Vision is an important sense for humans, and visual impairment/blindness has a huge impact in daily life. The retina is a nervous tissue that is essential for visual processing since it possesses light sensors (photoreceptors) and performs a pre-processing of visual information. Thus, retinal cell dysfunction or degeneration affects visual ability and several general aspects of the day-to-day of a person’s lives. The retina has a blood–retinal barrier, which protects the tissue from a wide range of molecules or microorganisms. However, several agents, coming from systemic pathways, reach the retina and influence its function and survival. Pesticides are still used worldwide for agriculture, contaminating food with substances that could reach the retina. Natural products have also been used for therapeutic purposes and are another group of substances that can get to the retina. Finally, a wide number of medicines administered for different diseases can also affect the retina. The present review aimed to gather recent information about the hazard of these products to the retina, which could be used to encourage the search for more healthy, suitable, or less risky agents.
- pesticides
- medicinal herbs
- natural products
- medicinal
- retina
1. Introduction
2. Natural Products
In an evaluation of retinas from the National Toxicology Program bioassay database, Yamashita and co-workers (2016) [59][19] demonstrated that retinas from a 2-year carcinogenicity study with kava kava extract (KKE) showed a significant increase in degeneration. KKE is derived from the root of the tropical shrub Piper methysticum, and it was originally used for ceremonial beverages in the South Pacific. Both males and females exhibited features of retinal degeneration after 0.3 g/kg or 1.0 g/kg KKE dose [59][19]. In a subsequent study, the group demonstrated that F344N rats dosed with KKE 1.0 g/kg for 90 days did not show signs of retinal degeneration. However, the RPE, only from the superior retina, had a reduced number of phagosomes [60][20]. The authors speculated that this result could indicate an impairment in photoreceptor outer segment phagocytosis by RPE cells, which could affect the health of the retina after the 2-year exposure observed in the previous study. Another compound that has been studied due to its potential anticancer properties [61][21] is hypericin, a polycyclic aromatic naphthodianthrone that occurs naturally. It has been previously shown that hypericin induced cell death of human and bovine isolated RPE cells [62,63][22][23]. A more recent study showed that an acute exposure of isolated bovine retinas to hypericin caused a reduction in the amplitude of the b-wave in an electroretinogram recording, indicating an impairment in retinal function. Embelia ribes possess significant potential in the prevention and treatment of several chronic diseases, including arthritis, bacterial infections, cancer, cardiovascular diseases, diabetes, neurological problems, and wound healing [64][24]. Hagenia abyssinica (Rosaceae) is one of the most-used medicinal plants for the treatment of diarrhea and to treat diabetes mellitus in some regions of Africa [65,66][25][26]. Post-hatched chicks orally received treatment either with a high dose of 0.25 g (5 g/kg per day) or a low dose of 0.025 g (0.5 g/kg per day) of Embelia ribes for 1 or 5 days or Hagenia abyssinica for 1 or 9 days. Both compounds impaired visual function (visual discrimination and stimulus detection in the peripheral visual field). High doses of both agents induced degeneration of the ganglion cell layer [67][27]. Several studies have shown a protective effect of curcumin in different pathological models, such as diabetic retinopathy and ischemia and light-, oxidative stress-, and N-Methyl-D-Aspartate (NMDA)-induced cell death, among others [68,69,70,71,72,73][28][29][30][31][32][33]. However, in one study, curcumin induced apoptosis in mouse-rat hybrid retina ganglion cells, called N18 [74][34]. Similarly, but with fewer studies, garlic or some of its bioactive compounds show a protective effect in several pathological models. Yet, 24-h exposure to diallyl disulfide (DADS) induced a dose-dependent reduction in N18 cell viability [75][35]. DADS induced increase in ROS, intracellular calcium and activation of the classic apoptosis mediator, caspase-3 [75][35]. Concerning natural products, it is important to note the huge number of studies showing protective effects of these substances in animal models of distinct retinal diseases. However, several of these natural herbs/medicines were not tested or fail to show protective effects or, even worse, can be toxic to retinal humans [76,77][36][37]. Therefore, clinical studies seem to be extremely important to confirm/reject data from animal models.3. Drugs and Medicine
Despite the BRB, the retina is susceptible to harmful effects of systemic, intravitreal, or topical drugs leading to dysfunction and retinal degeneration. Retinal drug toxicities can be expressed in many ways: disruption of RPE and photoreceptor complex, vascular damage, ganglion cell or optic nerve, cystoid macular edema, crystalline retinopathy, or ganglion cell or optic nerve injury (Figure 21).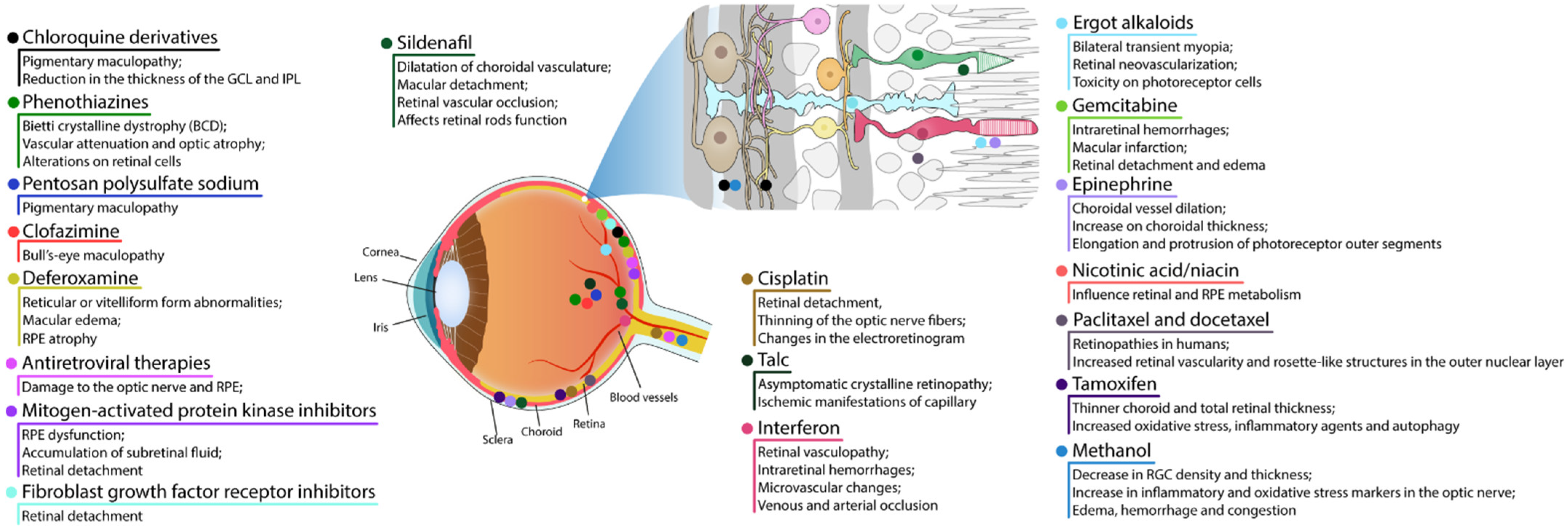
3.1. RPE and Photoreceptor Complex
The most common presentation of alteration in RPE and photoreceptor complex is pigmentary maculopathy (Figure 32).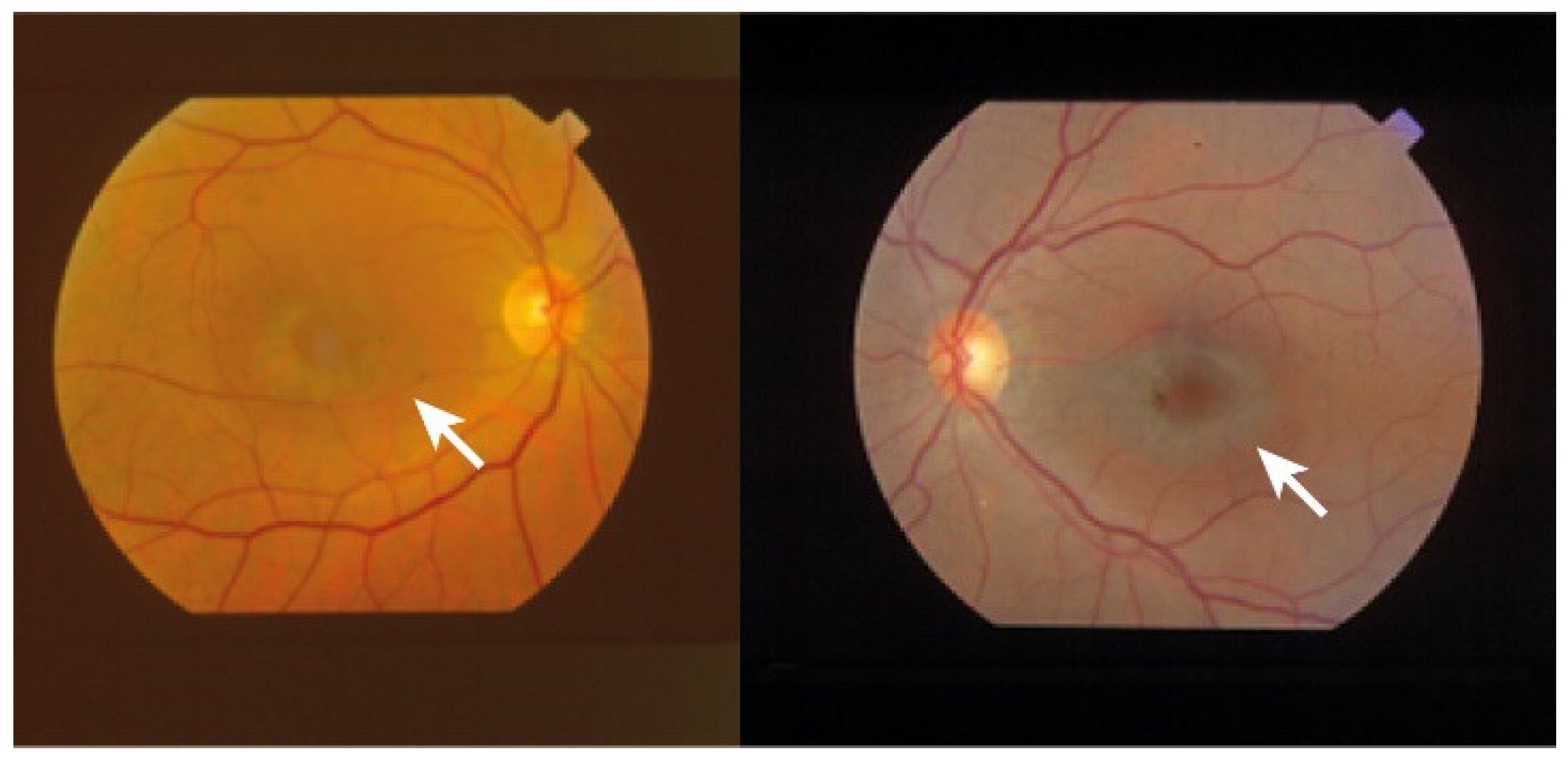
3.1.1. Chloroquine Derivatives
Chloroquine (CQ) and its derivative, hydroxychloroquine sulfate (HCQ), are immunomodulatory drugs that are prescribed for malarial prophylaxis and to treat autoimmune conditions such as rheumatoid arthritis or systemic lupus erythematosus. Both medications bind to melanin in the RPE and uveal tissue and can affect metabolic function. Prolonged use of the CQ derivatives typically results in a pigmentary maculopathy (Figure 43). The incidence of retinopathy in patients treated with CQ is approximately 10 to 20% [78][38].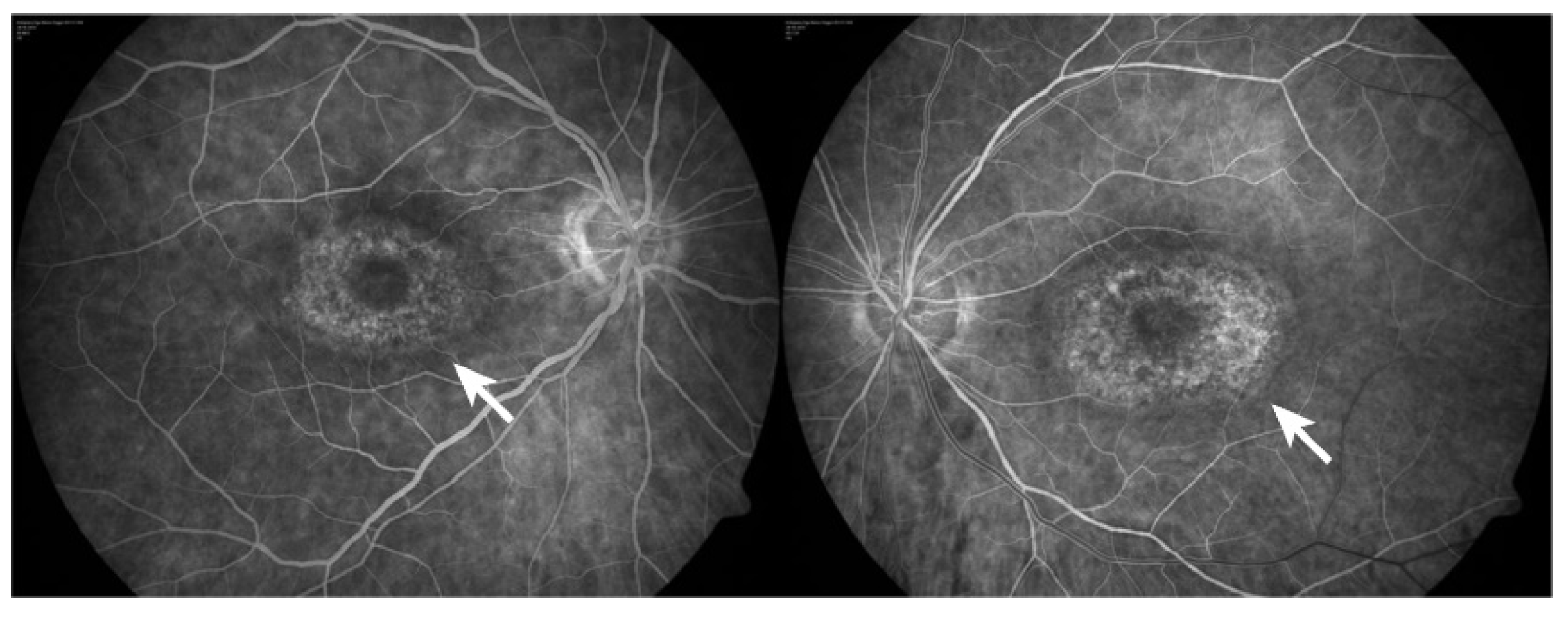
3.1.2. Phenothiazines
