Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Dalibor Mijaljica.
A broad range of topical antifungal formulations containing miconazole or terbinafine as actives are commonly used as efficacious choices for combating fungal skin infections. Their many benefits, owing to their specific mechanism of action, include their ability to target the site of infection, enhance treatment efficacy and reduce the risk of systemic side effects. Their proven efficacy, and positioning in the treatment of fungal skin infections, is enhanced by high patient compliance, especially when appropriate vehicles such as creams, ointments and gels are used.
- allylamines
- azoles
- ergosterol
- formulation
- fungi
1. Introduction: The Human Skin and Fungal Infections
Human skin is a dynamic and multifunctional organ that provides a physical barrier against the hostile external environment through its highly organised and intricate landscape [1,2,3][1][2][3]. The skin’s landscape is fundamentally composed of three well-integrated features that encompass the following: (1) a specialised, heterogeneous, interlocking, three-layered interface consisting of the outermost epidermis, middle dermis and innermost hypodermis [1,3,4,5][1][3][4][5]; (2) indispensable adnexal structures including sweat glands, sebaceous glands and hair follicles [1,3,4,5][1][3][4][5]; and (3) mechanical elasticity and stability [1,3,5][1][3][5]. Essentially, the skin protects our body from attack by harmful chemicals and pathogens, defends against ultraviolet (UV) radiation and mechanical insults, prevents dehydration and overhydration, controls thermoregulation and allows us to perceive the world around us through a highly innervated surface [3,6][3][6]. Although the skin functions defensively to keep harmful microbes out, it simultaneously provides a protective niche for its highly abundant, native resident microbiome [3]—a complex and diverse set of microorganisms, consisting primarily of bacteria [7,8,9][7][8][9] and fungi (mycobiota) [10[10][11],11], but also harbours parasites and viruses as well [12]. These skin inhabitants [13] can be commensal, symbiotic, opportunistic, or even pathogenic [3[3][7][8][9][10][11],7,8,9,10,11], and can interact with the skin in numerous ways. Some live predominantly in symbiosis and provide the skin with a variety of benefits (e.g., maintaining skin homeostasis), while others can be deleterious (e.g., lead to opportunistic infection, disease) [8[8][14],14], depending on a multitude of intrinsic (e.g., age, genetics, immunity, hydration, sebum levels, metabolism) [9,12][9][12] and extrinsic (e.g., hygiene and beauty routine, exposure to chemicals and sunlight, climate) factors [9,11,12,15][9][11][12][15].
The diverse environments found on the human skin can have a significant effect on the number or type of microorganisms that are found. For example, moist or humid body sites (e.g., the axilla, groin, toe web) usually create favourable conditions for fungal and microbial growth, and are therefore, often colonised by numerous microorganisms, while dry body sites (e.g., the forearms, legs) are not so microbiologically diverse. Similarly, areas of the skin with a considerable number of sebaceous glands (e.g., the head and neck) provide an optimal environment for many lipophilic microorganisms (e.g., the genus Malassezia) [12]. In addition, the skin’s microbiome can also be affected by exogenous factors: exposure of the skin to UV radiation from the sun, for instance, can disturb the genetic and structural variability of the skin’s microbiome, resulting in susceptibility to microbial infections or exacerbating already existing symptoms [12].
The numerous fungi that colonise the surface of the skin, including those generally innocuous (e.g., genera Malassezia) and those that are pathogenic (e.g., dermatophytes, genera Candida), are characterised by phylogenetic divergence [10,11,16,17,18][10][11][16][17][18]. Despite their differences, they all have a structurally complex hallmark feature known as the cell wall, which is primarily made of a variety of polysaccharides (e.g., glucans, chitin) and (glyco)proteins (e.g., NO-linked oligosaccharides) [19]. Their cell membrane is structurally quite unique as well, since it contains ergosterol, a major membrane lipid that is absent in animals and plants [20]. The fungal cell wall synthesis and maintenance involve a considerable number of biosynthetic and signaling pathways [19] and it safeguards the contents of the fungal cell from different stresses, gives it rigidity and integrity, and defines its structure and shape [19].
