
[122][123][184,185]. Hence, one can envision how the targeting of filopodia and the subsequent directed transport of RD3AD-siRNA/miRNA complexes from the filopodia to the ER would potentially allow for a very efficient trafficking route of exogenous siRNAs/miRNAs into the cellular RNA silencing machinery. In fact, recent preliminary findings have found that complexation of RD3AD with a synthetic fluorescently-labeled let-7b miRNA duplex could similarly localize the miRNA mimic to filopodia and direct its entry into cancer cells (
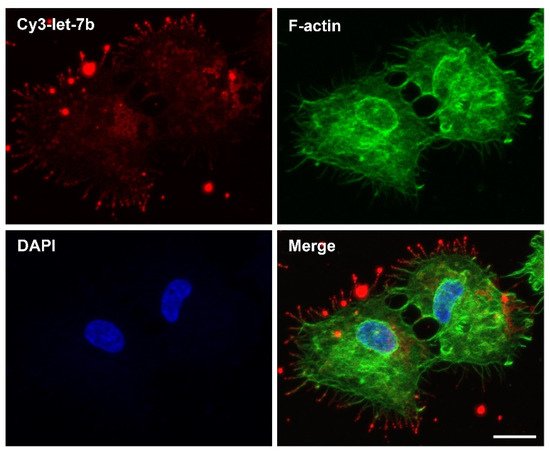
Figure 2). The significance is that one can, thus, potentially exploit filopodia-directed cell-entry machineries and subcellular-trafficking routes via CPPs for the development of more effective miRNA therapeutics.
Figure 2. RD3AD peptide-mediated localization of complexed let-7b miRNAs to filopodia and delivery into cancer cells. Confocal fluorescence microscopy analysis of CAL 27 oral cancer cells 2 hours post-treatment with a synthetic Cy3-labeled let-7b miRNA duplex (Cy3-let-7b; red) in complex with the RD3AD peptide. Filopodia (green) were stained with the F-actin label Alex Fluor 488 phalloidin, and nuclei (blue) were counterstained with DAPI. The merged images are also presented. Scale bar: 15 µm.