Thioridazine and chlorpromazine are used as antipsychotics to treat schizophrenia and other psychiatric disorders. The exact mechanism of retina lesion is not known, nevertheless it may involve enzyme disruption and abnormal rhodopsin synthesis. Chlorpromazine toxicity is rare, being more commonly observed with thioridazine due to its piperidyl side chain. Both chlorpromazine and thioridazine accumulate in the melanin of RPE and uveal tissue. In the early stage, there is RPE stippling in the posterior pole; at a later stage, nummular areas of RPE/choriocapillaris loss are seen from the posterior pole to mid-periphery. The late-stage mimics choroideremia or Bietti crystalline dystrophy (BCD), and vascular attenuation and optic atrophy are seen [84,85][42][43]. The early fundus abnormalities often advance despite discontinuation of the medication. The risk of retinopathy is more dependent on total daily dose rather than cumulative amount received. Thioridazine toxicity at dosages less than 800 mg/day is rare, though cases have been reported with lower doses over several years. Regardless of the dose, patients taking thioridazine should be regularly monitored for signs of toxicity [84,85][42][43]. A recent study using human retinal organoids, on day 150 of differentiation, showed that thioridazine (135 µM, 24 h) induced a wide range of alterations [86][44]. Thioridazine induced the expression of genes related to oxidative stress, inflammation, and cell death. Unsurprisingly, an increase in the percentage of cells labeling to chemokine (C-C motif) ligand 2 (CCL2), triggered and released in inflammatory conditions, and to α-crystallin (CRYAB), involved in the protection of cells from stress by binding misfolded proteins, was observed. Classical signals of glia activation were also demonstrated. Accordingly, a widespread reduction of retinal cells (photoreceptors, horizontal, amacrine, Müller glia, and retinal ganglion cells) accompanied by a decrease of approximately 50% in neuroepithelium thickness was shown [86][44].3.1.3. Pentosan Polysulfate Sodium
Pentosan polysulfate sodium (PPS) is used for the treatment of interstitial cystitis and is associated with a pigmentary maculopathy. Usually, toxicity is more common in women, after chronic use for over 15 years, and in patients exposed to more than 1500 g [87][45]. Common symptoms include blurred vision, difficulty reading, metamorphopsia, paracentral scotomas, and prolonged dark adaptation. Signs comprise parafoveal pigmented deposits at the level of the RPE, vitelliform deposits, and patchy paracentral RPE atrophy similar in appearance to pattern dystrophy [87,88,89][45][46][47]. Since PPS is an inhibitor of heparanase, used chronically, a study investigated the consequence of the absence of this enzyme in knockout mice (KO) [90][48]. Heparanase KO mice at 3-, 9- and 15-weeks-old showed lesions in the retina (central and peripheral), RPE folds, choroidal thickening, cells detached from RPE, increased ONL thickness, and retinal disorganization. The damage in RPE/choroid seemed to progress over time from moderate RPE/choroid changes in 3- and 9-week-old KO mice to severe choroid/RPE protrusions in 58% and 75% for 9- and 15-week-old KO, respectively. There were no signals of proliferation and recruitment of macrophages cells, thus concluding that the RPE protrusions are not related to inflammatory signals from recruited cells.3.1.4. Clofazimine
Clofazimine is a phenazine dye with anti-mycobacterial and anti-inflammatory action. It binds preferentially to mycobacterial DNA by inhibiting DNA replication and cell growth and is used to treat dapsone-resistant leprosy and autoimmune disorders such as psoriasis and lupus. Ocular side effects include bull’s-eye maculopathy. Drug discontinuation can halt progression but retinopathy does not regress [91][49].3.1.5. Deferoxamine
Deferoxamine (DFO) is used as a chelating agent to treat iron toxicity/overload. Signs of toxicity are reticular or vitelliform form abnormalities and/or macular edema due to RPE pump failure. Retinopathy includes several pattern dystrophy-like changes or minimal changes affecting the RPE–Bruch membrane–photoreceptor complex. Multimodal imaging confirms histology findings that photoreceptor outer-derived retinoids, fluorophores, and RPE displacement or clumping are entangled in DFO retinopathy, leading to unequivocal RPE atrophy in many cases of pattern dystrophy–like changes. Drug cessation can reverse established mild retinopathy. Nevertheless, when exposure is prolonged, RPE and outer retina damage may persist [92][50]. Nonetheless, iron chelant, including deferoxamine, has been shown to protect retinal cells in different degenerative models, such as NMDA-induced excitotoxicity [93][51], cell death promoted by oxidative stress in ARPE 19 cells [94[52][53],95], or 611 photoreceptor lineage cells [96][54] by blocking ferroptosis.3.1.6. Antiretroviral Therapies
Even though antiretroviral drugs can help arrest human immunodeficiency virus (HIV) progress or other infection-associated retinal disease, an undesirable rare retinal toxicity could occur [97][55]. Didanosine (DDI) is a nucleotide reverse transcriptase inhibitor used to treat individuals with acquired immunodeficiency syndrome (AIDS), probably because it inhibits polymerase (pol-γ), the enzyme responsible for replication and repair of mitochondrial DNA. DDI can cause mitochondrial dysfunction and toxicity resulting in damage to the optic nerve and RPE; peripheral field loss occurs with concentric loss/mottling of RPE (areas of chorioretinal atrophy), beyond arcades to mid-periphery, bilaterally symmetrical [98][56] (Figure 54).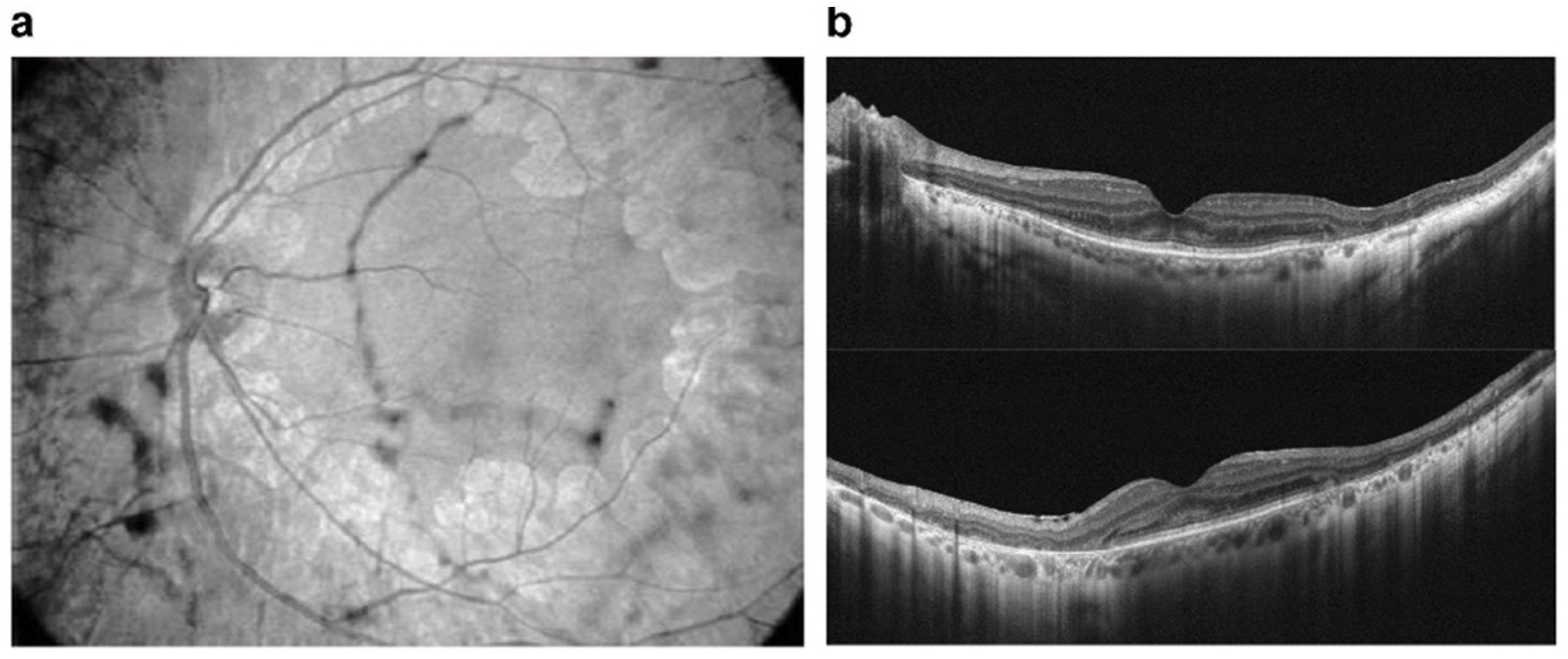
3.1.7. Mitogen-Activated Protein Kinase Inhibitors
Mitogen-activated protein kinase (MEK) inhibitors (trametinib (Mekinist), cobimetinib (Cotellic), binimetinib (Mektovi), and selumetinib (Koselugo)) are used to treat metastatic melanoma. The toxicity mechanism is thought to be due to RPE-induced dysfunction with subsequent accumulation of subretinal fluid. The most common structural abnormality found is bilateral multifocal serous retinal detachment with at least one focus involving the fovea. Onset can occur shortly after initiation of therapy. Visual symptoms are typically minimal with the fluid often spontaneously resolving. For persistent cases, discontinuation of the drug usually leads to complete resolution [99][57]. Human neuroretina shows phosphorylated ERK, which is inhibited by binimetinib treatment [100][58]. Phosphorylated ERK status is recovered after ceasing MEK inhibitor treatment both in ARPE 19 cells and primary neuroretina cells from human tumor eyes [100][58]. These results indicate that the disappearance of retinopathy with the discontinuation of binimetinib treatment is associated with the reactivation of ERK.3.1.8. Fibroblast Growth Factor Receptor Inhibitors