The ‘give-and-take’ interaction between the mycobiota, host skin cells, and the immune system is responsible for maintaining skin health, and a disruption of this delicate balance by altering skin barrier or invasive attack by harmful pathogens can lead to impaired and compromised skin function and subsequently result in fungal infection of the skin, known as mycosis [3]. Overall, there are two types of skin mycoses: (1) the common superficial type, and (2) the less common deep, invasive and systemic type [21,22][21][22]. The majority of superficial fungi that reside on the skin, hair and nails degrade keratin—a structural protein responsible for maintaining the skin’s structural stability and integrity [23]—and utilise it as a nutrient source for their growth [24,25,26][24][25][26]. When a fungal cell invades the skin surface—specifically the cornified top layer of the epidermis rich in keratin—, it produces keratinase enzyme, which degrades the tissue and ultimately causes skin inflammation that is often accompanied by pruritus (itch) [24,25,26][24][25][26]. These types of fungi are known as keratinophilic dermatophytes, which include several fungi under the genera Epidermophyton, Microsporum and Trichophyton, and can cause several contagious superficial fungal skin infections (dermatomycoses) of various body regions. These include Tinea corporis (ringworm, a localised or solitary ring-like rash on the body), Tinea pedis (athlete’s foot, a fungal infection between toes), Tinea unguium (fungal infection of the toenails and fingernails—onychomycosis), Tinea cruris (jock itch, a fungal infection of the groin area) and Tinea capitis (a fungal infection of the head or scalp) [24,25,26,27,28][24][25][26][27][28].
Genera Candida can cause severe fungal infections such as candidiases. Approximately, twenty species of Candida are responsible for human skin infections, and the most common species is the yeast-like fungus, Candida albicans, which can cause both superficial and systemic types of fungal infections [25,27,29][25][27][29]. The overgrowth of Candida albicans can cause severe mucosal and dermal thrush, nappy rash, and genital infection [25,30][25][30]. Hyperkeratosis (thickening of the epidermal outermost layer, the stratum corneum, often associated with a keratin abnormality) and epidermal hypertrophy (increased thickness of the keratinocyte layers) persistent with inflammation are diagnostic symptoms for fungal infection caused by the candida species [25].
Since fungi are classified as eukaryotes and, as such, have a complex cellular structure and organisation; many biochemical and cellular processes take place in a similar way as in human cells [31]. Therefore, it is challenging to develop antifungal/antimycotic drug formulations with high selectivity and efficacy, but low side effects in human cells [22]. However, the two key differences between fungal and human cells are the existence of both a cell wall [19] and ergosterol in fungi [20]. With this in mind, most, if not all, antifungals, including those used in topical vehicle formulations (e.g., creams, ointments, gels) [32[32][33],33], are aimed at degrading or disrupting the cell wall or cell membrane (and its individual components) of the fungus to inhibit its key biosynthetic pathways and mechanism of infection, and eventually cause fungal cell death [25]. There are five main classes of antifungals: (1) azoles (e.g., miconazole); (2) allylamines (e.g., terbinafine); (3) polyenes (e.g., nystatin); (4) echinocandins (e.g., caspofungin); and (5) antimetabolites (e.g., flucytosine) that are readily used to combat a spectrum of mycoses including dermatomycoses and candidiases [25,34,35][25][34][35]. Today, these antifungals [22,34,35][22][34][35] are often combined with corticosteroids (see Section 2) of varying potencies [36,37,38][36][37][38] in order to simultaneously alleviate inflammation [39[39][40][41],40,41], diminish the itch-scratch cycle [39[39][40][41][42],40,41,42], limit spreading and subsequently suppress fungal infection [39,40,41][39][40][41]. The combination of topical antifungal actives with corticosteroids represents a potentially hugely beneficial treatment strategy for fungal infection, as the inflammatory component of the infection can often exacerbate the condition and impede its resolution.
