2.3. Macula, Retinal Nerve Fiber Layer and Visual Pathways Involvement
Correlation between brain MRI lesions and impairment of visual pathways, macula and retinal nerve fiber layer (RNFL) was found by Langwinska-Wosko et al.
[37][44]. They compared 58 WD patients mean age 38.7 years, with or without brain lesions on MRI (39 MRI+ and 19 MRI−, respectively) and 30 healthy controls (mean age 39.6 years). Total RNFL measured spectral-domain optical coherence tomography (SD-OCT) was thinner in WD patients MRI+ than WD patients MRI− (
p = 0.001). Central macular thickness (CMT) was also significantly thinner in WD patients MRI+ than WD patients MRI− (
p < 0.001). No significant difference was found in RNFL or CMT between WD patients MRI− and controls. Latency of visual evoked potentials (PEV) and electroretinography (ERG) were prolonged in WD patients MRI+ compared to WD patients MRI− (
p < 0.001 and
p < 0.001, respectively). Interestingly, some WD patients MRI− had electrophysiological abnormalities. These results confirmed what had been already demonstrated by a German team in 2012
[38][45]. An Indian team has also shown prolonged latencies in PEV and ERG in WD patients with neurological manifestations compared to controls and the improvement of PEV and ERG latencies after treatment of WD
[39][46].
2.4. Eye Mobility
WD is responsible for eye movement abnormalities such as slow horizontal and vertical saccades
[23][40][41][23,49,50], abnormal vertical smooth pursuit
[42][51], increased antisaccadic latency and error rate
[43][52]. Ingster-Moati et al. found that 91% of 34 WD patients (mean age 29 years, 24 neurological forms, 9 hepatic forms and 1 asymptomatic patient) had abnormalities of ocular motility detected by electro-oculography. Here, 29 patients (85%) had an abnormal vertical smooth pursuit, 41% a vertical optokinetic nystagmus and 41% an impaired horizontal smooth pursuit. Among the 27 who underwent MRI, seven patients had normal brainstem and lenticular nuclei images despite the detection of ocular motility abnormalities
[42][51]. Brain MRI of WD patients showed a strong association between prolonged latencies of prosaccades and the brainstem atrophy (r = −0.53 and
p = 0,02 for horizontal latencies and r = 0.47 and