Fibroblast growth factor receptor (FGFR) inhibitors are chemotherapeutic agents used to treat cholangiocarcinoma and urothelial carcinoma [102][59]. Currently, FDA-approved drugs include erdafitinib, pemigatinib, and infigratinib [103][60]. FGFR inhibitors appear to cause serous retinal detachments, similar to MEK retinopathy. Few reports are available in the literature of FGFR retinopathy [104][61].3.1.9. Sildenafil
Sildenafil is used to treat erectile dysfunction and pulmonary artery hypertension. It blocks phosphodiesterase 5, an enzyme that promotes breakdown of cyclic guanosine monophosphate (cGMP). A possible side-effect is dilatation of choroidal vasculature and secondary serous macular detachment, and retinal vascular occlusion can occur [105][62]. Mice treated with sildenafil showed a reversible increase in maximal retina vessel dilatation and choroid effusion promptly after intravitreal injection and 30 min after intraperitoneal injection [106][63]. In 5% of mice, sildenafil provoked RGC loss and damage of optic nerve after 21 days of the treatment. In an in vivo approach to evaluate the effect of sildenafil in the mouse retina, QUEnch-assiSTed (QUEST) magnetic resonance imaging (MRI) was used in subretinal space using QUEST optical coherence tomography (OCT), while QUEST optokinetic tracking (OKT) was used for cone-based vision [107][64]. QuestMRI showed an increase in oxidative stress in a group treated with sildenafil when compared to a group exposed to sildenafil plus antioxidants. This effect was only detected in the peripheral superior retina [107][64]. Levels of ROS evaluated by DCF staining in freshly isolated retinal sections were higher all over the retina treated with sildenafil compared to saline, with more prominent labeling in the superior retina. ONL thickness was constant regardless of treatment with sildenafil or antioxidants. At 5 h post-sildenafil treatment, contrast sensitivity was significantly lower-than-normal and similar even in the presence of antioxidants.3.1.10. Cisplatin
Cisplatin (cisdiamminedichloroplatinum—CIS) has been used effectively for years as a chemotherapy drug in the treatment of solid tumors, metastases, and small cell cancers with unknown primary tumors [109][65]. Despite its success in inducing tumor death and remission of associated symptoms, several studies report side effects related to the visual system in patients treated with CIS. In these cases, partial loss of vision, retinal detachment, thinning of the optic nerve fibers, changes in the electroretinogram, occlusion of the middle retinal, and cilioretinal arteries have already been demonstrated [110,111,112,113][66][67][68][69]. At the cellular level, the cytotoxicity of CIS was confirmed in ocular tissue cells of different species. In rat retinas, a single intraperitoneal injection with CIS (16 mg/kg) increased the levels of MDA, as well as decreased the levels of reduced glutathione (GSH). Immunohistochemical assays revealed an increase in labeling for 8-hydroxy-2p-deoxyguanosine (marker of DNA damage) in horizontal cells and for endothelial nitric oxide synthase (eNO) in retinal blood vessel cells [114][70]. Blood samples from rats administered for 14 days with CIS (2.5 mg/kg) also revealed an increase in MDA, myeloperoxidase (MPO), and the levels of the total oxidant system, but also in the pro-inflammatory cytokines, including the tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β). At the same time, there was a reduction in GSH, SOD activity, and levels of the total antioxidant system [115,116,117][71][72][73]. After treatment with CIS, the retinal tissue showed degeneration, edema, and vascular congestion with disorganization of the retinal layers. Optic nerve tissue anomalies have also been observed, such as destruction, hemorrhage, edema, and an increase in the number of astrocytes and polymorphonuclear leukocytes [114,115,116,117][70][71][72][73].3.2. Retinal Vascular Damage
Many drugs can damage the retinal vasculature, by inducing a hypercoagulable state, or by particle clogging of blood vessels. Some might be used as intraoperative ocular medications as aminoglycosides, moxifloxacin, or vancomycin.3.2.1. Talc
Talc retinopathy is characterized by the presence of small, yellow crystals located in small retinal vessels and within different retinal layers. Ocular damage usually develops after chronic intravenous drug abuse and manifestations range from asymptomatic crystalline retinopathy to severe ischemic manifestations of capillary non perfusion. The presence of crystals is thought to be secondary to emboli derived from talc, which is an insoluble inert particulate filler material used in some oral (methylphenidate hydrochloride, methadone, pentazocine, and amphetamine), inhaled (crack cocaine), and intravenous (cocaine and heroin) preparations.3.2.2. Interferon
Interferons are classified into three major types—INF-α, INF-β, and IFN-γ—and have been used for treatment of different pathologies, including Kaposi sarcoma, hepatitis B and C, multiple sclerosis (MS), and malignant osteopetrosis. Systemic therapy has been associated with retinal vasculopathy characterized by cotton wool spots, intraretinal hemorrhages, microvascular changes including capillary drop-out, CME (typically in the posterior pole and peripapillary region), venous occlusion, or arterial occlusion, consistent with ischemic retinopathy [120][74]. The exact mechanism of toxicity is not known but may involve impairment of retinal microcirculation. Changes typically present 4–8 weeks after initiation of therapy and usually regress after treatment cessation [120][74]. Shed light on these molecules as central regulators and not just inflammatory markers. Among these three types of interferon, only IFN-γ was related to possible retinal injurious effects. Roche and collaborators (2018) reported that IFN-γ released by microglia induces pSTAT3 signaling in Müller cells and increases glial fibrillary acidic protein (GFAP) expression [121][75]. These results were explored in the context of the retinitis pigmentosa (rd10) mouse model, which increased GFAP staining