2. Topical Antifungal-Corticosteroid Combinations
2.1. Setting the Scene: The Need for Antifungal-Corticosteroid Combinations
During a superficial mycosis or dermatomycosis such as athlete’s foot or jock itch [24[24][25][26][27][28],25,26,27,28], the degradation of keratin via the release of an arsenal of hydrolytic enzymes (including keratinases such as subtilisin-like proteases and dipeptidyl peptidases) [43,44][43][44] triggers a skin-specific immune response [44]. Subsequently, it results in the release of pro-inflammatory mediators such as tumor necrosis factor (TNF) and interleukin 6 (IL-6) among many others [39,44][39][44]. This in turn causes inflammatory symptoms such as pruritus, erythema, swelling and burning at the site of infection. Not only can these symptoms cause substantial discomfort, scratching and even sporadic pain, they can also decrease patient adherence to treatment, ultimately leaving the skin damaged, compromised and susceptible to secondary fungal or bacterial infection, or bacterial superinfection [39]. As such, antifungal treatment strategies should ideally incorporate topical corticosteroids [39,40,41,45][39][40][41][45] to alleviate the inflammation associated with superficial mycoses [39,40,41,45][39][40][41][45]. Such strategies [39,40,41,45][39][40][41][45] should ideally contain an antifungal component to simultaneously treat the fungal infection and a corticosteroid component to manage varying degrees of inflammation and associated pruritus [39,40,41,45][39][40][41][45] (Figure 1). By reducing inflammation and pruritus, topical corticosteroids can reduce the spread of infection and the risk of secondary infection, and ultimately result in a faster and more desirable clinical outcome [39]. It is plausible that the initial suppression of the immune system caused by topical corticosteroid actives causes the infection to temporarily increase its growth rate, ultimately leading to increased uptake of the antifungal active, which in turn produces a more efficient antifungal action.
2.2. The Diversity and Use of Existing Antifungal-Corticosteroid Combinations
Although topical antifungals and corticosteroids are available in a broad range of single-active formulations, there are a relatively small number of topical formulations available that combine the two classes of active, especially for the treatment of superficial dermatomycoses of the feet, scalp, and other regions of the body, for both children and adults [39]. An initial twice-daily application of a topical antifungal plus corticosteroid for 1–2 weeks duration is recommended for localised inflammatory, superficial fungal infections of the body (excluding the areas of the body with thinner skin such as the face and groin) and feet [39]. For mild-to-moderate inflamed infections, an antifungal (preferably azoles such as miconazole and clotrimazole) in combination with a mild-to-moderate corticosteroid such as hydrocortisone or clobetasone is usually sufficient [39,41][39][41]. For example, a clotrimazole-hydrocortisone combination is the preferred treatment option in children with inflammatory superficial mycoses due to the tolerability and safety of topical hydrocortisone [39,46][39][46]. In fact, the efficacy and safety of hydrocortisone in combination with miconazole has long been established: treatment with Daktacort® cream (containing 2% miconazole plus 1% hydrocortisone) in patients with inflamed skin infections of mycotic or bacterial origin induced a significant improvement in suppressing inflammation and was superior to individual treatments with miconazole or hydrocortisone [47]. For severely inflamed infections, an antifungal (again, preferably azole class as isoconazole) in combination with a potent corticosteroid such as diflucortolone, betamethasone, or clobetasol, is recommended [39,41][39][41]. Furthermore, some of these topical combinations also contain antibacterial components such as neomycin and gentamycin to reduce the risk of bacterial superinfection [39,41][39][41]. Some studies [39,41,45][39][41][45] have shown that there is no significant difference in the resolution of Tinea cruris and Tinea corporis infection between treatments using an antifungal-corticosteroid combination (e.g., azole class antifungal-corticosteroid) and an antifungal alone (e.g., azole class) [39,41,45][39][41][45]. However, the key conclusion drawn from all of these studies is that an antifungal-corticosteroid combination demonstrates more rapid therapeutic activity and is more effective in achieving clinical resolution than a topical antifungal alone [39].2.3. Emerging Antifungal-Corticosteroid Combinations: Rationale and Formulation Design