p = 0.004 for vertical maximum speed in prosaccades, respectively). Impairment in eye movement is probably secondary to the lesions induced by the copper deposit in the brainstem as the nerve centers responsible for vertical and horizontal eye tracking are located in the midbrain and the pons, respectively.
3. Conclusions
Ocular manifestations may be the first presenting symptoms of WD, which must be recognized to prevent fatal outcomes.
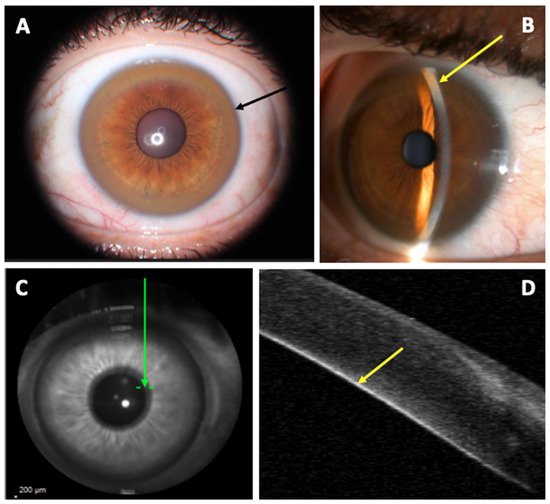
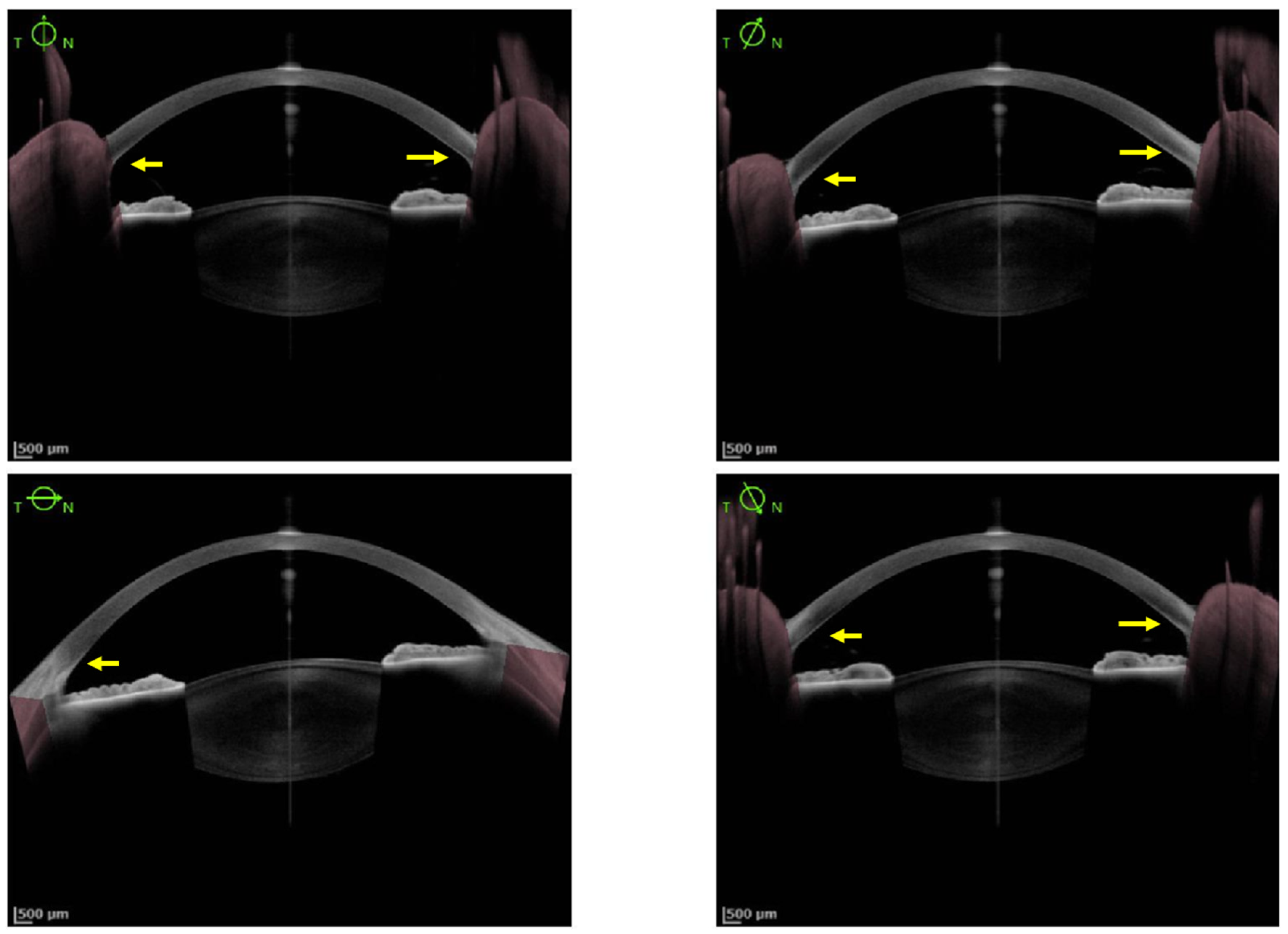
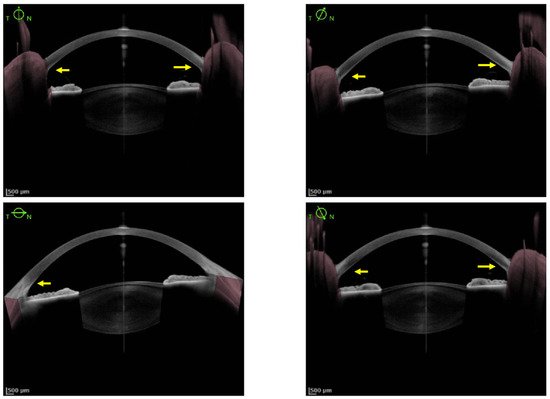
As KFR is an essential criterion for the diagnosis of WD, multiple methods have been studied to improve its diagnosis. So far, SLE is the gold standard for detection of KFR, but its Se is low and it requires experimented ophthalmologists. Since 2016, AS-OCT studies demonstrated a better Se and Sp to diagnose KFR compared to SLE. Its interpretation appears easier and more accessible for non-experimented ophthalmologists and non-ophthalmologist practitioners
[28][30][30,32]. The density of copper deposit in the cornea at the diagnosis and during the follow-up of WD could determine the severity of the disease and the response under chelator treatment
[28][30]. Pentacam HR Scheimpflug imaging using ImageJ software and calculation of the ratio between anterior and posterior peak signal presents also a good Se (96%) and specificity (Sp, 95%) to detect KFR. Nevertheless, the utilization of those methods to assess therapeutic efficacy needs further evaluation.
It appears that ophthalmological involvement is frequent in WD patients, in particular for the KFR and, to a lesser extent, sunflower cataracts. Other manifestations involving retinal and visual systems, eye mobility or other structures of the eye have been described with various frequencies. AV is nearly often preserved despite corneal or neurological involvement. The evolution of ophthalmologic manifestations seems to be correlated with decoppering treatment, especially for KFR and sunflower cataracts. New methods like AS-OCT and Scheimpflug imaging are alternatives to traditional SLE. These methods allow non-ophthalmologists to look for and quantify KFR more easily and are useful tools to follow the evolution of these abnormalities under chelating treatment. In the near future, the recent development of AI in the analysis of ophthalmic imaging will probably be helpful for the screening of WD anomalies.