throughout time. IFN- γ was also linked to the increase of BRAF-activated non-coding RNA (BANCR), a long non-coding RNA involved in the inflammatory context of RPE dysfunction associate to diseases like AMD, acting through signal transducer and activator of transcription 1 (STAT1) phosphorylation in ARPE-19 cells [122][76]. This increase was progressive, reaching up to 30 times higher concentration of the RNA when treated with 100 units/mL of IFN-γ. In addition, in ARPE-19 cells and in this context of inflammation and AMD progression, IFN-γ (50 ng/mL–48 h) induced cell death [123][77]. Controversially, in a context of diabetic retinopathy, diabetic mice lacking IFN-γ showed a more than two-time increase in the mRNA expression of vascular endothelial growth factor (VEGF), intercellular adhesion molecule 1 (ICAM-1), retinoic-acid-receptor-related orphan nuclear receptor gamma (ROR-γt), a transcription factor of immune cells T helper (Th) 17 cells, and a more than ten-times increase in splenic IL-17-producing CD4+ cells in comparison to diabetic mice [125][78]. Meanwhile, IFN-γ shows an anti-angiogenic effect in a mouse model of oxygen induced retinopathy (OIR) [126][79]. These data show a role for IFN-γ as a critical regulator of inflammation and a possibility to explore it as a tool given its role as an inflammatory cytokine regulated in eye disease.3.2.3. Ergot Alkaloids
Ergot alkaloids are mycotoxins produced by many fungal species of the Claviceps genus. There are four main types of ergot alkaloids: clavines, lysergic acids, lysergic acid amides, and ergopeptides. One of these ergopeptides is dihydroergotamine, which has been extensively used in the treatment of migraine. The antimigraine effect is mainly related to its agonist activity at 5-hydroxytryptamine receptor 1B (5-HT1B), 5-hydroxytryptamine receptor 1D (5-HT1D), and 5-hydroxytryptamine receptor 1F (5-HT1F) receptors [127][80]. One case report described that one patient that received for the first time the oral medication Cefalium, a medication used to treat migraine, which contains dihydroergotamine in the formula, presented some ocular anomalies such as acute bilateral transient myopia, retinal folds, and island of choroidal delay after one day of treatment. The interruption of the treatment was able to solve all clinical symptoms [128][81]. Dopaminergic agonists derived from ergot are a group of drugs consisting of bromocriptine, cabergoline, dihydroergocryptine, lisuride, and pergolide. They have been available on the market for many years and are mainly used to treat Parkinson’s disease, either alone or in combination with other medicines. A recent report described that knockout mice of rod transducin G protein subunit alpha transducin 1 (Gnat1), visual arrestin 1 (ARR1), or rhodopsin kinase 1 (GRK1) showed light damage and robust retinal inflammation after bright light exposure [129][82]. The pretreatment with metoprolol plus tamsulosin and bromocriptine protected the retina in all genetic knockout mice [129][82]. Abnormalities of angiogenesis are very common in age-related macular degeneration and proliferative diabetic retinopathy. In a study with a zebrafish animal model, retinal neovascularization induced by cobalt chloride promoted hypoxia. Pre-incubation with bromocriptine, cabergoline, pergolide, and all ergot-derived D2 dopamine receptor agonists significantly inhibited abnormalities of angiogenesis, decreasing mRNA expression levels of vascular endothelial growth factor Aa (VEGFAA) [130][83]. Lysergic acid diethylamide (LSD) is a potent synthetic psychedelic drug that can be derived from the ergot alkaloids. Visual changes are some of the effects after LSD use. One study investigated the effect of LSD on macrophage activation state and its toxicity to photoreceptor cells in vitro. They showed that the treatment of macrophage cultures with LSD induced a change to a pro-inflammatory profile [131][84]. LSD treatment of co-cultured macrophages with photoreceptors induced an increase in the oxidative stress markers and toxicity on photoreceptor cells [131][84]. Another study demonstrated that C57BL/6 mice treated with LSD had a decrease in electroretinography response and the loss of photoreceptor cells. This cell death of photoreceptors was mediated by upregulation of p-JAK1/p-STAT1 pathway [132][85].3.2.4. Gemcitabine
Gemcitabine, a pyrimidine nucleoside analog, is a chemotherapy drug used as a treatment for different types of cancer, including bladder and breast cancer. Some studies have reported retinopathy associated with the use of this chemotherapeutic agent. One study demonstrated that one patient had several issues related to use of gemcitabine, such as a decrease in vision with appearance of cotton wool spots and intraretinal hemorrhages [133][86]. Another case report described that one patient had macular infarction after chemotherapy with gemcitabine and carboplatin [134][87]. More recently gemcitabine-associated retinal pathologies, such as presence of bilateral peripheral exudative retinal detachment, retinal edema, and Elschnig’s spots, were also described [135][88].3.3. Cystoid Macular Edema
Cystoid macular edema (CME) may occur after the treatment with fingolimod Gilenya for MS, topical prostaglandin analogs (e.g., latanoprost) for ocular hypertension or glaucoma, nicotinic acid (niacin) for lipid disorders, and/or paclitaxel treatment or DFO for iron toxicity/overload. Drug cessation results in resolution of the CME, although topical and local steroids or topical non-steroidal anti-inflammatory drugs have been used to facilitate resolution.3.3.1. Epinephrine
Epinephrine, an endogenous molecule that can be used to treat cardiac arrest and anaphylaxis in a hospital environment, may also influence retinal physiology. Systemic adrenergic stimulation with isoproterenol, a β1-and β2-adrenergic receptors agonist, impact RPE renin expression, with implications on retinal pathophysiology [136][89]. The influence of stimulating the adrenergic system in the retina is not only related to changes in the renin–angiotensin system, but the stimulation can also affect RPE ion transport. Treatment with epinephrine induced a small, but rapid increase in what the authors called “short-circuit current” (current required to reduce the potential across the epithelial membrane to zero) [137][90]. Angiographic changes such as choroidal vessel dilation, increase on choroidal thickness, disruption and effacement of the ellipsoid zone, and elongation and protrusion of photoreceptor outer segments have been reported after epinephrine treatment for 8 weeks in cynomolgus monkey [138][91].3.3.2. Nicotinic Acid/Niacin