Due to their superiority to other antifungals, azoles (e.g., miconazole) and allylamines (e.g., terbinafine) are widely used in antifungal-corticosteroid combinations. Both types of antifungals are used at different potencies (e.g., clotrimazole 1%, miconazole/miconazole nitrate 2%, terbinafine/terbinafine hydrochloride 1%) and different dosing regimens [45,48][45][48], and both seek to disrupt different parts or intermediate stages of the ergosterol synthesis pathway, resulting in changes to the cell membrane that in turn inhibit fungal growth and thus lead to fungal cell death [20,49,50][20][49][50]. The potency of the corticosteroids used in these types of combination can vary (e.g., hydrocortisone/hydrocortisone acetate 0.5% or 1%, clobetasone/clobetasone butyrate 0.05%, clobetasol/clobetasol propionate 0.05%, mometasone furoate 0.1%), so careful consideration should be given to the appropriate potency for the condition, alongside their efficacy and safety profile [45,51][45][51] (Figure 2).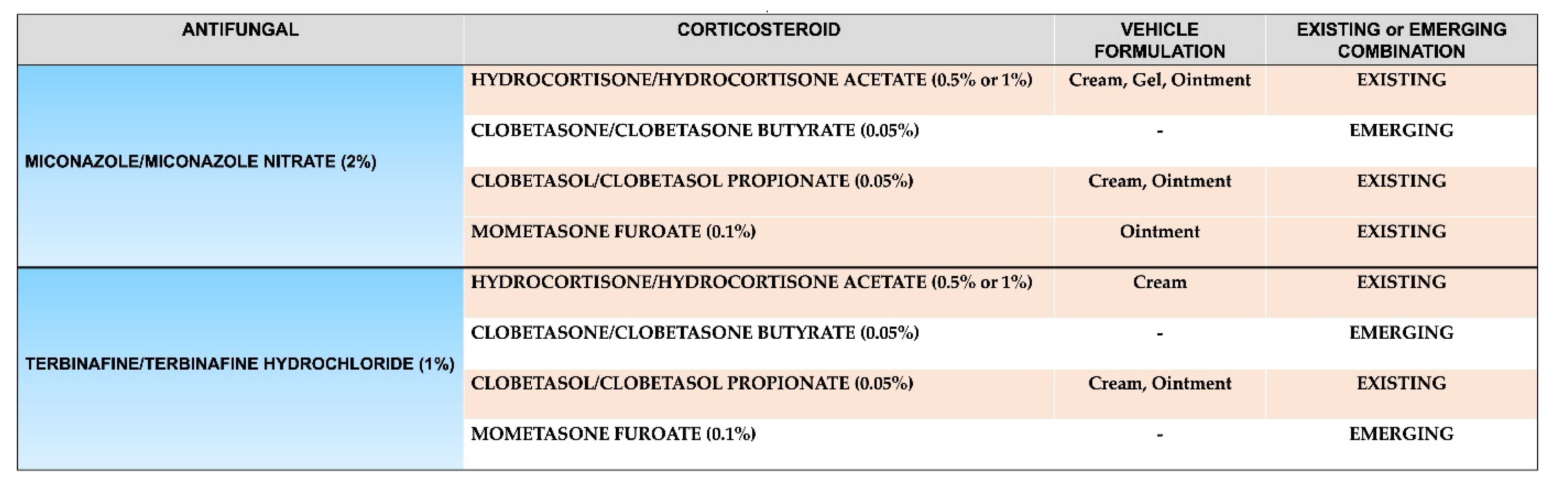
2.4. Choosing a Topical Vehicle Formulation for the Delivery of Antifungal-Corticosteroid Combinations
The ideal topical antifungal-corticosteroid combination [39,41,45,51,52][39][41][45][51][52] is one that produces a rapid and effective anti-inflammatory response, a high-resolution rate, a low relapse rate, has high patient compliance, a short duration of action, and causes minimal or no adverse effects [45]. The choice of topical antifungal-corticosteroid combination administered to a patient should be specific and tailored according to individual needs and must be used appropriately and according to treatment recommendations and guidelines [39,41][39][41]. Single-active vehicle formulations such as creams, ointments and gels that contain either antifungals or corticosteroids alone have been widely used in the treatment of various skin conditions [36,45,48,51,53][36][45][48][51][53]. The choice of vehicle formulation will depend on the specific characteristics of the fungal infection (i.e., the body area infected, the total surface area of the infection and degree of inflammation, and the overall severity) on the one hand and the specific characteristics of the patient (i.e., age and the presence of underlying comorbidities, if any) on the other [39]. The most commonly used vehicle formulations for topical antifungal-corticosteroid combinations are creams, ointments and gels [41,45][41][45].2.4.1. Creams
Creams are emulsions of oil dispersed in water, or less commonly water dispersed in oil. They have good hydrating and emollient qualities; their ability to absorb into the superficial layers of the skin and their relatively high water content makes them cosmetically appealing. Creams are generally less efficacious than ointments when it comes to hydrating capacity due to shorter contact time, and also tend to not be the vehicle of choice for topical drug delivery for the same reason. However, creams are useful in intertriginous areas of the body (e.g., the axilla of the arm) where ointments may not be used. However, creams do not provide the occlusive effects that ointments provide [32,33][32][33].2.4.2. Ointments