Nicotinic acid, also known as vitamin B, and its derivatives such as nicotinamide, can influence retinal and RPE metabolism. For instance, there is an established method for culturing ARPE-19 cells that uses nicotinamide to stimulate cell growth and differentiation [139][92].3.3.3. Paclitaxel and Docetaxel
Paclitaxel and docetaxel are antineoplastic agents of the taxane class of drugs used in the therapy of many solid tumors, including breast and lung cancer. They act by promoting and stabilizing microtubule assembly, while preventing physiological microtubule depolymerization/disassembly in the absence of GTP. This leads to a significant inhibition of cellular mitosis and cell death [140][93]. There are several case studies reported in the literature linking retinopathies, such as phototoxic maculopathy and cystoid macular edema, induced by paclitaxel and docetaxel [141,142,143,144][94][95][96][97], but there are few reports about studies with these compounds in animal models or in vitro models. In C57BL/6J littermate pups, it was seen that paclitaxel treatment was able to reduce the number of retinal vascular branches in a dose-dependent manner during mouse retinal development in vivo [145][98]. Another study in a rat model showed that a single intraperitoneal injection of paclitaxel led to an increased retinal vascularity and rosette-like structures in the outer nuclear layer, a lesser number of astrocytes and oligodendrocytes, and some signs of cellular necrosis [146][99].3.4. Crystalline Retinopathy
Tamoxifen
Tamoxifen is a selective estrogen receptor modulator and has been used to treat breast cancer. Retinopathy induced by tamoxifen is characterized by crystalline deposits and pseudocystic foveal cavitations. These findings are like macular telangiectasia type 2, suggesting a similar pathogenesis involving Müller cell dysfunction. Tamoxifen is associated with thinner choroid and total retinal thickness, suggesting that there were structural changes in patients without symptoms that could be early signs of RPE and photoreceptor damage. Toxicity is dependent on dose and length of use and typically manifests after 2-3 years. Visual function and macular edema typically improve after drug cessation, though the crystalline deposits remain [147,148][100][101] (Figure 65).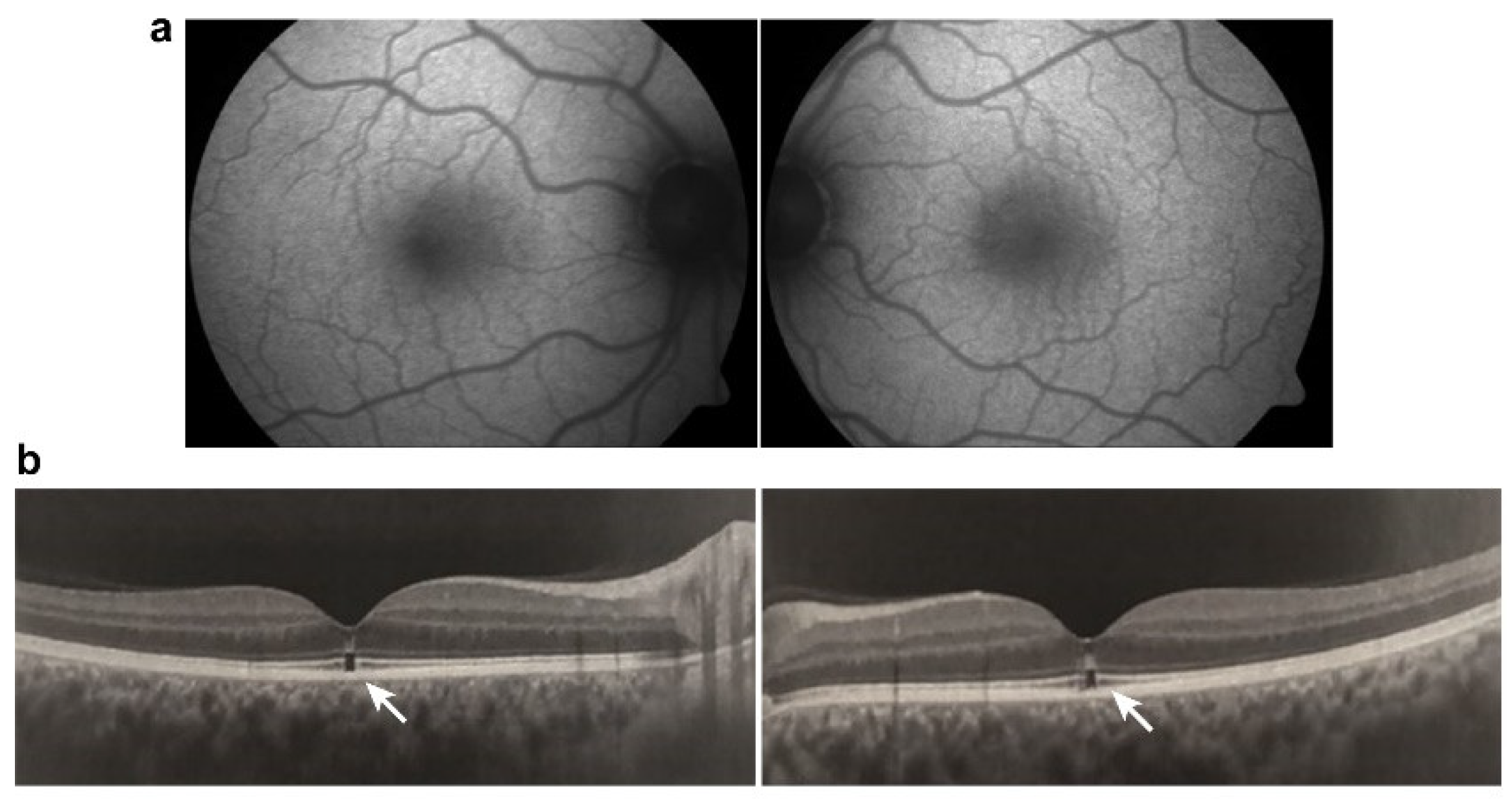
3.5. Damage to Ganglion Cell Layer or Optic Nerve
Methanol
Methanol intoxication is a very debilitating condition, most commonly found in developing countries where ingestion of contaminated alcohol in beverages could lead to a variety of systemic symptoms that range from mild intoxication, such as abdominal pain, nausea, vomiting, headache, general weakness, dyspnea and nervous system disturbances, including the retina, to more severe intoxication leading to renal failure, cardiovascular alterations, rhabdomyolysis, convulsions, coma and eventually death [151][103]. Visual symptoms might appear hours after ingestion, and they vary widely from progressive decrease in vision to dyschromatopsia, scotoma, and photophobia, accompanied by hyperemic and edematous optic disk acutely, while atrophy and pallor can be observed chronically [152][104]. The molecule itself is not the most alarming toxic agent, but rather, its metabolite, formic acid, represents a serious threat, being able to bind and inhibit cytochrome c oxidase, an enzyme of the mitochondrial respiratory chain, and therefore, inhibiting oxidative phosphorylation, causing ATP deficiency, and increasing oxidative stress, damaging a series of cellular components [151][103]. A decrease in RGC density and thickness alongside increased caspase-3 was also observed in a rat orally administrated methanol-induced toxicity model, an effect reversed by citicoline (1 g/kg/day) [155][105]. Very recently, Dorgau and colleagues (2022) aimed to establish a retinal organoid model to study drug toxicity and contribute to the development of treatment strategies [86][44]. The retinal organoids assemble from human pluripotent stem cells (hPSCs) and are arranged in a layered structure, expressing biomarkers for key cell types. Methanol treatment (32 mM–24 h) reduced the staining for middle/long wavelength cone opsin to almost zero, and although it did not change expressing markers for ganglion cells, it did reduce the number of active RGCs by half in response to white light pulses. Moreover, in a rat methanol-induced toxicity study (3 g/kg—oral, for 7 days), Rutin (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside) or taxifolin (3,3′,4′,5,7-pentahydroxiflavanone), flavonoids found in vegetables and fruits, given after methanol ingestion, could prevent methanol induced increase in inflammatory markers (IL-1β, NF-κB and TNF-α) and oxidative stress markers (8-OHdG, MDA, MPO) in the optic nerve, alongside restoration of histologic pattern and avoidance of edema, hemorrhage, and congestion, suggesting an attenuation of optic neuropathy [156,157][106][107].References
- Wässle, H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 2004, 5, 747–757.
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84.
- De Campos, V.S.; Calaza, K.C.; Adesse, D. Implications of TORCH Diseases in Retinal Development-Special Focus on Congenital Toxoplasmosis. Front. Cell Infect. Microbiol. 2020, 10, 585727.
- Thoreson, W.B.; Dacey, D.M. Diverse Cell Types, Circuits, and Mechanisms for Color Vision in the Vertebrate Retina. Physiol. Rev. 2019, 99, 1527–1573.
- Fain, G.; Sampath, A.P. Rod and cone interactions in the retina. F1000Research 2018, 7, 657.
- Coughlin, B.A.; Feenstra, D.J.; Mohr, S. Müller cells and diabetic retinopathy. Vis. Res. 2017, 139, 93–100.
- Newman, E.A. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos. Trans. R. Soc. B 2015, 370, 1672.
- Reichenbach, A.; Bringmann, A. Glia of the human retina. Glia 2020, 68, 768–796.
- Carpi-Santos, R.; de Melo Reis, R.A.; Gomes, F.C.A.; Calaza, K.C. Contribution of Müller Cells in the Diabetic Retinopathy Development: Focus on Oxidative Stress and Inflammation. Antioxidants 2022, 11, 617.
- Subirada, P.V.; Paz, M.C.; Ridano, M.E.; Lorenc, V.E.; Vaglienti, M.V.; Barcelona, P.F.; Luna, J.D.; Sánchez, M.C. A journey into the retina: Müller glia commanding survival and death. Eur. J. Neurosci. 2018, 47, 1429–1443.
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40.
- Santos, A.M.; Calvente, R.; Tassi, M.; Carrasco, M.C.; Martín-Oliva, D.; Marín-Teva, J.L.; Navascués, J.; Cuadros, M.A. Embryonic and postnatal development of microglial cells in the mouse retina. J. Comp. Neurol. 2008, 506, 224–239.
- Li, F.; Jiang, D.; Samuel, M.A. Microglia in the developing retina. Neural. Dev. 2019, 14, 12.
- Kinuthia, U.M.; Wolf, A.; Langmann, T. Microglia and Inflammatory Responses in Diabetic Retinopathy. Front. Immunol. 2020, 11, 564077.
- Yu, C.; Roubeix, C.; Sennlaub, F.; Saban, D.R. Microglia versus Monocytes: Distinct Roles in Degenerative Diseases of the Retina. Trends Neurosci. 2020, 43, 433–449.
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881.
- Ye, X.; Wang, Y.; Nathans, J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol. Med. 2010, 16, 417–425.
- George, S.M.; Lu, F.; Rao, M.; Leach, L.L.; Gross, J.M. The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems. Prog. Retin. Eye Res. 2021, 85, 100969.
- Yamashita, H.; Hoenerhoff, M.J.; Peddada, S.D.; Sills, R.C.; Pandiri, A.R. Chemical Exacerbation of Light-induced Retinal Degeneration in F344/N Rats in National Toxicology Program Rodent Bioassays. Toxicol. Pathol. 2016, 44, 892–903.
- Yamashita, H.; Hoenerhoff, M.J.; Shockley, K.R.; Peddada, S.D.; Gerrish, K.E.; Sutton, D.; Cummings, C.A.; Wang, Y.; Julie, F.F.; Behl, M.; et al. Reduced Disc Shedding and Phagocytosis of Photoreceptor Outer Segment Contributes to Kava Kava Extract-induced Retinal Degeneration in F344/N Rats. Toxicol. Pathol. 2018, 46, 564–573.
- Dong, Q.; Hu, N.; Yue, H.; Wang, H. Inhibitory Activity and Mechanism Investigation of Hypericin as a Novel α-Glucosidase Inhibitor. Molecules 2021, 26, 4566.
- Harris, M.S.; Sakamoto, T.; Kimura, H.; He, S.; Spee, C.; Gopalakrishna, R.; Gundimeda, U.; Yoo, J.S.; Hinton, D.R.; Ryan, S.J. Hypericin inhibits cell growth and induces apoptosis in retinal pigment epithelial cells: Possible involvement of protein kinase C. Curr. Eye Res. 1996, 15, 255–262.
- Wielgus, A.R.; Chignell, C.F.; Miller, D.S.; Van Houten, B.; Meyer, J.; Hu, D.N.; Roberts, J.E. Phototoxicity in human retinal pigment epithelial cells promoted by hypericin, a component of St. John’s wort. Photochem. Photobiol. 2007, 83, 706–713.
- Devi Daimary, U.; Girisa, S.; Parama, D.; Verma, E.; Kumar, A.; Kunnumakkara, A.B. Embelin: A novel XIAP inhibitor for the prevention and treatment of chronic diseases. J. Biochem. Mol. Toxicol. 2022, 36, e22950.
- Kifle, Z.D.; Belayneh, Y.M. Antidiabetic and Anti-hyperlipidemic Effects of the Crude Hydromethanol Extract of Hagenia abyssinica (Rosaceae) Leaves in Streptozotocin-Induced Diabetic Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 4085–4094.
- Kifle, Z.D.; Atnafie, S.A.; Yimer Tadesse, T.; Belachew, T.F.; Kidanu, B.B. Methanolic Crude Extract of Hagenia abyssinica Possesses Significant Antidiarrheal Effect: Evidence for In Vivo Antidiarrheal Activity. Evid. Based Complement. Alternat. Med. 2021, 2021, 9944629.
- Low, G.; Rogers, L.J.; Brumley, S.P.; Ehrlich, D. Visual deficits and retinotoxicity caused by the naturally occurring anthelmintics, Embelia ribes and Hagenia abyssinica. Toxicol. Appl. Pharmacol. 1985, 81, 220–230.
- Mandal, M.N.; Patlolla, J.M.; Zheng, L.; Agbaga, M.P.; Tran, J.T.; Wicker, L.; Kasus-Jacobi, A.; Elliott, M.H.; Rao, C.V.; Anderson, R.E. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic. Biol. Med. 2009, 46, 672–679.
- Vasireddy, V.; Chavali, V.R.; Joseph, V.T.; Kadam, R.; Lin, J.H.; Jamison, J.A.; Kompella, U.B.; Reddy, G.B.; Ayyagari, R. Rescue of photoreceptor degeneration by curcumin in transgenic rats with P23H rhodopsin mutation. PLoS ONE 2011, 6, e21193.
- Wang, L.; Li, C.; Guo, H.; Kern, T.S.; Huang, K.; Zheng, L. Curcumin inhibits neuronal and vascular degeneration in retina after ischemia and reperfusion injury. PLoS ONE 2011, 6, e23194.
- Xie, T.; Chen, X.; Chen, W.; Huang, S.; Peng, X.; Tian, L.; Wu, X.; Huang, Y. Curcumin is a Potential Adjuvant to Alleviates Diabetic Retinal Injury via Reducing Oxidative Stress and Maintaining Nrf2 Pathway Homeostasis. Front. Pharmacol. 2021, 12, 796565.
- López-Malo, D.; Villarón-Casares, C.A.; Alarcón-Jiménez, J.; Miranda, M.; Díaz-Llopis, M.; Romero, F.J.; Villar, V.M. Curcumin as a Therapeutic Option in Retinal Diseases. Antioxidants 2020, 9, 48.
- Chandrasekaran, P.R.; Madanagopalan, V.G. Role of Curcumin in Retinal Diseases-A review. Graefes. Arch. Clin. Exp. Ophthalmol. 2022, 260, 1457–1473.
- Lu, H.F.; Lai, K.C.; Hsu, S.C.; Lin, H.J.; Yang, M.D.; Chen, Y.L.; Fan, M.J.; Yang, J.S.; Cheng, P.Y.; Kuo, C.L.; et al. Curcumin induces apoptosis through FAS and FADD, in caspase-3-dependent and -independent pathways in the N18 mouse-rat hybrid retina ganglion cells. Oncol. Rep. 2009, 22, 97–104.
- Lin, H.L.; Yang, J.S.; Yang, J.H.; Fan, S.S.; Chang, W.C.; Li, Y.C.; Chung, J.G. The role of Ca2+ on the DADS-induced apoptosis in mouse-rat hybrid retina ganglion cells (N18). Neurochem. Res. 2006, 31, 383–393.
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177.
- Zhang, H.W.; Zhang, H.; Grant, S.J.; Wan, X.; Li, G. Single herbal medicine for diabetic retinopathy. Cochrane Database Syst. Rev. 2018, 12, Cd007939.
- Yusuf, I.H.; Sharma, S.; Luqmani, R.; Downes, S.M. Hydroxychloroquine retinopathy. Eye 2017, 31, 828–845.
- Godinho, G.; Madeira, C.; Falcão, M.; Penas, S.; Dinah-Bragança, T.; Brandão, E.; Carneiro, Â.; Santos-Silva, R.; Falcão-Reis, F.; Beato, J. Longitudinal Retinal Changes Induced by Hydroxychloroquine in Eyes without Retinal Toxicity. Ophthalmic. Res. 2021, 64, 290–296.
- Yang, J.; Guo, Z.; Liu, X.; Liu, Q.; Wu, M.; Yao, X.; Liu, Y.; Cui, C.; Li, H.; Song, C.; et al. Cytotoxicity Evaluation of Chloroquine and Hydroxychloroquine in Multiple Cell Lines and Tissues by Dynamic Imaging System and Physiologically Based Pharmacokinetic Model. Front. Pharmacol. 2020, 11, 574720.
- Nguyen Hoang, A.T.; Lee, H.; Lee, S.J. Casein kinase I inhibitor D4476 influences autophagy and apoptosis in chloroquine-induced adult retinal pigment epithelial-19 cells. Exp. Eye Res. 2022, 218, 109004.
- Corradetti, G.; Violanti, S.; Au, A.; Sarraf, D. Wide field retinal imaging and the detection of drug associated retinal toxicity. Int. J. Retin. Vitr. 2019, 5, 26.
- Richa, S.; Yazbek, J.C. Ocular adverse effects of common psychotropic agents: A review. CNS Drugs 2010, 24, 501–526.
- Dorgau, B.; Georgiou, M.; Chaudhary, A.; Moya-Molina, M.; Collin, J.; Queen, R.; Hilgen, G.; Davey, T.; Hewitt, P.; Schmitt, M.; et al. Human Retinal Organoids Provide a Suitable Tool for Toxicological Investigations: A Comprehensive Validation Using Drugs and Compounds Affecting the Retina. Stem. Cells Transl. Med. 2022, 11, 159–177.