On the other hand, ointments generally consist of a ‘greasy’ hydrophobic base, usually white soft paraffin, which forms an occlusive layer over the skin. Ointments are effective in enhancing the percutaneous absorption of topical actives by increasing the hydration, and thus barrier function, of the skin. Their long contact time also usually makes them the vehicle of choice for topical drug delivery, including antifungal-corticosteroid combinations. Compared to creams and gels, ointments are generally the least spreadable of the three. The greasy nature of ointments can sometimes limit patient compliance and they are not always cosmetically appealing, particularly on hair-bearing skin [32,33][32][33].2.4.3. Gels
Gels, which include hydrogels, are usually water-based, and consist of transparent lattices of organic macromolecules. They tend to be thick and liquefy on contact with warm skin, providing a pleasant sensation. They dry to form a thin film that does not stain or leave behind a greasy texture and surface evaporation of the water within the formulation creates a cooling effect, which can be beneficial for skin prone to pruritus. These features make gels one of the more cosmetically pleasing topical vehicles. Gels are both easy to apply and wash off. They are particularly suitable for use in sebum-rich oily areas, such as the face, and also in hairy areas of the body [32,33,54][32][33][54].2.5. Misuse of Topical Antifungal-Corticosteroid Combinations
While the combination of topical antifungals with corticosteroids is an obvious choice in terms of treatment efficacy, it does still pose questions around safety, especially if misuse or even overuse occurs due to inadequate training or regulatory guidance on use. In India for example, combination formulations containing topical antifungals and corticosteroids are easily available over the counter (OTC) and are widely used without strict enforcement of existing drug regulations or supervision by a trained healthcare professional [41]. Furthermore, many of these antifungal-corticosteroid combinations are significantly cheaper than single-active antifungal formulations, and provide quick symptomatic relief, meaning they are often the go-to choice, even if the fungal infection being treated does not have any associated inflammation. These combination formulations account for over 50% of the sales of all topical corticosteroids available on the Indian market [41]. Some local or even systemic side effects have been documented, most often linked to misuse such as the prolonged use of medium to high-potency corticosteroids (e.g., clobetasol), especially on the face, or use on large surface body areas [40,41][40][41]. However, it seems that the issue is not with these topical combinations themselves and any inherent safety concerns, rather, the issue of safety relates to the education of both patients and healthcare professionals as to appropriate usage, and also regulatory oversight to minimise (or even eradicate if possible) misuse and potential overuse.References
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The applied anatomy of human skin: A model for regeneration. Wound Med. 2020, 28, 100179.
- Lefèvre-Utile, A.; Braun, C.; Haftek, M.; Aubin, F. Five functional aspects of the epidermal barrier. Int. J. Mol. Sci. 2021, 22, 11676.
- Swaney, M.H.; Kalan, L.R. Living in your skin: Microbes, molecules, and mechanisms. Infect. Immun. 2021, 89, e00695-20.
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and physiology of the skin. J. Dermatol. Nurses Assoc. 2011, 3, 203–213.
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98.
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the skin’s immune system. Int. J. Mol. Sci. 2019, 20, 1811.
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155.
- Flowers, L.; Grice, E.A. The skin microbiota: Balancing risk and reward. Cell Host Microbe 2020, 28, 190–200.
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in exploring and manipulating the human skin microbiome. Microbiome 2021, 9, 125.
- Tiew, P.Y.; Mac Aogain, M.; Ali, N.A.B.M.; Thng, K.X.; Goh, K.; Lau, K.J.X.; Chotirmall, S.H. The mycobiome in health and disease: Emerging concepts, methodologies and challenges. Mycopathologia 2020, 185, 207–231.
- Zhu, T.; Duan, Y.-Y.; Kong, F.-Q.; Galzote, C.; Quan, Z.-X. Dynamics of skin mycobiome in infants. Front. Microbiol. 2020, 11, 1790.