- Wang, D.; Au, A.; Gunnemann, F.; Hilely, A.; Scharf, J.; Tran, K.; Sun, M.; Kim, J.H.; Sarraf, D. Pentosan-associated maculopathy: Prevalence, screening guidelines, and spectrum of findings based on prospective multimodal analysis. Can. J. Ophthalmol. 2020, 55, 116–125.
- Hanif, A.M.; Armenti, S.T.; Taylor, S.C.; Shah, R.A.; Igelman, A.D.; Jayasundera, K.T.; Pennesi, M.E.; Khurana, R.N.; Foote, J.E.; O’Keefe, G.A.; et al. Phenotypic Spectrum of Pentosan Polysulfate Sodium-Associated Maculopathy: A Multicenter Study. JAMA Ophthalmol. 2019, 137, 1275–1282.
- Leung, E.H.; Sharma, S.; Levie-Sprick, A.; Lee, G.D.; Cho, H.; Mukkamala, K. Pentosan Polysulfate Sodium-Associated Pigmentary Retinopathy: Risk Factors and Fundus Findings. Clin. Ophthalmol. 2021, 15, 4809–4816.
- Van Bergen, T.; Etienne, I.; Jia, J.; Li, J.P.; Vlodavsky, I.; Stitt, A.; Vermassen, E.; Feyen, J.H.M. Heparanase Deficiency Is Associated with Disruption, Detachment, and Folding of the Retinal Pigment Epithelium. Curr. Eye Res. 2021, 46, 1166–1170.
- Khan, M.J.; Papakostas, T.; Kovacs, K.; Gupta, M.P. Drug-induced maculopathy. Curr. Opin. Ophthalmol. 2020, 31, 563–571.
- Viola, F.; Barteselli, G.; Dell’Arti, L.; Vezzola, D.; Mapelli, C.; Villani, E.; Ratiglia, R. Multimodal imaging in deferoxamine retinopathy. Retina 2014, 34, 1428–1438.
- Sakamoto, K.; Suzuki, T.; Takahashi, K.; Koguchi, T.; Hirayama, T.; Mori, A.; Nakahara, T.; Nagasawa, H.; Ishii, K. Iron-chelating agents attenuate NMDA-Induced neuronal injury via reduction of oxidative stress in the rat retina. Exp. Eye Res. 2018, 171, 30–36.
- Totsuka, K.; Ueta, T.; Uchida, T.; Roggia, M.F.; Nakagawa, S.; Vavvas, D.G.; Honjo, M.; Aihara, M. Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells. Exp. Eye Res. 2019, 181, 316–324.
- Liu, B.; Wang, W.; Shah, A.; Yu, M.; Liu, Y.; He, L.; Dang, J.; Yang, L.; Yan, M.; Ying, Y.; et al. Sodium iodate induces ferroptosis in human retinal pigment epithelium ARPE-19 cells. Cell Death Dis. 2021, 12, 230.
- Chen, C.; Chen, J.; Wang, Y.; Liu, Z.; Wu, Y. Ferroptosis drives photoreceptor degeneration in mice with defects in all-trans-retinal clearance. J. Biol. Chem. 2021, 296, 100187.
- Abalem, M.F.; Carricondo, P.C.; Rao, R.C. Bullseye Retinopathy from Antiretroviral Therapy. Ophthalmology 2017, 124, 1539.
- Hu, X.; Calton, M.A.; Tang, S.; Vollrath, D. Depletion of Mitochondrial DNA in Differentiated Retinal Pigment Epithelial Cells. Sci. Rep. 2019, 9, 15355.
- Urner-Bloch, U.; Urner, M.; Jaberg-Bentele, N.; Frauchiger, A.L.; Dummer, R.; Goldinger, S.M. MEK inhibitor-associated retinopathy (MEKAR) in metastatic melanoma: Long-term ophthalmic effects. Eur. J. Cancer 2016, 65, 130–138.
- Van Dijk, E.H.C.; Duits, D.E.M.; Versluis, M.; Luyten, G.P.M.; Bergen, A.A.B.; Kapiteijn, E.W.; de Lange, M.J.; Boon, C.J.F.; van der Velden, P.A. Loss of MAPK Pathway Activation in Post-Mitotic Retinal Cells as Mechanism in MEK Inhibition-Related Retinopathy in Cancer Patients. Medicine 2016, 95, e3457.
- Pellino, A.; Loupakis, F.; Cadamuro, M.; Dadduzio, V.; Fassan, M.; Guido, M.; Cillo, U.; Indraccolo, S.; Fabris, L. Precision medicine in cholangiocarcinoma. Transl. Gastroenterol. Hepatol. 2018, 3, 40.
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684.
- Alekseev, O.; Ojuok, E.; Cousins, S. Multifocal serous retinopathy with pemigatinib therapy for metastatic colon adenocarcinoma. Int. J. Retin. Vitr. 2021, 7, 34.
- Etminan, M.; Sodhi, M.; Mikelberg, F.S.; Maberley, D. Risk of Ocular Adverse Events Associated with Use of Phosphodiesterase 5 Inhibitors in Men in the US. JAMA Ophthalmol. 2022, 140, 480–484.
- Zahavi, A.; Weiss, S.; Vieyra, M.; Nicholson, J.D.; Muhsinoglu, O.; Barinfeld, O.; Zadok, D.; Goldenberg-Cohen, N. Ocular Effects of Sildenafil in Naïve Mice and a Mouse Model of Optic Nerve Crush. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1987–1995.
- Berkowitz, B.A.; Podolsky, R.H.; Lins Childers, K.; Saadane, A.; Kern, T.S.; Roberts, R.; Olds, H.; Joy, J.; Richards, C.; Rosales, T.; et al. Sildenafil-evoked photoreceptor oxidative stress in vivo is unrelated to impaired visual performance in mice. PLoS ONE 2021, 16, e0245161.
- Haugnes, H.S.; Bosl, G.J.; Boer, H.; Gietema, J.A.; Brydøy, M.; Oldenburg, J.; Dahl, A.A.; Bremnes, R.M.; Fosså, S.D. Long-term and late effects of germ cell testicular cancer treatment and implications for follow-up. J. Clin. Oncol. 2012, 30, 3752–3763.
- Alkan, A.; Talaz, S. Cilioretinal artery occlusion associated with cisplatin. J. Oncol. Pharm. Pract. 2019, 25, 969–971.
- Dulz, S.; Asselborn, N.H.; Dieckmann, K.P.; Matthies, C.; Wagner, W.; Weidmann, J.; Seidel, C.; Oing, C.; Berger, L.A.; Alsdorf, W.; et al. Retinal toxicity after cisplatin-based chemotherapy in patients with germ cell cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1319–1325.
- Langevin, S.; Chang, J.S.; Chang, S. Serous retinopathy associated with cisplatin treatment. Retin. Case. Brief Rep. 2019, 13, 211–214.
- Khadka, S.; Byanju, R.; Poon, S. Chemotherapy-Induced Central Retinal Artery Occlusion in Gestational Trophoblastic Neoplasia: Case Report. Int. Med. Case. Rep. J. 2020, 13, 431–435.
- Fındık, H.; Tumkaya, L.; Yılmaz, A.; Gökhan Aslan, M.; Okutucu, M.; Akyildiz, K.; Mercantepe, T. The protective effects of astaxanthin against cisplatin-induced retinal toxicity. Cutan. Ocul. Toxicol. 2019, 38, 59–65.
- Taşlı, N.G.; Uçak, T.; Karakurt, Y.; Keskin Çimen, F.; Özbek Bilgin, A.; Kurt, N.; Süleyman, H. The effects of rutin on cisplatin induced oxidative retinal and optic nerve injury: An experimental study. Cutan. Ocul. Toxicol. 2018, 37, 252–257.
- Karakurt, Y.; Uçak, T.; Tasli, N.; Ahiskali, I.; Şipal, S.; Kurt, N.; Süleyman, H. The effects of lutein on cisplatin-induced retinal injury: An experimental study. Cutan. Ocul. Toxicol. 2018, 37, 374–379.
- Sunar, M.; Yazici, G.N.; Mammadov, R.; Kurt, N.; Arslan, Y.K.; Süleyman, H. Coenzyme Q10 effect on cisplatin-induced oxidative retinal injury in rats. Cutan. Ocul. Toxicol. 2021, 40, 312–318.
- Wu, C.M.; Su, F.H.; Muo, C.H.; Huang, J.C.; Wu, M.M.; Yeh, C.C. Analysis of Different Types of Interferon-Associated Retinopathy in Patients with Chronic Hepatitis C Virus Infection Treated with Pegylated Interferon Plus Ribavirin. Viruses 2021, 13, 475.
- Roche, S.L.; Ruiz-Lopez, A.M.; Moloney, J.N.; Byrne, A.M.; Cotter, T.G. Microglial-induced Müller cell gliosis is attenuated by progesterone in a mouse model of retinitis pigmentosa. Glia 2018, 66, 295–310.
- Kutty, R.K.; Samuel, W.; Duncan, T.; Postnikova, O.; Jaworski, C.; Nagineni, C.N.; Redmond, T.M. Proinflammatory cytokine interferon-γ increases the expression of BANCR, a long non-coding RNA, in retinal pigment epithelial cells. Cytokine 2018, 104, 147–150.
- Wei, T.T.; Zhang, M.Y.; Zheng, X.H.; Xie, T.H.; Wang, W.; Zou, J.; Li, Y.; Li, H.Y.; Cai, J.; Wang, X.; et al. Interferon-γ induces retinal pigment epithelial cell Ferroptosis by a JAK1-2/STAT1/SLC7A11 signaling pathway in Age-related Macular Degeneration. FEBS J. 2022, 289, 1968–1983.
- Taguchi, M.; Someya, H.; Inada, M.; Nishio, Y.; Takayama, K.; Harimoto, K.; Karasawa, Y.; Ito, M.; Takeuchi, M. Retinal changes in mice spontaneously developing diabetes by Th17-cell deviation. Exp. Eye Res. 2020, 198, 108155.
- Jung, I.; Jung, D.; Zha, Z.; Jeong, J.; Noh, S.; Shin, J.; Park, J.K.; Kim, K.S.; Jeong, Y.; Hur, J.; et al. Interferon-γ inhibits retinal neovascularization in a mouse model of ischemic retinopathy. Cytokine 2021, 143, 155542.
- Silberstein, S.D.; McCrory, D.C. Ergotamine and dihydroergotamine: History, pharmacology, and efficacy. Headache 2003, 43, 144–166.
- Arana, L.A.; Bach, M.B.; Vedana, G.; Volkmann, M.A.; Arana, J. Cefalium-induced bilateral transient myopia, retinal folds, and focal choroidal delay. Retin. Case. Brief Rep. 2018, 12, 118–121.
- Leinonen, H.; Choi, E.H.; Gardella, A.; Kefalov, V.J.; Palczewski, K. A Mixture of U.S. Food and Drug Administration-Approved Monoaminergic Drugs Protects the Retina from Light Damage in Diverse Models of Night Blindness. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1442–1453.
- Kasica, N.; Święch, A.; Saładziak, K.; Mackiewicz, J.; Osęka, M. The Inhibitory Effect of Selected D(2) Dopaminergic Receptor Agonists on VEGF-Dependent Neovascularization in Zebrafish Larvae: Potential New Therapy in Ophthalmic Diseases. Cells 2022, 11, 1202.
- Hu, Q.D.; Xu, L.L.; Gong, Y.; Wu, G.H.; Wang, Y.W.; Wu, S.J.; Zhang, Z.; Mao, W.; Zhou, Y.S.; Li, Q.B.; et al. Lysergic acid diethylamide causes photoreceptor cell damage through inducing inflammatory response and oxidative stress. Cutan. Ocul. Toxicol. 2018, 37, 233–239.
- Chen, K.; He, X.; Li, C.; Ou, Y.; Li, Y.; Lai, J.; Lv, M.; Li, X.; Ran, P.; Li, Y. Lysergic acid diethylamide causes mouse retinal damage by up-regulating p-JAK1/p-STAT1. Cutan. Ocul. Toxicol. 2020, 39, 106–110.
- Jhaj, G.; Jhaj, R.; Shrier, E.M. Gemcitabine-Induced Retinopathy. Retina 2017, 37, e130–e131.
- Behera, U.C.; Modi, R.R.; Sheth, J.; Singh, A. Bilateral macular infarction after gemcitabine and carboplatin chemotherapy. Int. Ophthalmol. 2018, 38, 2195–2198.
- Loscalzo, F.; Balbarrey, M.; Grigera, J.D. Gemcitabine-Associated Retinopathy with Bilateral Exudative Retinal Detachment and Elschnig’s Spots. Ophthalmic. Surg. Lasers Imaging Retin. 2022, 53, 222–226.
- Martins, J.R.; Reichhart, N.; Kociok, N.; Stindl, J.; Foeckler, R.; Lachmann, P.; Todorov, V.; Castrop, H.; Strauß, O. Systemic ß adrenergic stimulation/sympathetic nerve system stimulation influences intraocular RAS through cAMP in the RPE. Exp. Eye Res. 2019, 189, 107828.
- Skarphedinsdottir, S.B.; Eysteinsson, T.; Árnason, S.S. Mechanisms of Ion Transport Across the Mouse Retinal Pigment Epithelium Measured In Vitro. Investig. Ophthalmol. Vis. Sci. 2020, 61, 31.
- Cheong, K.X.; Barathi, V.A.; Teo, K.Y.C.; Chakravarthy, U.; Tun, S.B.B.; Busoy, J.M.; Ho, C.E.H.; Agrawal, R.; Takahashi, K.; Cheung, C.M.G. Choroidal and Retinal Changes After Systemic Adrenaline and Photodynamic Therapy in Non-Human Primates. Investig. Ophthalmol. Vis. Sci. 2021, 62, 25.
- Hazim, R.A.; Volland, S.; Yen, A.; Burgess, B.L.; Williams, D.S. Rapid differentiation of the human RPE cell line, ARPE-19, induced by nicotinamide. Exp. Eye Res. 2019, 179, 18–24.
- Kaya, M.; Atas, F.; Gulsum Guc, Z.; Oztop, I.; Durak, I.; Saatci, A.O. A cross-sectional optical coherence tomography study in patients on taxane-based therapy and a case report with the literature review. Cutan. Ocul. Toxicol. 2020, 39, 287–293.
- Nghiem-Buffet, S.; Cohen, S.Y.; Giocanti-Auregan, A. Docetaxel Retinopathy: A Case Report. Case. Rep. Ophthalmol. 2017, 8, 21–25.
- Torrado, L.A.; Fivgas, G.D. Unilateral cystoid macular edema and bilateral subfoveal hyperreflectivity following docetaxel chemotherapy: A case report. Am. J. Ophthalmol. Case. Rep. 2020, 20, 100995.
- Tapia Quijada, H.E.; Quijada Fumero, E.; Mesa Lugo, F.I.; Serrano García, M.; Betancor Caro, N. Nepafenac for cystoid macular oedema secondary to paclitaxel. Arch. Soc. Esp. Oftalmol. 2021, 96, 434–437.
- Malcolm, J.; Lune Wong, C.O.; Ching, J.; Saidkasimova, S. Paclitaxel may be a risk factor for retinal phototoxicity. Am. J. Ophthalmol. Case. Rep. 2022, 25, 101292.
- Lin, P.K.; Salvador, J.; Xie, J.; Aguera, K.N.; Koller, G.M.; Kemp, S.S.; Griffin, C.T.; Davis, G.E. Selective and Marked Blockade of Endothelial Sprouting Behavior Using Paclitaxel and Related Pharmacologic Agents. Am. J. Pathol. 2021, 191, 2245–2264.
- Cinici, E.; Dilekmen, N.; Kutlu, Z.; Dincer, B.; Cinici, O.; Balta, H.; Calık, I. Carvone protects against paclitaxel-induced retinal and optic nerve cytotoxicity: A histopathological study. Cutan. Ocul. Toxicol. 2019, 38, 290–293.
- Kim, H.A.; Lee, S.; Eah, K.S.; Yoon, Y.H. Prevalence and Risk Factors of Tamoxifen Retinopathy. Ophthalmology 2020, 127, 555–557.
- Doshi, R.R.; Fortun, J.A.; Kim, B.T.; Dubovy, S.R.; Rosenfeld, P.J. Pseudocystic foveal cavitation in tamoxifen retinopathy. Am. J. Ophthalmol. 2014, 157, 1291–1298.
- Wang, X.; Zhao, L.; Zhang, Y.; Ma, W.; Gonzalez, S.R.; Fan, J.; Kretschmer, F.; Badea, T.C.; Qian, H.H.; Wong, W.T. Tamoxifen Provides Structural and Functional Rescue in Murine Models of Photoreceptor Degeneration. J. Neurosci. 2017, 37, 3294–3310.
- Liberski, S.; Kaluzny, B.J.; Kocięcki, J. Methanol-induced optic neuropathy: A still-present problem. Arch. Toxicol. 2022, 96, 431–451.
- Klein, K.A.; Warren, A.K.; Baumal, C.R.; Hedges, T.R., 3rd. Optical coherence tomography findings in methanol toxicity. Int. J. Retin. Vitr. 2017, 3, 36.
- Laksmita, Y.A.; Sidik, M.; Siregar, N.C.; Nusanti, S. Neuroprotective Effects of Citicoline on Methanol-Intoxicated Retina Model in Rats. J. Ocul. Pharmacol. Ther. 2021, 37, 534–541.
- Taşlı, N.G.; Çimen, F.K.; Karakurt, Y.; Uçak, T.; Mammadov, R.; Süleyman, B.; Kurt, N.; Süleyman, H. Protective effects of Rutin against methanol induced acute toxic optic neuropathy: An experimental study. Int. J. Ophthalmol. 2018, 11, 780–785.
- Ahiskali, I.; Pinar, C.L.; Kiki, M.; Cankaya, M.; Kunak, C.S.; Altuner, D. Effect of taxifolin on methanol-induced oxidative and inflammatory optic nerve damage in rats. Cutan. Ocul. Toxicol. 2019, 38, 384–389.