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human skin microbiome: Impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms 2021, 9, 543.
- Dorrestein, P.C.; Gallo, R.L.; Knight, R. Microbial skin inhabitants: Friends forever. Cell 2016, 165, 771–772.
- Limon, J.J.; Skalski, J.H.; Underhill, D.M. Commensal fungi in health and disease. Cell Host Microbe 2017, 22, 156–165.
- Dimitriu, P.A.; Iker, B.; Malik, K.; Leung, H.; Mohn, W.W.; Hillebrand, G.G. New insights into the intrinsic and extrinsic factors that shape the human skin microbiome. mBio 2019, 10, e00839-19.
- White, T.C.; Findley, K.; Dawson, T.L., Jr.; Scheynius, A.; Boekhout, T.; Cuomo, C.A.; Xu, J.; Saunders, C.W. Fungi on the skin: Dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 2014, 4, a019802.
- Talaga, K.; Krzyściak, P. Non-lipophilic mycobiota of human skin. Acta Micol. 2015, 50, 1068.
- Hilles, A.R.; Mahmood, S.; Kaderi, M.A.; Hashim, R. Review of fungal skin infections and their invasion. Fungal Territory 2019, 2, 3–5.
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 2020, 10, 2993.
- Dupont, S.; Lemetais, G.; Ferreira, T.; Cayot, P.; Gervais, P.; Beney, L. Ergosterol biosynthesis: A fungal pathway for life on land? Evolution 2012, 66, 2961–2968.
- Ghosh, A.; Gharti Magar, D.; Thapa, S.; Nayak, N.; Talwar, O.P. Histopathology of important fungal infections–a summary. J. Pathol. Nepal 2019, 9, 1490–1496.
- Ivanov, M.; Ćirić, A.; Stojković, D. Emerging antifungal targets and strategies. Int. J. Mol. Sci. 2022, 23, 2756.
- Zhang, X.; Yin, M.; Zhang, L.-J. Keratin 6, 16 and 17-critical barrier alarmin molecules in skin wounds and psoriasis. Cells 2019, 8, 807.
- Ridzuan, P.M.; Nazira, C.M.; Ruth, M.; Abdul Rassip, C.N.; Nur Raihan, M.H.; Ismail, S.A.; Rahman, N.I.; Suzima, E.A.; Azhan, H. Mini review on dermatomycosis. J. Sci. Math. Lett. 2019, 8, 6–15.
- Kaur, N.; Bains, A.; Kaushik, R.; Dhull, S.B.; Melinda, F.; Chawla, P. A Review on antifungal efficiency of plant extracts entrenched polysaccharide-based nanohydrogels. Nutrients 2021, 13, 2055.
- Jartarkar, S.R.; Patil, A.; Goldust, Y.; Cockerell, C.J.; Schwartz, R.A.; Grabbe, S.; Goldust, M. Pathogenesis, immunology and management of dermatophytosis. J. Fungi 2021, 8, 39.
- Kaushik, N.; Pujalte, G.G.; Reese, S.T. Superficial fungal infections. Prim. Care 2015, 42, 501–516.
- Kovitwanichkanont, T.; Chong, A.H. Superficial fungal infections. Aust. J. Gen. Pract. 2019, 48, 706–711.
- De Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; de Sousa Cartágenes, M.D.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Front. Microbiol. 2018, 9, 1351.
- Nobile, C.J.; Johnson, A.D. Candida albicans biofilms and human disease. Annu. Rev. Microbiol. 2015, 69, 71–92.
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Cellular, genomic and metabolic complexity. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1198–1232.
- Mayba, J.N.; Gooderham, M.J. A guide to topical vehicle formulations. J. Cutan. Med. Surg. 2018, 22, 207–212.
- Barnes, T.M.; Mijaljica, D.; Townley, J.P.; Spada, F.; Harrison, I.P. Vehicles for drug delivery and cosmetic moisturizers: Review and comparison. Pharmaceutics 2021, 13, 2012.
- Prasad, R.; Shah, A.H.; Rawal, M.K. Antifungals: Mechanism of action and drug resistance. In Yeast Membrane Transport: Advances in Experimental Medicine and Biology; Ramos, J., Sychrová, H., Kschischo, M., Eds.; Springer Nature: Basel, Switzerland, 2016; Volume 892, pp. 327–349.
- Gintjee, T.J.; Donnelley, M.A.; Thompson, G.R., 3rd. Aspiring antifungals: Review of current antifungal pipeline developments. J. Fungi 2020, 6, 28.
- Ference, J.D.; Last, A.R. Choosing topical corticosteroids. Am. Fam. Phys. 2009, 79, 135–140. Available online: https://www.aafp.org/afp/2009/0115/p135.html (accessed on 11 March 2022).
- Mehta, A.B.; Nadkarni, N.J.; Patil, S.P.; Godse, K.V.; Gautam, M.; Agarwal, S. Topical corticosteroids in dermatology. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 371–378.
- Aung, T.; Aung, S.T. Selection of an effective topical corticosteroid. Aust. J. Gen. Pract. 2021, 50, 651–655.
- Schaller, M.; Friedrich, M.; Papini, M.; Pujol, R.M.; Veraldi, S. Topical antifungal-corticosteroid combination therapy for the treatment of superficial mycoses: Conclusions of an expert panel meeting. Mycoses 2016, 59, 365–373.
- Verma, S.; Madhu, R. The great Indian epidemic of superficial dermatophytosis: An appraisal. Indian J. Dermatol. 2017, 62, 227–236.
- Rana, P.; Ghadlinge, M.; Roy, V. Topical antifungal-corticosteroid fixed-drug combinations: Need for urgent action. Indian J. Pharmacol. 2021, 53, 82–84.
- Harrison, I.P.; Spada, F. Breaking the itch-scratch cycle: Topical options for the management of chronic cutaneous itch in a dermatitis. Medicines 2019, 6, 76.
- Mercer, D.K.; Stewart, C.S. Keratin hydrolysis by dermatophytes. Med. Mycol. 2019, 57, 13–22.
- Burstein, V.L.; Beccacece, I.; Guasconi, L.; Mena, C.J.; Cervi, L.; Chiapello, L.S. Skin immunity to dermatophytes: From experimental infection models to human disease. Front. Immunol. 2020, 11, 605644.
- El-Gohary, M.; van Zuuren, E.J.; Fedorowicz, Z.; Burgess, H.; Doney, L.; Stuart, B.; Moore, M.; Little, P. Topical antifungal treatments for Tinea cruris and Tinea corporis. Cochrane Database Syst. Rev. 2014, 8, CD009992.
- Lenane, P.; Macarthur, C.; Parkin, P.C.; Krafchik, B.; DeGroot, J.; Khambalia, A.; Pope, E. Clobetasol propionate, 0.05%, vs hydrocortisone, 1%, for alopecia areata in children: A randomized clinical trial. JAMA Dermatol. 2014, 150, 47–50.
- Mertens, R.L.; Morias, J.; Verhamme, G. A double-blind study comparing Daktacort, miconazole and hydrocortisone in inflammatory skin infections. Dermatologica 1976, 15, 228–235.
- Crawford, F.; Hollis, S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst. Rev. 2007, 3, CD001434.
- Meis, J.F.; Verweij, P.E. Current management of fungal infections. Drugs 2001, 61, 13–25.
- Martinez, L.; Falson, P. Multidrug resistance ATP-binding cassette membrane transporters as targets for improving oropharyngeal candidiasis treatment. Adv. Cell. Mol. Otolaryngol. 2014, 2, 23955.
- Spada, F.; Barnes, T.M.; Greive, K.A. Comparative safety and efficacy of topical mometasone furoate with other topical corticosteroids. Aust. J. Dermatol. 2018, 59, e168–e174.
- European Patent Office. Topical Composition Comprising Terbinafine and Hydrocortisone. Available online: https://patents.google.com/patent/EP1656125A2/en (accessed on 11 March 2022).
- Carlos, G.; Uribe, P.; Fernandez-Penas, P. Rational use of topical corticosteroids. Aust. Prescrib. 2013, 36, 158–161.
- Harrison, I.P.; Spada, F. Hydrogels for atopic dermatitis and wound management: A superior drug delivery vehicle. Pharmaceutics 2018, 10, 71.
More
